Introduction
PACE4 is one of the neuroendocrine-specific
mammalian subtilisin-related endoproteases and a calcium-dependent
serine proteinase. PACE4 can cleave precursor proteins at basic
residues within the motif (K/R)-(X)n-(K/R)↓, where n=0, 2, 4, 6 and
X is any amino acid except Cys (1). Eight isoforms of PACE4 have been
reported: PACE4A-I, PACE4A-II, PACE4B, PACE4C, PACE4CS, PACE4D,
PACE4 E-I and PACE4E-II (2). These
isoforms are distributed in different tissues in humans, and have
been shown to function in the secretory pathway (3).
Breast cancer has become a common tumor, threatening
women’s physical and mental health. It is a complex tumor, and one
of its most common causes is genetic mutations. A number of reports
have provided evidence for an association between genes such as
BRCA-1, BRCA-2, p53, PTEN etc., with breast cancer
(4,5). The common features of cancer cells,
namely, increased proliferation, migration and tissue invasion,
lead to uncontrolled division and allow cells to invade surrounding
tissues as well as basement membranes, thereby accelerating
malignant growth and cancer progression. The protein PACE4 is
strongly involved in malignancy, due to its activity that allows to
generate biologically active proteins. There is evidence that
aberrant expression of PACE4 is involved in different types of
cancer. For example, overexpression of PACE4 in murine cells leads
to increased invasiveness both in vitro and in vivo
(6), and enhances tumor malignant
phenotypes in breast cancer (7).
Another report indicated that PACE4 plays an important role in
enhancing the progression of prostate cancer (8). The critical role of PACE4 in
promoting proceedings of tumors renders it an attractive target for
cancer treatment.
PACE4 was shown to play a pivotal role in the
extracellular matrix (ECM) (9).
Numerous proteins such as growth factors, cell adhesion molecules,
and oncogene products are active in the ECM. All these proteins are
important in the occurrence and progression of cancer. Thus, PACE4
may also be involved in cancer. A previous study indicated that
α1-PDX and ppPACE4 (PACE4 prosegment) can increase cell motility,
migration and invasion (7). In
this context, we aimed to explore the direct effect of PACE4 in
breast cancer cells and further investigate the roles of PACE4 in
this type of cancer.
In the present study, an RNA interference approach
was adopted to silence the expression of the PACE4 gene in
the MDA-MB-231 cell line and thereby, explore the role of PACE4 in
breast cancer. We studied the proliferation, migration and invasion
of MDA-MB-231 cells following siRNA transfection, and also detected
cell-cycle changes following the reduction in the expression of
PACE4. Breast cancer is a tumor related to hormones, and
thus, the relationship between PACE4 and 17β-estradiol was also
investigated. In order to elucidate the mechanism underlying the
PACE4 effects on MDA-MB-231 cells, the expression of genes involved
in cell growth, invasion and adhesion, such as matrix
metalloproteinase-9 (MMP-9), insulin like growth factor 2
(IGF-2) and myelin protein zero-like 2 (MPZL2), was
also studied following siRNA-mediated inhibition of PACE4
expression.
Materials and methods
Cell culture
Human breast cancer MDA-MB-231 and MCF-7 cells were
obtained from the Shanghai Institutes for Biological Sciences
(Shanghai, China) and cultured in HyClone™ Dulbecco’s modified
Eagle’s medium (DMEM) containing 10% HyClone™ fetal bovine serum
(HyClone), as well as Gibco® penicillin (100 IU/ml) and
streptomycin (100 μg/ml) in a 37°C, 5% CO2 incubator
(all from Thermo Fisher Scientific, Rockford, IL, USA).
Small interfering RNA transfection
Three pairs of siRNAs were used in order to achieve
the most effective inhibition of PACE4 expression
(si-PACE4-1 to -3). The siRNAs were synthesized by GenePharma
(Shanghai, China) and target the human PACE4 transcript
(GenBank accession no., NM_002570.3), at positions 1,011, 1,196,
and 2,118 of the sequence. The cultured MDA-MB-231 cells were
transfected with the siRNAs using the HiPerFect transfection
reagent (Qiagen, Hilden, Germany) according to the manufacturer’s
protocol, and three siRNA concentrations (50, 100 and 150 nM) were
tested. Cells transfected with an unrelated siRNA pair
(5′-CUCCGAACGUGUCACGUTT-3′) and cells cultured with the
transfection reagent alone were used as the negative control (NC)
and the mock, respectively.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from the cells using the
E.Z.N.A.® Total RNA Kit II (Omega Bio-Tek, Norcross, GA,
USA) according to the manufacturer’s protocol. The extracted RNA
was reverse transcribed using the ReverTra Ace® qPCR RT
kit (Toyobo, Osaka, Japan) according to the manufacturer’s
protocol. The relative expression of PACE4 following siRNA
transfection was analyzed by qPCR. The PCR was performed three
times using the SYBR Green kit (Roche Diagnostics, Mannheim,
Germany) and the following primers: PACE4 forward (F), 5′-AAG CAA
GGG AAG TTG AAA GA-3′, and reverse (R), 5′-CAC TGA AGG TGT GGT
ACG-3′; glyceraldehyde 3-phosphate dehydrogenase (GAPDH; used to
standardize the level of expression of PACE4) F, 5′-CAC CAT
CTT CCA GGA GC-3′, and R, 5′-AGT GGA CTC CAC GAC-3′; MMP9 F, 5′-CCC
TGC CAG TTT CCA TT-3′, and R, 5′-CCA TCA CCG TCG AGT CA-3′; IGF-2
F, 5′-GCT TCT ACT TCA GCA GGC-3′, and R, 5′-GTC CCT CTC GGA CTT
GG-3′; MPZL2 F, 5′-CTC TAA CAG TGA CCT GGA ATT T-3′, and R, 5′-GAA
GGA TGG AGG CAT CG-3′. The qPCR reactions were performed on a
LightCycler 480 (Roche Diagnostics GmbH, Mannheim, Germany), with
the following cycling conditions: an initial denaturation step at
95°C for 15 sec, 45 cycles at 95°C for 10 sec and 60°C for 30 sec,
followed by 10 sec at 72°C for final extension. The comparative
threshold cycle (Ct) method was used to analyze the relative
expression of mRNA. PCR products were subjected to 1% agarose gel
electrophoresis to confirm the specificity of the
amplification.
Western blotting
Total protein was extracted by incubation on ice for
30 min with RIPA lysis buffer (Beyotime Institute of Biotechnology,
Jiangsu, China) containing protease inhibitors. The preparation was
centrifuged at 14,000 × g for 30 min at 4°C. The protein
concentration was measured with the Bio-Rad protein assay system
(Hercules, CA, USA). Following incubation at 100°C for 5 min with
loading buffer, 30 μg of the total protein were resolved by sodium
dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to
nitrocellulose membranes (Amersham, Piscataway, NJ, USA) by wet
transfer, and blocked in 10 nM Tris-buffered saline (TBS)
containing 0.1% (v/v) Tween-20 (TBST) and 5% (w/v) skim milk. Next,
the membranes were incubated overnight at 4°C with specific
anti-human primary antibodies (anti-PACE4 at 2,000-fold dilution,
or anti-GAPDH at 1,000-fold dilution). Following a wash with
phosphate-buffered saline (PBS) containing 0.1% (v/v) Tween-20
(PBST), the membranes were incubated with the corresponding
secondary antibody (goat anti-rabbit immunoglobulin G-HRP; 1:5,000
dilution) for 1 h at 37°C. All antibodies were purchased from Abcam
(Cambridge, UK). Detection was performed using an enhanced
chemiluminescence (ECL) kit (KeyGen Biotech, Nanjing, China). The
film was scanned and analyzed with Quantity One Analysis software
(Bio-Rad).
Cell proliferation assay
The human breast cancer MDA-MB-231 cells were seeded
onto 96-well culture plates and incubated until they reached 80%
confluence. The cultured cells were transfected with si-PACE4 (150
nM). After growing for 24, 48 and 72 h, 20 μl of
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
5 mg/ml) were added to each well, and the incubation was continued
for an additional 4 h at 37°C. Then, the MTT solution was removed,
and 150 μl of dimethyl sulfoxide (Solarbio, Beijing, China) were
added and incubated at room temperature for 10 min to allow
extraction of the MTT-formazan products. The absorbance was
measured at 490 nm with a UV/VIS spectrophotometer (Yuanxi-UV5500,
Shanghai, China) and the measurements were repeated three
times.
Cell migration and invasion assays
Cell migration was measured by a wound healing
assay. The cells were plated onto 24-well plates and scratched
linearly in multiple areas with a cell scraper. Then, the cells
were transfected with 150 nM siRNA targeting PACE4, or with
the negative and the transfection reagent control. The same area of
each wound was examined at 0 and 24 h to quantify the cells that
had migrated into the wound.
The cell invasion assay was performed in a
Costar® Transwell appparatus coated with a Matrigel
filter (Corning, NY, USA). Cells were plated at a density of
3×104 cells/well, and incubated with 150 nM of siRNA in
the upper chamber of the apparatus for 8 h, while the lower
compartment was filled with DMEM with 10% FBS. The upper and the
lower chamber were filled with DMEM without FBS and incubated for
12 h. The non-invading cells at the upper surface of the filter
were scraped with cotton swabs, and the invading cells were stained
Giemsa (Solabio, China). The number of cells that had invaded
through the membrane was quantified in 5 random fields of a
fluorescence microscope (ECLIPSE50i, Nikon, Tokyo, Japan) at ×100
magnification.
Cell cycle changes detected by flow
cytometry
Cells were plated in 6-well culture plates at a
density of 1×105 cells/ml in 2 ml of DMEM, and were
transfected with siRNA as described above. The cells were then
harvested at 24 h by addition of trypsin (0.25%; Solarbio),
incubation for 5 min and centrifugation at 500 g for 10 min. The
cells were washed twice with PBS, fixed overnight with 70% cold
ethanol, and stained for 30 min at 37°C with 1 ml of propidium
iodide solution (50 μg/ml; Beyotime Institute of Biotechnology),
containing 50 μg/ml RNase A (MacGene Biotechnology, Beijing,
China). The cell number at different phases of the cell cycle was
determined on a FACScan flow cytometer (BD Biosciences, Franklin
Lakes, NJ, USA), and the DNA content was analyzed with the ModFitLT
V3.0 software (BD Biosciences).
PACE4 expression induced by
17β-estradiol
MDA-MB-231 and MCF-7 cells were cultured for 3 days
in phenol red-free DMEM medium (MacGene Biotechnology) containing
10% certified charcoal-stripped-FBS (Biological Industries, Kibbutz
Beit-Haemek, Israel), and were next treated with 10−10,
10−9, 10−8, 10−7 and
10−6 mol/l of 17β-estradiol (Sigma-Aldrich, St. Louis,
MO, USA) for 24 or 36 h. Cells cultured in phenol red-free DMEM
medium were used as the control. The mRNA level of PACE4 was
quantified by RT-qPCR, as described above.
Statistical analysis
Data were expressed as the mean ± standard deviation
(SD). All results were confirmed in at least three independent
experiments. Significant differences among three or more datasets
were analyzed by a one-way analysis of variance (ANOVA) using the
SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). P-values <0.05
were considered to indicate statistically significant
differences.
Results
PACE4 is successfully silenced by
siRNA
To select the most effective PACE4-specific
siRNA, RT-qPCR (data not shown) and western blot analyses were
performed to detect the expression of PACE4 at the mRNA and protein
level. We used three pairs of siRNA at concentrations 50, 100, 150
nM, and three periods of transfection, 24, 36 and 48 h. The most
efficient silencing of PACE4 was achieved with 150 nM
(Fig. 1B and C) of siRNA-1,
incubated with the cells for 24 h (Fig. 1A). These conditions were used in
the following experiments.
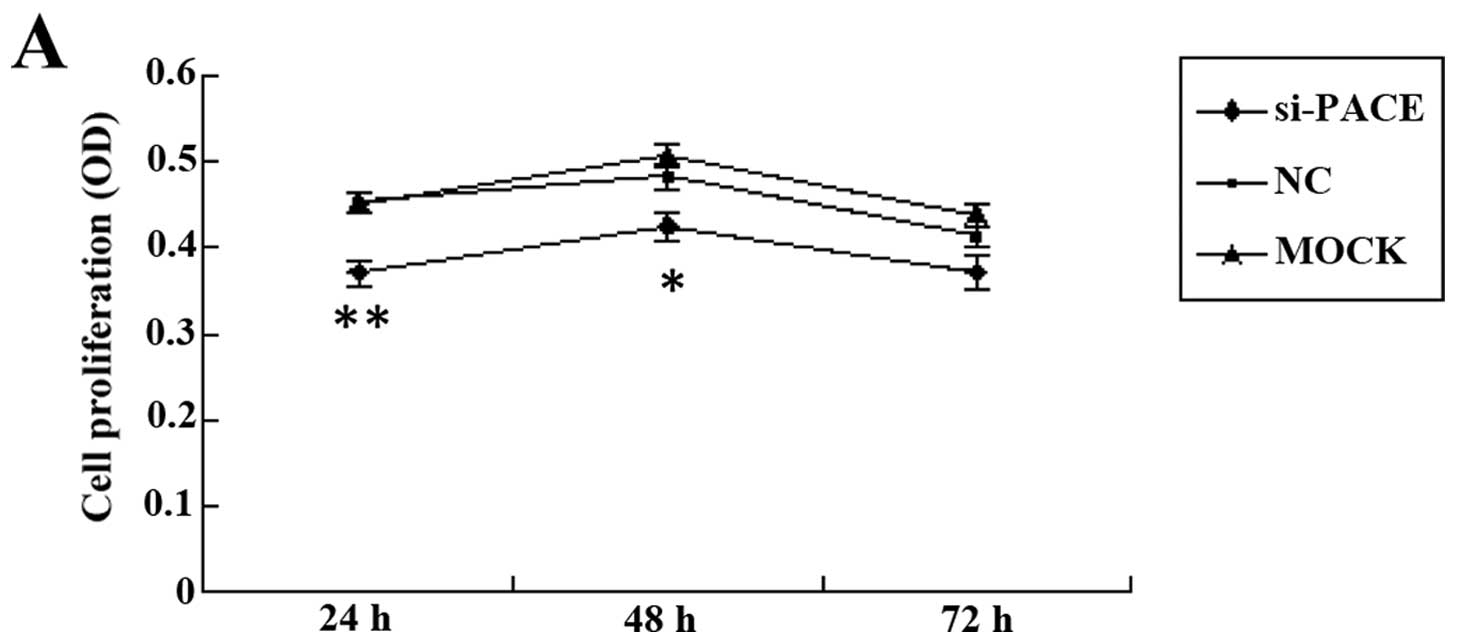
PACE4 silencing decreases the MDA-MB-231
proliferation rate
To analyze cell proliferation following
siRNA-mediated silencing of PACE4, the MTT assay was
performed. The results showed that the growth rate of the cells
transfected with the si-PACE4 is slower than that of the NC and
mock control groups (Fig. 2A).
This finding indicated that the human breast cancer cell line
MDA-MB-231 is sensitive to reduced levels of PACE4, since
silencing of the gene had an inhibitory effect on the growth of
these cells.
Silencing of PACE4 reduces MDA-MB-231
cell migration and invasion
Cell migration (Fig.
2B) and invasion (Fig. 2D)
assays were performed to investigate whether PACE4 affects cell
migration and invasion. The results showed that, following
PACE4 silencing, cell migration and invasion were both
reduced compared to the NC and mock controls. The number of cells
that migrated into the wound (Fig.
2C) and invaded through the Matrigel filter (Fig. 2E) were quantified, and were both
found to be significantly decreased compared to the controls.
PACE4 may induce G0/G1 phase arrest in
breast cancer cells
Flow cytomety was used to investigate the effects of
the PACE4 knockdown on the cell cycle. Two types of results
are shown in Fig. 3: typical
images of flow cytometry showing the cell-cycle phase distribution
of the cells (Fig. 3A), and the
results of quantification and statistical analyses of cell-cycle
phase distribution data at 24 h (Fig.
3B). These results showed that, following transfection with
si-PACE4 for 24 h, the populations of cells at the G0/G1 phase
remained increased, which indicates that PACE4 may induce G0/G1
arrest in breast cancer cells.
The expression of PACE4 in response to
17β-estradiol treatment in MDA-MB-231 and MCF-7 cells
An earlier study showed that the PACE4 gene
expression level significantly correlates to the concentration of
the cells in estrogen receptors (10). Based on this finding, we
investigated whether the expression of PACE4 is affected by
17β-estradiol using RT-qPCR in the human MDA-MB-231 and MCF-7 cell
lines. The results showed that the mRNA level of PACE4 is
not significantly different between treatments with different
concentrations of 17β-estradiol (Fig.
4).
The MMP9, IGF-2 and MPZL2 gene levels are
decreased following transfection with si-PACE4
To investigate the mechanism underlying the
PACE4 silencing-mediated effects on the breast cancer cell
line MDA-MB-231, the expression of the MMP9, IGF-2
and MPZL2 genes was studied following siRNA transfection.
qRT-PCR experiments were performed, and the results showed that all
three genes show significantly reduced expression following
transfection with si-PACE4 (Fig.
5).
Discussion
In recent years, the relationship between PACE4 and
cancer has been extensively studied. Overexpression of PACE4 was
demonstrated in skin, lung and prostate cancer (8,11,12).
However, other studies reported markedly reduced PACE4 expression
levels in human ovarian endometrial cancer cells (13,14).
As to breast cancer, PACE4 was found overexpressed, and the study
of Lapierre et al (7)
demonstrated that overexpression of the prosegment ppPACE4 in the
breast cancer cell line MDA-MB-231 significantly enhances cell
motility, migration and invasion of collagen in vitro,
although the role of PACE4 in these processes was not fully
clarified. The abnormal expression of PACE4 has overall become an
emerging research focus in numerous cancer studies.
In the present study, we transfected different pairs
of siRNA targeting the PACE4 gene (si-PACE4-1 to -4) in the
breast cancer cell line MDA-MB-231, and examined the degree of
PACE4 silencing by RT-qPCR and western blotting. The results
showed that the expression of PACE4 is efficiently silenced by
si-PACE4. The highest transfection efficiency was achieved at 24 h
of transfection with 150 nM of si-PACE4-1. The MTT assay also
indicated that cell growth is prominently decreased at 24 h of
siRNA transfection compared to the control groups. PACE4 has been
shown to play a vital role in the activation of numerous growth
factors, such as IGF-2. IGF-2 is involved in tumor progression by
stimulating cell proliferation, and can be activated by PACE4 at
Arg104 (14). We presumed that
PACE4 may promote cell proliferation through activation of growth
factors such as IGF-2. To confirm this, we studied the expression
of the IGF-2 gene by RT-qPCR following transfection of
MDA-MB-231 cells with si-PACE4, and the result showed that
IGF-2 expression is significantly decreased in the
si-PACE4-transfected cells. We also found that silencing of
PACE4 blocks the cell cycle transition from the G0/G1 to the
S phase. Taken together, these results indicated that si-PACE4 may
inhibit cell proliferation.
MMPs are enzymes involved in a number of
pathological processes, and play a vital role in tumor invasion and
metastasis, by damaging almost all the proteins of the ECM. MMP9
was identified as an important target of PACE4, degrading the ECM
and eventually resulting in enhanced invasiveness (15). To investigate the role of PACE4 in
the process(es) enhancing the invasive ability of breast cancer
MDA-MB-231 cells, we studied the expression of the MMP9 gene
by RT-qPCR. THis analysis showed that, following PACE4
silencing, the MMP9 level is significantly decreased. This
result is consistent with the findings of Lapierre et al
(7), who reported that
overexpression of ppPACE4 can increase the MMP9 activity.
The MPZL2 protein, which contains a putative serine
phosphorylation site and is specifically regulated during T cell
maturation, is believed to mediate cell adhesion through a
homophilic interaction (16). The
results of another study by our lab on rheumatoid arthritis
(unpublished data) also indicated that MPZL2 may be an important
target of PACE4. In this study, RT-qPCR detection of the
MPZL2 mRNA level showed that its expression is significantly
decreased upon si-PACE4 transfection of MDA-MB-231 cells. All the
above suggest that MPZL2 may represent an important protein in cell
differentiation.
It is well known that the occurrence and development
of breast cancer is closely related to estrogens (17). In this study, we chose MCF-7 cells
(estrogen receptor-positive cells) and MDA-MB-231 cells (estrogen
receptor-negative cells) to explore the relationship between
PACE4 gene expression and estrogen content. However, in
contrast to a previous study showing that PACE4 expression
significantly correlates to the estrogen receptor content (10), our results did not identify any
association between these two parameters.
In conclusion, our study showed that silencing of
PACE4 can decrease proliferation, migration and invasion in
breast cancer cells. An earlier report proposed that PACE4
inhibition may disrupt the function of numerous proteins critical
for tumorigenesis, and that attenuating PACE4 overexpression in
cancer may constitute a therapeutic approach (18). Therefore, the PACE4 gene may
be a promising inhibition target in the context of breast cancer
treatment.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (81102275), the
Science Promotion Foundation of Shandong (2012GSF12115), the
Shandong Scientific Instrument Equipment Promotion Transformation
Project (2011SJGZ26), and the Natural Science Foundation of
Shandong Province (ZR2011CQ028).
References
|
1
|
Seidah NG and Chrétien M: Proprotein and
prohormone convertases: a family of subtilases generating diverse
bioactive polypeptides. Brain Res. 848:45–62. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nagahama M, Taniguchi T, Hashimoto E, et
al: Biosynthetic processing and quaternary interactions of
proprotein convertase SPC4 (PACE4). FEBS Lett. 434:155–159. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mains RE, Berard CA, Denault JB, et al:
PACE4: a subtilisin-like endoprotease with unique properties.
Biochem J. 321:587–593. 1997.PubMed/NCBI
|
|
4
|
Jensen RA, Thompson ME, Jetton TL, et al:
BRCA1 is secreted and exhibits properties of a granin. Nat Genet.
12:303–308. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Walerych D, Napoli M, Collavin L and Del
Sal G: The rebel angel: mutant p53 as the driving oncogene in
breast cancer. Carcinogenesis. 33:2007–2017. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bassi DE, Zhang J, Cenna J, et al:
Proprotein convertase inhibition results in decreased skin cell
proliferation, tumorigenesis, and metastasis. Neoplasia.
12:516–526. 2010.
|
|
7
|
Lapierre M, Siegfried G, Scamuffa N, et
al: Opposing function of the proprotein convertases furin and PACE4
on breast cancer cells’ malignant phenotypes: role of tissue
inhibitors of metalloproteinase-1. Cancer Res. 67:9030–9034.
2007.PubMed/NCBI
|
|
8
|
D’Anjou F, Routhier S, Perreault JP, et
al: Molecular validation of PACE4 as a target in prostate cancer.
Transl Oncol. 4:157–172. 2011.PubMed/NCBI
|
|
9
|
Tsuji A, Sakurai K, Kiyokage E, et al:
Secretory proprotein convertases PACE4 and PC6A are heparin-binding
proteins which are localized in the extracellular matrix. Potential
role of PACE4 in the activation of proproteins in the extracellular
matrix. Biochim Biophys Acta. 1645:95–104. 2003. View Article : Google Scholar
|
|
10
|
Cheng M, Watson PH, Paterson JA, et al:
Pro-protein convertase gene expression in human breast cancer. Int
J Cancer. 71:966–971. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hubbard FC, Goodrow TL, Liu SC, et al:
Expression of PACE4 in chemically induced carcinomas is associated
with spindle cell tumor conversion and increased invasive ability.
Cancer Res. 57:5226–5231. 1997.PubMed/NCBI
|
|
12
|
Schalken JA, Roebroek AJ, Oomen PP, et al:
fur gene expression as a discriminating marker for small cell and
nonsmall cell lung carcinomas. J Clin Invest. 80:1545–1549. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fu Y, Campbell EJ, Shepherd TG and
Nachtigal MW: Epigenetic regulation of proprotein convertase PACE4
gene expression in human ovarian cancer cells. Mol Cancer Res.
1:569–576. 2003.PubMed/NCBI
|
|
14
|
Duguay SJ, Jin Y, Stein J, et al:
Post-translational processing of the insulin-like growth factor-2
precursor. Analysis of O-glycosylation and endoproteolysis. J Biol
Chem. 273:18443–18451. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bassi DE, Lopez De Cicco R, Cenna J, et
al: PACE4 expression in mouse basal keratinocytes results in
basement membrane disruption and acceleration of tumor progression.
Cancer Res. 65:7310–7319. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guttinger M, Sutti F, Panigada M, et al:
Epithelial V-like antigen (EVA), a novel member of the
immunoglobulin superfamily, expressed in embryonic epithelia with a
potential role as homotypic adhesion molecule in thymus
histogenesis. J Cell Biol. 141:1061–1071. 1998. View Article : Google Scholar
|
|
17
|
Dong S, Zhang Z and Takahara H:
Estrogen-enhanced peptidylarginine deiminase type IVgene (PADI4)
expression in MCF-7 cells is mediated estrogen
receptor-alpha-promoted transfactors activator protein-1, nuclear
factor-Y, and Sp1. Mol Endocrinol. 21:1617–1629. 2007. View Article : Google Scholar
|
|
18
|
Fu J, Bassi DE, Zhang J, et al: Enhanced
UV-induced skin carcinogenesis in transgenic mice overexpressing
proprotein convertases. Neoplasia. 15:169–179. 2013.PubMed/NCBI
|