Introduction
The incidence of diabetes mellitus (DM) is
increasing worldwide relentlessly and rapidly. Type 2 diabetes
mellitus (T2DM) accounts for 90–95% of all cases. It was estimated
that 92.4 million adults in China (20 years and older) suffered
from diabetes in 2010 while a further 148 million people were in
the pre-diabetic state (1).
Diabetic nephropathy (DN) is the primary microvascular complication
of DM, and is also a major cause of chronic renal failure. It is
estimated that approximately 50% of DM patients will suffer
complications with DN within ten years of diagnosis, and the
proportion of cases of DN in end-stage renal failure (ESRF) has
been increasing every year (2). In
the United States, DN is the leading cause of ESRF (3). In addition, the speed of development
to ESRF in patients with DN is approximately 14 times as fast as
that of normal kidney diseases (4).
The pathogenesis of DN is complex and has not yet
been fully elucidated. Hyperglycemia has been proven to be the
major driving force of the progression of DN (5). A previous study has indicated that
oxidative stress is one of the mechanisms by which hyperglycemia
causes DN (6). Oxidative stress
refers to the imbalance between the production of reactive oxygen
species (ROS) and the ability of endogenous antioxidative systems
to scavenge these ROS (7). It is a
systemic process which may occur in any tissue. Under normal
conditions, ROS produced in the cellular metabolism is efficiently
scavenged by the intrinsic antioxidant defense mechanisms. However,
in the hyperglycemic state of DM, excessive ROS are produced in
renal tissue due to the damaged scavenging ability. Excessive ROS
leads to renal histological changes and functional abnormalities of
oxidizing lipids, proteins and nucleic acids. A previous study has
revealed that oxidative stress plays a critical role in the
development and progression of DN (8). Interventions could ameliorate DN
through the reduction of oxidative stress and the increase of renal
antioxidant enzyme activity (9).
Inflammation is a further significant risk factor of
DN, and has been scrutinized in recent years. Currently,
anti-inflammatory drugs are used in the management of DN. Studies
have reported that kidney inflammation is crucial in promoting the
development and progression of DN (10). Inflammation may be a key factor
which is activated by the metabolic, biochemical and hemodynamic
derangements known to exist in the diabetic kidney (11). Hyperglycemia, advanced
glycoxidation end products (AGEs) and immune complexes in DM
decrease the release of chemokines and the upregulation of cell
adhesion molecules (12). These
events promote the infiltration of renal monocytes and lymphocytes.
One study on drugs which have significant effects on DN has
reported that these drugs work through their anti-inflammatory
effects (13). However, existing
drugs only partially alleviate the inflammatory response, and do
not deter the progress of DN. If efficacy and safety are taken into
consideration, there are few anti-inflammation drugs that truly
function in the treatment of DN patients. Therefore, it is of
particular urgency to identify chemicals which are safe and
effective to combat DN.
Grape seed proanthocyanidin extract (GSPE) is a
potent antioxidant extracted from grape seeds and skins. GSPE is
reported to be highly bioavailable and provides significantly
greater protection against the damage of oxidative stress than
vitamins C and E and β-carotene (14). GSPE may be rapidly absorbed by the
gastrointestinal tract and reaches a peak level after 45 min. It
has a half-life of 5 h. Fourteen percent of it is evacuated by the
biliary system in 11 h while 70% is evacuated in the form of
CO2, urine and feces after 24 h. Another study
demonstrated that GSPE mediated a protective effect against
doxorubicin-induced cardiac injury through antioxidant,
anti-inflammatory and antiapoptotic mechanisms (15). Therefore, the use of GSPE against
oxidative stress and inflammation offers the possibility of a
protective effect on renal injury induced by DM. Previous studies
have demonstrated that GSPE protects against renal injury induced
by T1DM (16). However, there is
limited information on its effects on renal injury induced by T2DM.
This study aims to identify its effects and possible
mechanisms.
Materials and methods
Materials and reagents
GSPE (lot no. 1003007-24) was donated by JF-Natural
Limited Company (Tianjin, China). The proanthocyanidin content was
95% when analyzed using high-performance liquid chromatography with
gas chromatography-mass spectrometry detection. It contained 56%
dimers, 12% trimers, 6.6% tetramer and small amounts of other
polymeric forms. The high-carbohydrate/high-fat diet was purchased
from Beijing Ke’ao Limited Company (Beijing, China), and included
66% basal diet, 15% lard, 10% sucrose, 6% casein and 3% egg
yolk.
Commercial kits used for the determination of
superoxide dismutase (SOD), catalase (CAT), malondialdehyde (MDA)
and protein in kidney were purchased from Jiancheng Institute of
Biotechnology (Nanjing, China). The antibodies of tumor necrosis
factor-α (TNF-α), monocyte chemoattractant protein-1 (MCP-1),
intercellular adhesion molecule-1 (ICAM-1) and tissue inhibitor of
metalloproteinase-1 (TIMP-1) were purchased from Zhongshanjinqiao
Biotechnology Limited Company (Beijing, China). Matrix
metalloproteinase-9 (MMP-9) was purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Ethanol, acetaldehyde
and isopropanol were of analytical grade and purchased from Beijing
Chemical Company (Beijing, China).
Animals and treatment
Seventy male Sprague-Dawley rats (6 weeks old,
weighing 200–250 g) were obtained from the Animal Service of the
Health Science Center, Peking University, China. The rats were kept
in a filter-protected, air-conditioned room with controlled
temperature (21–25°C), relative air humidity (40–50%) and a 12-h
light/12-h dark cycle (lights on between 07.30 and 19.30). Animal
treatment and maintenance were carried out strictly in accordance
with the Principles of Laboratory Animal Care (NIH publication no.
85–23, 1985) and the guidelines of the Peking University Animal
Research Committee.
Experimental design
Rats were randomly divided into two groups. Group 1
(n=12, normal control) was fed the basal diet. Group 2 (n=58) was
fed a high-carbohydrate/high-fat diet for 4 weeks. Then after
fasting for 15 h, rats in group 2 were intraperitoneally injected
with streptozotocin (STZ; 0.01 mol/l, 30 mg/kg·bw). Another STZ
injection was administered after 7 days. STZ was freshly dissolved
in ice-cold citrate buffer (pH 4.5). Rats in group 1 were injected
with the citrate buffer vehicle at the same time. Fourteen days
after the STZ injection, the fasting serum glucose of rats in group
2 was tested. Diabetes was defined as fasting serum glucose
>11.1 mmol/l in caudal vein blood and rats having the symptoms
of polyuria, polyphagia and emaciation. A total of 48 rats in group
2 satisfied the above conditions and were used in the next
experiments. The diabetic rats (n=48) were randomly divided into
four groups (n=12 in each): one DM control group and three groups
for low (125 mg/kg·bw), medium (250 mg/kg·bw) and high (500
mg/kg·bw) doses of GSPE. Distilled water was used as the solvent
for GSPE. The rats in the GSPE intervention groups were given GSPE
intragastrically while the others received an equal volume of
distilled water during the experimental period. The study lasted
for a total of 16 weeks. Food consumption, water intake and urine
volume of rats were measured through metabolic cages. Systolic
blood pressures of all rats were measured. Blood was collected from
the femoral artery and the rats were then sacrificed by cervical
dislocation after collecting 24 h volume urine. Serum was separated
by centrifugation at 3,000 rpm for 15 min. Portions of the kidney
were then obtained for biochemical assays.
Biochemical assays
Systolic blood pressure was measured by the BP-98A
intelligent non-invasive blood pressure monitor (Softron
Biotechnology Limited Company, Beijing, China). The levels of
fasting blood glucose (FBG) and high-sensitivity c-reactive protein
(hs-CRP) in serum were detected using an automatic biochemistry
analyzer (Hitachi, Tokyo, Japan), as were serum creatinine (SCr)
and blood urea nitrogen (BUN) in urine. SOD and CAT activity and
the MDA level in renal tissues were determined by detection kits
according to the manufacturer’s instructions.
Western blot analysis
Total protein was extracted from the frozen renal
tissues using RIPA lysis buffer (1% Triton X-100, 1% deoxycholate,
0.1% SDS) and 1 mM phenylmethanesulfonyl fluoride. Following
ultrasonication for 5 min, extracts were centrifuged at 12,000 rpm
for 15 min at 4°C, and the supernatants containing protein were
retained. The concentrations of protein were measured with the
bicinchoninic acid method (Beyotime®, Institute of
Biochemistry, China). Then 40-μg protein samples were resolved on
15% Tris-glycine polyacrylamide minigels for TNF-α, MCP-1 and
TIMP-1 and 5% for ICAM-1 and MMP-9, and then transferred to
polyvinylidene fluoride (PVDF) membranes. Membranes were blocked
with 5% skimmed milk in TBST for 1 h. Primary antibodies were used
overnight at 4°C and dilutions were as follows: TNF-α (1:200),
MCP-1 (1:200), TIMP-1 (1:200), MMP-9 (1:200) and ICAM-1 (1:500),
all from Santa Cruz. Secondary antibodies were used at the
following dilutions: goat antibody to mouse IgG (1:5000) and goat
anti-rabbit (1:5000), both from ZSGB-BIO, Beijing, China. Signals
were visualized using the enhanced chemiluminescence system and
analyzed densitometrically using Image Pro Plus version 6.0
software (Media Cybernetics, Inc., Silver Spring, MD, USA). PVDF
membranes were reprobed with β-actin (at 1:1000, Cell Signaling
Technology, Inc., Beverly, MA, USA) to verify equal protein loading
on the gel.
Statistical analysis
The data are expressed as the mean ± standard
deviations. The analyses were carried out with SPSS 13.0
statistical software (SPSS, Inc., Chicago, IL, USA). Data were
first tested for homogeneity and then evaluated by means of a
one-way ANOVA test followed by Tukey’s post hoc least significant
difference test, if variances were equal, or Tamhane’s T3 test, if
variances were not equal. P<0.05 was considered to indicate a
statistically significant result.
Results
Body weight, food consumption, water
intake and urine volume values
As shown in Table
I, the body weight was lower in DM rats than in normal rats at
the end of the study (P<0.05). Food consumption, water intake
and urine volume were increased in DM rats compared with normal
rats throughout the study period (P<0.01 for each). However,
GSPE treatment slightly increased body weight and decreased food
consumption, water intake and urine volume in a dose-dependent
manner.
 | Table IEffect of grape seed proanthocyanidin
extract on body weight, food intake, water intake and urine volume
in rats after 16 weeks. |
Table I
Effect of grape seed proanthocyanidin
extract on body weight, food intake, water intake and urine volume
in rats after 16 weeks.
| Parameters | Normal rats | DM rats | Low GSPE rats | Medium GSPE
rats | High GSPE rats |
|---|
| Initial body weight
(g) | 425.70±5.87 | 442.
23±10.15a | 452.43±12.78 | 440.22±8.17 | 454.50±9.42 |
| Final body weight
(g) | 585.38±13.23 | 415.78±9.45a | 434.53±10.89 |
447.65±12.74c |
450.63±11.67c |
| Initial food intake
(g/d) | 16.86±0.88 | 23.43±1.24a | 19.16±1.56 | 21.13±0.98 | 20.16±1.42 |
| Final food intake
(g/d) | 15.50±0.56 | 24.67±1.55b | 27.50±1.01 | 20.75±1.32c | 18.14±1.42c |
| Initial water
intake (ml/d) | 40.21±4.32 | 80.76±5.05a | 78.14±9.97 | 82.14±7.74 | 86.13±6.94 |
| Final water intake
(ml/d) | 35.77±5.11 |
121.67±11.67b | 115.71±8.65 | 78.59±5.67d | 71.57±5.43d |
| Initial urine
volume (ml/d) | 7.57±1.73 | 36.50±8.39b | 33.26±6.38 | 40.58±2.32 | 38.85±3.59 |
| Final urine volume
(ml/d) | 6.71±1.95 | 100.80±9.96b | 90.13±10.55 | 70.83±10.50c | 55.75±5.12d |
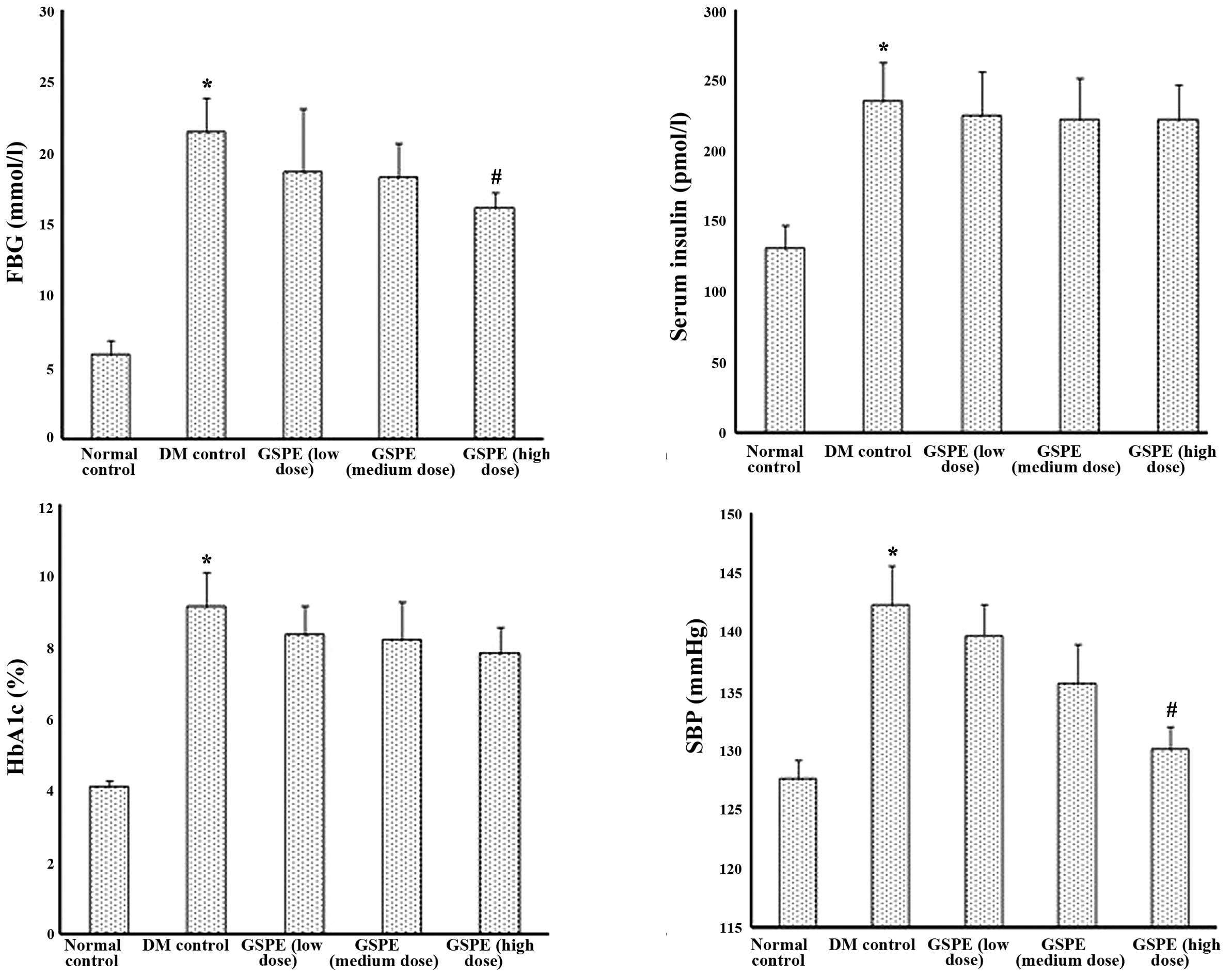
Fasting blood glucose, serum insulin,
HbA1c and systolic blood pressure values
As shown in Fig. 1,
FBG, serum insulin, HbA1c and systolic blood pressure were markedly
increased in DM control rats compared with normal control rats
(P<0.05). Following administration of GSPE (particularly at a
dose of 500 mg/kg·bw), the above parameters were decreased to a
certain extent.
Renal function index values
BUN, SCr, creatinine clearance (CCr), 24-h urine
protein and kidney index (kidney weight/body weight) were used to
evaluate renal injury. From Table
II it may be noted that some renal injury occurred in DM
control rats including renal hypertrophy and renal dysfunction
(P<0.01 or P<0.05 compared with normal control rats).
However, GSPE treatment was able to improve these renal function
parameters (P<0.01 or P<0.05).
 | Table IIEffect of grape seed proanthocyanidin
extract on 24-h urine protein, blood urea nitrogen, serum
creatinine, creatinine clearance and renal mass in rats. |
Table II
Effect of grape seed proanthocyanidin
extract on 24-h urine protein, blood urea nitrogen, serum
creatinine, creatinine clearance and renal mass in rats.
| Parameters | Normal rats | DM rats | Low GSPE rats | Medium GSPE
rats | High GSPE rats |
|---|
| 24-h urine protein
(mg) | 77.96±3.83 | 524.76±8.75b | 539.50±6.30 | 445.39±4.59c | 231.04±4.07d |
| BUN (mmol/l) | 4.38±0.26 | 12.25±5.09b | 7.97±0.89 | 7.15±0.77 | 5.12±1.56d |
| SCr (μmol/l) | 30.38±8.39 | 48.47±10.32a | 43.12±9.66 | 39.63±11.21 | 31.12±9.95d |
| CCr (mmol/l) | 2.03±0.23 | 6.79±1.04b | 6.03±0.53 | 5.07±0.60 | 3.35±1.01d |
| Kidney weight/body
weight (%) | 0.46±0.02 | 0.76±0.02b | 0.82±0.02 | 0.74±0.04 | 0.54±0.02d |
MMP-9 and TIMP-1 expression in renal
tissues
As shown in Fig. 2,
low-dose streptozotocin and a high-carbohydrate/high-fat diet
treatment significantly decreased the expression of MMP-9 and
increased the expression of TIMP-1 compared with that observed in
the normal control rats (P<0.05). The 500 mg/kg·bw GSPE
treatment markedly increased the expression of MMP-9 and reduced
the expression of TIMP-1 when compared with diabetic control rats
(P<0.05).
SOD and CAT activity and MDA levels in
renal tissues
Parameters of oxidative stress are shown in Table III. The activity of SOD and CAT
in the kidneys of the DM control rats was significantly lower than
that in normal control rats (P<0.01) while MDA levels were
significantly higher (P<0.05). The SOD and CAT activity in
high-dose GSPE rats was significantly higher than that in DM
control rats (P<0.01 and P<0.05, respectively). The MDA
levels in high-dose GSPE-treated rats were significantly lower than
those in DM control rats (P<0.01).
 | Table IIIEffect of grape seed proanthocyanidin
extract on superoxide dismutase and catalase activity and
malondialdehyde levels in renal tissues of rats. |
Table III
Effect of grape seed proanthocyanidin
extract on superoxide dismutase and catalase activity and
malondialdehyde levels in renal tissues of rats.
| Parameters | Normal rats | DM rats | Low GSPE rats | Medium GSPE
rats | High GSPE rats |
|---|
| SOD (U/mg·pro) | 69.45±5.27 | 53.71±3.45b | 60.72±10.10 | 62.58±4.35 | 67.78±7.11d |
| CAT (U/mg·pro) | 38.46±2.75 | 28.01±2.64b | 30.70±2.76 | 32.38±5.14 | 35.65±2.32c |
| MDA
(nmol/mg·pro) | 0.59±0.18 | 1.15±0.31a | 0.82±0.16 | 0.72±0.16 | 0.53±0.25d |
Serum high sensitivity c-reactive protein
levels and expression of TNF-α, MCP-1 and ICAM-1 in renal
tissues
Parameters of inflammation were determined as shown
in Figs. 3 and 4. Compared with normal control rats,
serum hs-CRP in DM control rats was significantly increased
(P<0.01), while in GSPE medium- and high-dose groups it was
significantly lower than in DM control rats (P<0.01).
Inflammatory cytokines, including TNF-α, MCP-1 and ICAM-1, were all
significantly elevated in the kidneys of DM control rats when
compared with normal controls (P<0.05 for each). However, GSPE
(particularly at a dose of 500 mg/kg·bw) decreased their expression
when compared with the DM control group (P<0.05 for each).
Discussion
Due to its poor prognosis and the rapidly increasing
number of cases, type 2 DN has raised great concern. In this study,
we successfully built a T2DM model of rats using a
high-carbohydrate/high-fat diet for 4 weeks and two low-dose
streptozotocin injections. Following 16 weeks of treatment, GSPE
was demonstrated to improve renal function, decrease the levels of
FBG, serum insulin, HbA1c and systolic blood pressure, and suppress
oxidative stress and inflammatory response.
At the end of this study, nephritic structural and
functional damages were observed in diabetic control rats as
demonstrated by the BUN, SCr, endogenous CCr, 24-h proteinuria,
kidney weight/body weight, and expression of MMP-9 and TIMP-1.
Increased glomerular filtration rate and proteinuria are the main
physical signs of DN. BUN, SCr, CCr and 24-h urine protein are the
main indicators of renal function. In the present study, the BUN,
SCr, CCr and 24-h urine protein of DM rats were significantly
higher than those in normal rats, which implied glomerular
hyperfiltration and renal dysfunction in the DM rats. However, GSPE
improved these renal function parameters. This is similar to the
results observed in a previous study by Li et al (17). These authors also indicated that
GSPE was capable of decreasing proteinuria, attenuating the
progression of nephropathy in diabetic rats.
Glomerular extracellular matrix (ECM) accumulation
and base-membrane thickness are the main pathological changes in DN
(18). MMP-9 is considered to be
essential for ECM turnover in kidneys, and the downregulation of
MMP-9 levels has been demonstrated in streptozotocin-induced
diabetic rats (19,20). Previous studies have indicated that
an imbalance between MMPs and their inhibitors (TIMPs) contributes
to nephropathy and that they may be potential targets for
therapeutic interventions (21,22).
In the present study, decreased MMP-9 activity and increased TIMP-1
activity were observed in diabetic rats. GSPE promoted the
upregulation of MMP-9 and downregulation of TIMP-1, which may be a
potential mechanism of the protective effect. This finding may be
significant as there are few reports on MMPs and their inhibitors
in studies involving GSPE in DN.
Hyperglycemia is a typical feature of DM, which is
also one of the most common symptoms. Long-term hyperglycemia is
the main cause of DN, thereby imposing a great risk to patients.
One prospective diabetes study from the UK demonstrated that the
risk of microalbuminuria and clinical DN in DM patients could be
significantly reduced if intensive treatment was administered to
control the levels of blood glucose (23). Pinent et al (24) observed that GSPE had a hypoglycemic
effect on STZ-induced diabetic rats by delaying the absorption of
glucose in intestines. In addition, El-Alfy et al (25) demonstrated that GSPE could lower
hyperglycemia in mice induced by alloxan monohydrate in an
antagonistic manner. GSPE, as an effective antioxidant, protects
islet tissue from damage, and may also promote residual islet B
cells to secrete insulin. A previous study revealed that 250
mg/kg·bw GSPE could not decrease FBG and HbAlc levels in diabetic
rats (26). In the present study,
we noted that 500 mg/kg·bw GSPE slightly decreased the levels of
FBG.
Hypertension is always accompanied by hyperglycemia,
and is another major cause of DN. Glomerular capillary pressure due
to high blood pressure may cause increased capillary tension and
thickening of the arterial wall, thus leading to ischemia and
endothelial cell damage. Then mesangial cells and matrix
proliferate and promote glomerular metabolism and compensatory
hypertrophy, which eventually results in glomerulosclerosis. This
damage could cause the blood pressure to increase in turn, creating
a vicious cycle thereafter. Therefore, controlling the blood
pressure to a normal level is also essential in the treatment of
DN. In our study, the systolic blood pressure in GSPE-treated rats
was significantly lower than that in normal rats, which indicated
that GSPE was able to reduce blood pressure. Cui et al
(27) fed ouabain-induced
hypertensive rats with GSPE and noted a lowered blood pressure,
making GSPE a potential natural reagent in hypertension treatment.
Li et al (17) also
observed a similar result. GSPE may enhance the production and
release of nitric oxide, reduce the expression of endothelin-1 in
the vascular endothelium, and thus have an effect on hypertension
(28).
Increased oxidative stress in DN induces a number of
ROS, including peroxide hydrogen (H2O2),
superoxide anion (O2−) and hydroxyl radical (·OH). ROS
activate nuclear transcription factor-κB and activator protein-1 by
activating protein kinase C (PKC) and mitogen-activated protein,
which results in large amounts of cytokines and growth factors in
the kidney (29,30). Microvascular endothelial cells are
damaged as a result, and a number of negative consequences induced
by oxidative stress, such as mitochondrial DNA deletions, will
accelerate the development of DN (31,32).
Numerous studies have suggested that antioxidant therapy reduces
renal injury in DN (33–35). SOD is the main antioxidant enzyme
in vivo, while CAT is an enzyme scavenger decomposing
hydrogen peroxide into hydrogen and water, and MDA is the most
abundant by-product of lipid peroxidation. These three may be
considered as indicators of oxidative stress. The present results
reveal that DM rats possessed a higher MDA level and lower SOD and
CAT activity levels in the kidney than those of normal control
rats, which confirmed the involvement of oxidative stress in renal
injury induced by DM. It was also noted that GSPE increased the
activity of SOD and CAT, but decreased the levels of MDA. In other
words, GSPE reduced the levels of ROS and protected the kidneys
from injury induced by oxidative stress, which may be one of the
potential mechanisms by which it exerts its beneficial effects on
DN.
Possibly the most significant results in this study
were the notable anti-inflammatory effects of GSPE and its pivotal
role in the treatment of DN. An increasing number of inflammatory
signal pathways and cytokines are being investigated and deemed new
molecular targets for treating DN. In the progress of DN, certain
pro-inflammatory cytokines and ROS become activated, which induces
mesangial cells to secrete type IV collagen, laminin and
fibronectin, and leads to glomerulosclerosis (36,37).
Moreover, inflammation is involved in the activation of certain
signal transduction pathways in vivo, including the polyol,
AGE, ROS and PKC pathways, which promotes the expression of the
specific cytokines and induces the expression of the gene within
the cell as well as protein dysfunction, eventually leading to the
occurrence of microvascular complications (38). The present study revealed a severe
inflammatory reaction in the serum and kidney. The levels of TNF-α,
MCP-1 and ICAM-1 were all significantly higher than those of normal
controls. Previous studies have reported that renal injury was
attenuated when their levels were decreased (39–41).
One of mechanisms of protecting the kidney may be that GSPE
decreased the levels of inflammatory cytokines.
In addition, inflammation and oxidative stress are
not assumed to take effect separately. They are interrelated and
interactive with each other in a number of diseases, including
hypertension and cardiovascular disease (42,43).
However, information on the correlation between oxidative stress
and inflammation in DN is limited. Further studies are necessary to
identify whether either of these play a significant role in the
treatment of DN.
In summary, this study indicated that oxidative
stress and inflammatory reaction may, at least in part, cause renal
injury in T2DM rats. GSPE may alleviate renal injury and exert its
antioxidative effect in vivo by increasing the activity of
SOD and CAT, decreasing the levels of MDA, and decreasing the
levels of inflammatory cytokines including hs-CRP, TNF-α, MCP-1 and
ICAM-1. However, there are several inflammatory pathways in
vitro. Further studies are required to investigate the exact
inflammatory pathways by which GSPE protects against renal injury,
as well as its specific mechanism of action.
Acknowledgements
This study was supported by research grants from the
National Natural Science Foundation of China (81102116 and
81072293).
Abbreviations:
|
FBG
|
fasting blood glucose
|
|
hs-CRP
|
high-sensitivity c-reactive
protein
|
|
SCr
|
serum creatinine
|
|
CCr
|
endogenous creatinine clearance
rate
|
|
BUN
|
blood urea nitrogen
|
|
SOD
|
superoxide dismutase
|
|
CAT
|
catalase
|
|
MDA
|
malondialdehyde
|
|
TNF-α
|
tumor necrosis factor-α
|
|
TIMP-1
|
tissue inhibitor of
metalloproteinase-1
|
|
ICAM-1
|
intercellular adhesion molecule-1
|
|
MCP-1
|
monocyte chemoattractant protein-1
|
|
MMP-9
|
matrix metalloproteinase-9
|
References
|
1
|
Yang W, Lu J, Weng J, et al: Prevalence of
diabetes among men and women in China. New Engl J Med.
362:1090–1101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ceriello A: New insights on oxidative
stress and diabetic complications may lead to a “causal”
antioxidant therapy. Diabetes Care. 26:1589–1596. 2003.
|
|
3
|
Collins AJ, Kasiske B, Herzog C, et al:
Excerpts from the United States Renal Data System 2003 Annual Data
Report: atlas of end-stage renal disease in the United States. Am J
Kidney Dis. 42:A5–A7. S1–S230. 2003.
|
|
4
|
Caramori ML, Fioretto P and Mauer M: Low
glomerular filtration rate in normoal buminuric type 1 diabetic
patients: an indicator of more advanced glomerular lesions.
Diabetes. 52:1036–1040. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wada J and Makino H: Inflammation and the
pathogenesis of diabetic nephropathy. Clin Sci (Lond). 124:139–152.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ha H, Yu MR and Kim KH: Melatonin and
taurine reduce early glomerulopathy in diabetic rats. Free Radic
Biol Med. 26:944–950. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li SY, Fu ZJ and Lo AC: Hypoxia-induced
oxidative stress in ischemic retinopathy. Oxid Med Cell Longev.
2012:e4267692012.
|
|
8
|
Pan HZ, Zhang L, Guo MY, et al: The
oxidative stress status in diabetes mellitus and diabetic
nephropathy. Acta Diabetol. 47:71–76. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mansouri E, Panahi M, Ghaffari MA, et al:
Effects of grape seed proanthocyanidin extract on oxidative stress
induced by diabetes in rat kidney. Iran Biomed J. 15:100–106.
2011.PubMed/NCBI
|
|
10
|
Kanasaki K, Taduri G and Koya D: Diabetic
nephropathy: the role of inflammation in fibroblast activation and
kidney fibrosis. Front Endocrinol (Lausanne). 4:72013.PubMed/NCBI
|
|
11
|
Lim AK and Tesch GH: Inflammation in
diabetic nephropathy. Mediators Inflamm. 2012:146–154. 2012.
|
|
12
|
Rivero A, Mora C, Muros M, et al:
Pathogenic perspectives for the role of inflammation in diabetic
nephropathy. Clin Sci (Lond). 116:479–492. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gui D, Huang J, Guo Y, et al:
Astragaloside IV ameliorates renal injury in streptozotocin-induced
diabetic rats through inhibiting NF-κB-mediated inflammatory genes
expression. Cytokine. 61:970–977. 2013.PubMed/NCBI
|
|
14
|
Zhang XY, Li WG, Wu YJ, et al:
Proanthocyanidin from grape seeds enhances doxorubicin-induced
antitumor effect and reverses drug resistance in
doxorubicin-resistant K562/DOX cells. Can J Physiol Pharm.
83:309–318. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Boghdady NA: Antioxidant and antiapoptotic
effects of proanthocyanidin and ginkgo biloba extract against
doxorubicin-induced cardiac injury in rats. Cell Biochem Funct.
31:344–351. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mansouri E, Panahi M, Ghaffari MA and
Ghorbani A: Effects of grape seed proanthocyanidin extract on
oxidative stress induced by diabetes in rat kidney. Iran Biomed J.
15:100–106. 2011.PubMed/NCBI
|
|
17
|
Li X, Xiao Y, Gao H, et al: Grape seed
proanthocyanidins ameliorate diabetic nephropathy via modulation of
levels of AGE, RAGE and CTGF. Nephron Exp Nephrol. 111:e31–e41.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kolset SO, Reinholt FP and Jenssen T:
Diabetic nephropathy and extracellular matrix. J Histochem
Cytochem. 60:976–986. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Furness PN: Basement membrane synthesis
and degradation. J Pathol. 183:1–3. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu K, Setty S, Mauer SM, et al: Altered
kidney matrix gene expression in early stages of experimental
diabetes. Acta Anat (Basel). 158:155–165. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han SY, Jee YH, Han KH, et al: An
imbalance between matrix metalloproteinase-2 and tissue inhibitor
of matrix metalloproteinase-2 contributes to the development of
early diabetic nephropathy. Nephrol Dial Transplant. 21:2406–2416.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hadler-Olsen E, Winberg JO, Reinholt FP,
et al: Proteases in Plasma and Kidney of db/db Mice as Markers of
Diabetes-Induced Nephropathy. ISRN Endocrinol. 2011:e8326422011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Efficacy of atenolol and captopril in
reducing risk of macrovascular and microvascular complications in
type 2 diabetes: UKPDS 39. UK Prospective Diabetes Study Group.
BMJ. 317:713–720. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pinent M, Blay M, Bladé MC, et al: Grape
seed-derived procyanidins have an antihyperglycemic effect in
streptozotocin-induced diabetic rats and insulin-omimetic activity
in insulin-sensitive cell lines. Endocrinology. 145:4985–4990.
2004. View Article : Google Scholar
|
|
25
|
El-Alfy AT, Ahmed AA and Fatani AJ:
Protective effect of red grape seeds proanthocyanidins against
induction of diabetes by alloxan in rats. Pharmacol Res.
52:264–270. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li BY, Cheng M, Gao HQ, et al:
Back-regulation of six oxidative stress proteins with grape seed
proanthocyanidin extracts in rat diabetic nephropathy. J Cell
Biochem. 104:668–679. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cui Xiaopei, Liu Xiangju, Feng Hua, et al:
Grape seed proanthocyanidin extracts enhance endothelial nitric
oxide synthase expression through 5′-AMP activated protein
kinase/Surtuin 1-Krüpple like factor 2 pathway and modulate blood
pressure in ouabain induced hypertensive rats. Biol Pharm Bull.
35:2192–2197. 2012.PubMed/NCBI
|
|
28
|
Wu S, Guo R, Guo R, et al: Effects of
grape seed proanthocyanidin extracts on renal vascular hypertension
in rats. Chin J Pathophysiol. 27:593–595. 2011.
|
|
29
|
Ha H and Lee HB: Reactive oxygen species
as glucose signaling molecules in mesangial cells cultured under
high glucose. Kidney Int Suppl. 77:S19–S25. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Scivittaro V, Ganz MB and Weiss MF: AGEs
induce oxidative stress and activate protein kinase C-beta (II) in
neonatal mesangial cells. Am J Physiol Renal Physiol.
278:F676–F683. 2000.PubMed/NCBI
|
|
31
|
Tomlinson DR: Mitogen-activated protein
kinases as glucose transducers for diabetic complications.
Diabetologia. 42:1271–1281. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Suzuki S, Hinokio Y, Komatu K, et al:
Oxidative damage to mitochondrial DNA and its relationship to
diabetic complications. Diabetes Res Clin Pract. 45:161–168. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kedziora K, Szram S, Komatowski T, et al:
Effect of vitamin E and vitamin C supplementation on antioxidative
state and renal glomendar basement membrane thickness in diabetic
kidney. Nephron Exp Nephrol. 95:134–143. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bhatti F, Mankhey RW, Asico L, et al:
Mechanisms of antioxidant and pro-oxidant effects of alpha-lipoic
acid in the diabetic and nondiabetic kidney. Kidney Int.
67:1371–1380. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Naito Y, Uchiyama K, Aoi W, et al:
Prevention of diabetic nephropathy by treatment with astaxanthm in
diabetic db/db mice. Biofactors. 20:49–59. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tam FW, Riser BL, Meeran K, et al: Urinary
monocyte chemoattractant protein-1 (MCP-1) and connective tissue
growth factor (CCN2) as prognostic markers for progression of
diabetic nephropathy. Cytokine. 47:37–42. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Navarro-González JF, Jarque A, Muros M, et
al: Tumor necrosis factor-alpha as a therapeutic target for
diabetic nephropathy. Cytokine Growth Factor Rev. 20:165–173.
2009.PubMed/NCBI
|
|
38
|
Rivero A, Mora C, Muros M, et al:
Pathogenic perspectives for the role of inflammation in diabetic
nephropathy. Clin Sci (Lond). 116:479–492. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chow FY, Nikolic-Paterson DJ, Ma FY, et
al: Monocyte chemoattractant protein-1-induced tissue inflammation
is critical for the development of renal injury but not type 2
diabetes in obese db/db mice. Diabetologia. 50:471–480. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Navarro JF, Mora C, Muros M, et al:
Urinary tumour necrosis factor-alpha excretion independently
correlates with clinical markers of glomerular and
tubulointerstitial injury in type 2 diabetic patients. Nephrol Dial
Transpl. 21:3428–3434. 2006. View Article : Google Scholar
|
|
41
|
Okada S, Shikata K, Matsuda M, et al:
Intercellular adhesion molecule-1-deficient mice are resistant
against renal injury after induction of diabetes. Diabetes.
52:2586–2593. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Anatoliotakis N, Deftereos S, Bouras G, et
al: Myeloperoxidase: expressing inflammation and oxidative stress
in cardiovascular disease. Curr Top Med Chem. 13:115–138. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Crowley SD: The cooperative roles of
inflammation and oxidative stress in the pathogenesis of
hypertension. Antioxid Redox Signal. 20:102–120. 2014. View Article : Google Scholar : PubMed/NCBI
|