Introduction
Colorectal cancer (CRC) is one of the most prevalent
cancers worldwide (1). Although
the treatment of CRC has improved, the mortality rate of CRC
patients remains high. The first course of treatment for primary
CRC is surgical resection and adjuvant chemotherapy; however, once
metastasis occurs it is almost incurable. Previous studies have
determined that 90% of mortalities due to cancer are associated
with metastasis (2,3). In addition, metastasis is the most
common cause of mortality in patients with advanced stage CRC
(4). Although irinotecan,
oxaliplatin and fluorouracil-based chemotherapy regimens have
improved the survival rate of patients with metastatic CRC in the
last decade (5), there remains a
medical requirement for more effective and well-tolerated therapies
for CRC.
An increasing number of studies suggest that there
are numerous natural compounds which act as cancer preventative and
therapeutic agents, in addition to multiple prescription drugs that
are derived from natural plant species (6–8).
Trillium tschonoskii Maxim. is a perennial herb belonging to
the Trilliaceae family, which is found in mid-western China
(9). T. tschonoskii Maxim.
has traditionally been used by the residents of China for the
treatment of a number of conditions, including hypertension,
headache, neurasthenia, giddiness, cancer and for ameliorating
pain. Previous studies have demonstrated that numerous bioactive
compounds, including steroidal saponins and steroidal glycosides,
are found in a number of species in the Trillium genus,
including Trillium erectum (10,11),
Trillium kamtschaticum (12) and T. tschonoskii. A previous
study extracted a steroidal saponin, Paris saponin VII (PS VII),
from T. tschonoskii and evaluated its growth-inhibitory
effect on HT-29 and SW-620 cells in a murine xenograft tumor model.
In addition, the protective effects of PS VII against
AOM/DSS-induced colitis-associated carcinogenesis were investigated
in ICR mice.
In the present study, the anti-metastatic activities
of PS VII were investigated in two metastatic CRC cell lines. The
present study aimed to explore the molecular mechanisms for the
effects of PS VII on metastatic colorectal cancer, in the hope to
provide a basis for the future development of PS VII as a novel
anti-colorectal cancer agent, for both primary and metastatic
diseases.
Materials and methods
Materials and chemicals
PS VII, with a purity of >99%, was isolated from
the root and rhizome of T. tschonoskii Maxim. which was
obtained from the Qinba Mountains (Ankang, China) (13). The chemical structure of PS VII is
shown in Fig. 1. PS VII was
dissolved in dimethylsulfoxide (DMSO) at 1 M and stocked at −20°C
in aliquots. Trypan blue, Triton X-100, pyruvate, penicillin G and
streptomycin were obtained from Sigma (St. Louis, MO, USA).
RPMI-1640 and fetal bovine serum (FBS) were purchased from Corning
Inc. (Corning, NY, USA). Rabbit polyclonal anti-MMP-2 and MMP-9
antibodies were purchased from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). Matrigel was purchased from BD Biosciences
(Franklin Lakes, NJ, USA). Materials and chemicals for
electrophoresis were obtained from Bio-Rad Laboratories (Hercules,
CA, USA). All other chemicals were of analytical reagent grade and
purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai,
China).
Cell line and culture conditions
SW620 and LoVo human colon carcinoma cell lines
[American Type Culture Collection (ATCC), Rockville, MD, USA] were
cultured in RPMI-1640 medium, and human umbilical vein endothelial
cells (HUVECs; which were supplied by Prof. Zhou Siyuan, Department
of Pharmacology) were cultured in Dulbecco’s modified Eagle’s
medium. For cell maintenance, the basal medium was supplemented
with 10% FBS, 100 U/ml penicillin and 100 U/ml streptomycin. The
solvent control contained an equivalent amount of DMSO
corresponding to the highest concentration of PS VII that was
used.
Cell viability assay
The effects of PS VII on cell viability were
determined using an MTT assay. Cells were seeded in a 96-well plate
at a density 5,000 cells per well and incubated with or without PS
VII for 24 h at 37°C. After incubation, 20 μl MTT solution (5
mg/ml) was added to each well and the plate was incubated for a
further 4 h at 37°C. The supernatants were aspirated carefully and
200 μl DMSO was added, and then the plate was subjected to
vibration for 20 sec. The optical density (OD) of the cell
suspension was measured at a wavelength of 490 nm using a
microplate reader (iMark model 680; Bio-Rad Laboratories). The
inhibition rate was calculated using the following formula:
Inhibition = (1-ODtreatment group/ODcontrol
group) × 100. All experiments were performed in
triplicate.
Cell-matrix and cell-cell attachment
assays
Cells contained in dishes were pretreated with or
without PS VII (0.3 and 1 μM) for 24 h. Following pretreatment, the
cells were detached from the dishes via incubation with 15 mM EDTA,
washed twice with phosphate-buffered saline (PBS) and once with
serum-free medium (SFM), counted and seeded into 96-well plates at
a density of 5,000 cells per well. For cell-matrix attachment
assays (13), the 96-well plates
(Costar, Corning Inc., Corning, NY, USA) were precoated with
Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) or bovine serum
albumin as a background control, incubated for 1 h at room
temperature (RT) and 1 h at 37°C, and washed with PBS, prior to the
assay. Plates with CRC cells were incubated for 1 h at 37°C, cells
were washed twice with PBS and attached cells were assessed using
the MTT method. For cell-cell attachment assays (14) HUVECs were plated in growth medium
at a density of 30,000 cells per well and incubated at 37°C in 5%
CO2 for 24 h prior to the assay. The plates containing
the CRC cells were incubated in a humidified atmosphere for 1 h,
washed four times with warm SFM, stained with a 0.25% rose
bengal-PBS solution and incubated for 5 min at RT. Subsequently,
cells were washed twice with SFM. Rose bengal was dissolved in a
95% ethanol-PBS solution (1:1) and detected using a microplate
reader. Duplicate wells containing only HUVECs were included in
each experiment as background controls. After subtracting the OD
value of background wells, the rate of attachment was determined as
the ratio of attaching cells to that of the non-washed group.
Wound-healing assay
Cells were seeded in 6-well plates at a density of
5×105 cells per well. Once the cells reached 90%
confluence, a wound area was carefully created by scraping the cell
monolayer with a sterile 200-μl pipette tip, from one end of the
well to the other. The detached cells were removed by washing with
PBS. Subsequently, the cells were incubated at different
concentrations of PS VII (0.3 and 1 μM). Migration of cells into
the wounded region was observed using an Olympus CK-2 inverted
microscope (Olympus, Tokyo, Japan) and images were captured at 0
and 24 h at ×100 magnification. The wound area was measured using
the image processing program ImageJ (NIH, Bethesda, Maryland, USA).
The cell wound closure rate was calculated using the following
equation: Wound closure = [1-(wound area at Tt/wound
area at T0) × 100, where Tt is the time
passed since wounding and T0 is the time the wound was
created. The experiments were performed in triplicate.
Cell invasion determinations
Cell invasion was determined using Matrigel-coated
Transwell cell culture chambers (8 μm pore size) (Millipore,
Billerica, MA, USA) as described previously (15,16).
Cells were maintained for 24 h in SFM, trypsinized and resuspended
in serum-free RPMI-1640 medium. Following resuspension, cells were
placed in the upper chamber of the Transwell insert
(5×104 cells per well) and incubated with different
concentrations of PS VII (0.3 or 1 μM). RPMI-1640 medium containing
10% FBS was added to the lower chamber. The cells were incubated at
37°C in an incubator supplemented with 5% CO2 for 24 h;
non-invasive cells in the upper chamber were removed by wiping with
a cotton swab and invasive cells were fixed with 4% formaldehyde in
PBS and stained with 1% crystal violet in 2% ethanol. Images of
cells on the lower surface of the filter were captured under a
light microscope (TS100; Nikon Corporation, Tokyo, Japan; ×100
magnification). The inserts were washed with 33% acetic acid. The
absorbance of the washing buffer at 570 nm was determined for each
well using a microplate reader. Cell-free inserts which only
contained medium were included in duplicate throughout each
experiment as OD background controls. The reported OD data
represent the mean background-corrected values ± standard deviation
(SD) obtained from three independent experiments in duplicate.
Gelatin zymography
The zymographic analysis was adapted from Surgucheva
et al (17). Cells were
seeded in a 24-well plate at a density of 5×105 cells
per well and incubated with PS VII (0.3 or 1 μM) for 24 h at 37°C.
Following incubation, conditional medium was harvested and then
electrophoresed on 15% denaturing sodium dodecyl sulphate (SDS)
polyacrylamide gels containing 1 mg/ml gelatin (Sangon, Shanghai,
China). Gels were washed twice in rinsing buffer (50 mM Tris-HCl, 5
mM CaCl2, 2.5% Triton-X 100, 1 μM ZnCl2 and
0.05% NaN3) for 1 h and then incubated for 24 h at 37°C
in the rinsing buffer without Triton-X 100, so that renaturation of
the enzyme could occur. Gels were stained with Coomassie blue R-250
(Bio-Rad Laboratories) and destained with 5% acetic acid containing
10% methanol. Gelatinolytic activities were visualized as clear
bands against a blue background.
Western blotting
Following treatment with PS VII (0.3 and 1 μM) for
24 and 48 h, cells were washed twice with PBS and treated with
extraction buffer (50 mM Tris-Cl, pH 7.5, 150 mM NaCl, 0.1% SDS, 1%
NP-40, and 0.5% deoxycholic acid). The cell extractions were
collected, centrifuged at 10,000 × g for 15 min at 4°C and the cell
lysates collected as supernatants. The cell lysates were subjected
to SDS-PAGE and transferred to nitrocellulose membranes (Millipore,
Bedford, MA, USA). The membranes were blocked with 5% (w/v) non-fat
milk in PBS containing 0.1% Tween-20, and then blotted with primary
antibody. Subsequently, the membranes were incubated with an
appropriate secondary antibody (horseradish peroxidase-conjugated
goat anti-mouse or anti-rabbit IgG). The immuno-detected proteins
were then revealed using enhanced chemiluminescence
(ChemiScope5000; Clinx Science Instruments, CO., Ltd., Shanghai,
China).
Statistics
Results are expressed as the mean ± SD. Two group
comparisons were evaluated using Student’s t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
PS VII inhibits the viability of CRC
cells
The viability of SW620 and LoVo cells treated with
PS VII at different concentrations (0.1, 0.3, 1, 3, 10 or 30 μM)
for 24 h was determined using an MTT reduction assay. As shown in
Fig. 2, PS VII reduced the cell
viability rate in a concentration-dependent manner. Higher
concentrations of PS VII inhibited the growth of CRC cells
directly. Hence, in order to observe the effect of PS VII on the
attachment, migration and invasion of CRC cells more accurately and
clearly, concentrations for the following experiments were selected
which demonstrated no obvious cytotoxicity to the proliferation of
the cells (0.3 and 1 μM).
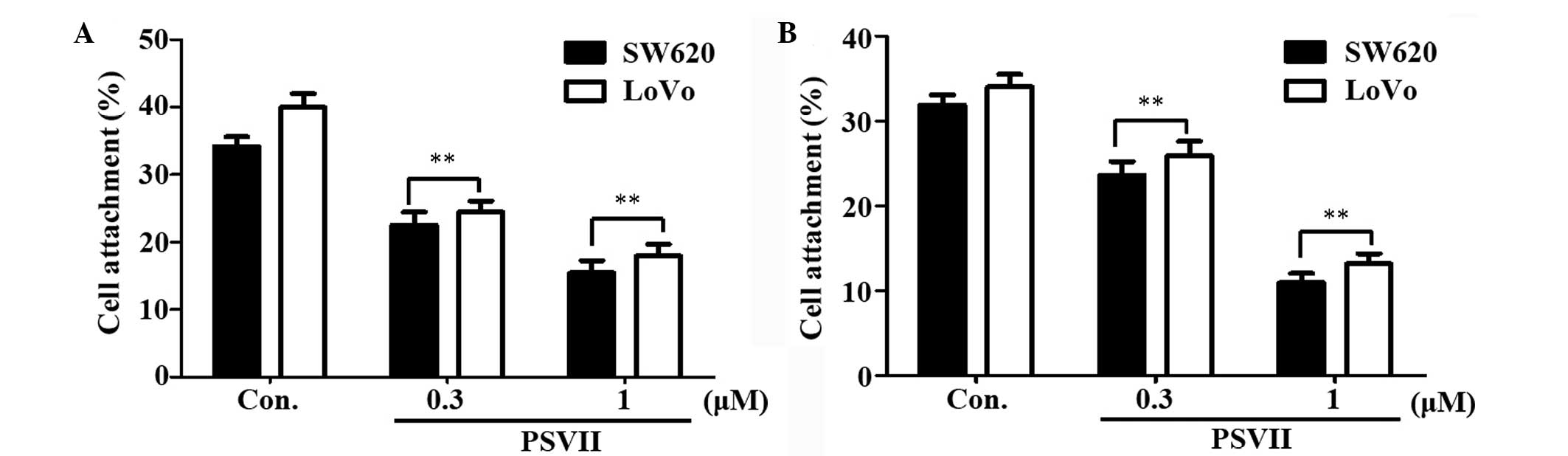
PS VII inhibits cell attachment
To determine whether PS VII affects the attachment
ability of CRC cells, the attachment of the cells to Matrigel and
vascular endothelial cells was assessed. Matrigel is an
extracellular matrix (ECM) which is composed of laminin, collagen
type IV, nidogen and heparan sulfate glycoprotein. As shown in
Fig. 3A, pretreatment with 0.3 and
1 μM PS VII for 24 h reduced the attachment of SW620 and LoVo cells
to Matrigel following incubation for 1 h, compared with that of the
control cells. The potential effect of PS VII on the attachment of
CRC cells to vascular endothelial cells was investigated via
incubation with HUVECs. As shown in Fig. 3B, SW620 and LoVo cells that were
pretreated with 0.3 and 1 μM PS VII for 24 h demonstrated an
impaired ability to attach to HUVECs after incubation for 1 h,
compared with that of the control cells.
PS VII inhibits the migration of SW620
and LoVo cells in vitro
One characteristic of tumor metastasis is the
increased migratory ability of tumor cells. The inhibition of
migration of SW620 and LoVo cells by PS VII was investigated using
wound-healing assays. Cells were incubated with different
concentrations of PS VII for 24 h. Higher concentrations of PS VII
(1 μM) were observed to significantly increase the inhibition of
cell migration in the two cell lines compared with that of the
control cells (Fig. 4).
PS VII inhibits the invasion of SW620 and
LoVo cells in vitro
In order to determine the inhibitory effect of PS
VII on the invasion of SW620 and LoVo cells across the ECM, the
cells that invaded through the Matrigel-coated polycarbonate filter
in the Transwell chamber were analyzed. The results are presented
in Fig. 5. The majority of SW620
and LoVo cells invaded from the upper to the lower chamber in the
control group; however, the presence of PS VII inhibited the
penetration of the Matrigel-coated filter by SW620 and LoVo cells
(Fig. 5A). This inhibitory effect
was greatest at a concentration of 1 μM. The quantification of
cells in the lower chamber from Fig.
5B indicated that PS VII significantly inhibited SW620 and LoVo
cell invasion and that this inhibitory effect was
concentration-dependent.
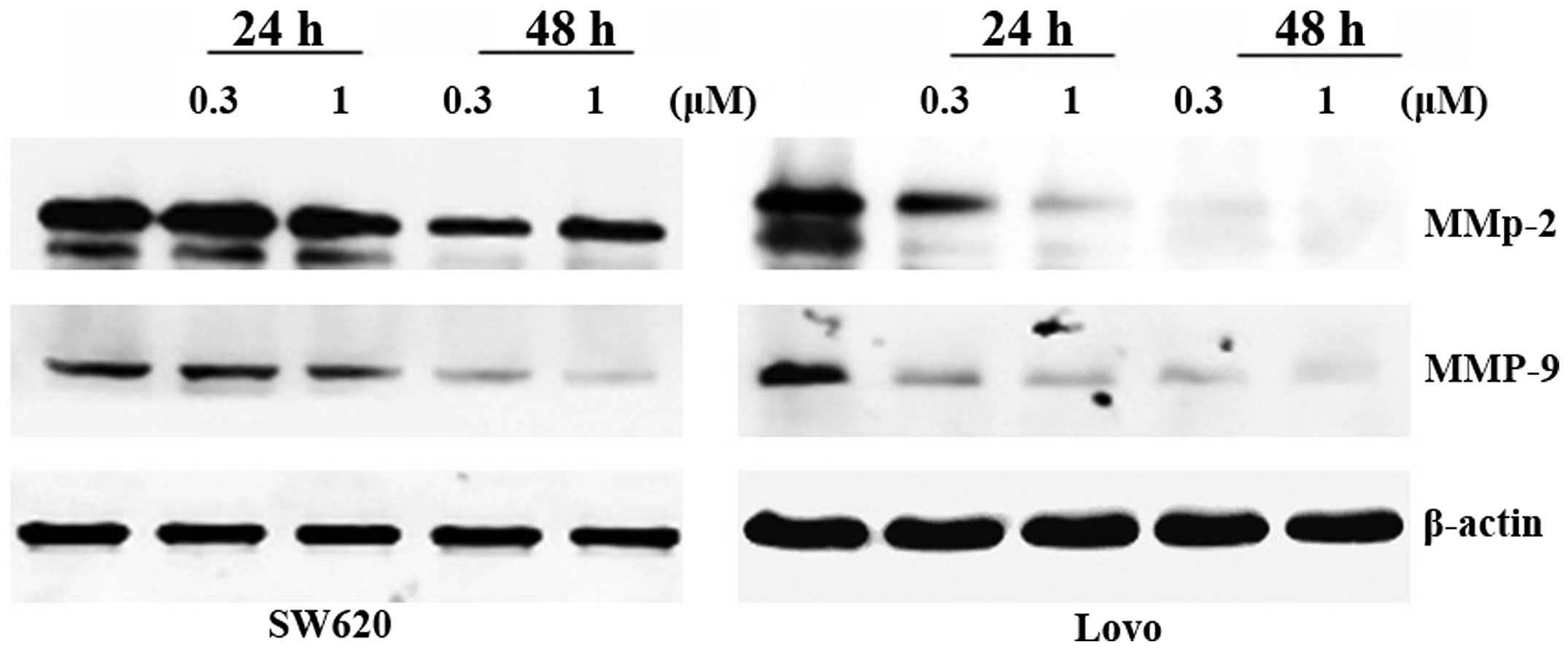
PS VII affects the expression levels of
metastasis-related proteins in SW620 and LoVo cells
The expression of MMPs is crucial to ECM
degradation, which is required for cell invasion. Hence, the effect
of PS VII on the expression of MMPs was investigated by western
blot analysis. The results demonstrated that PS VII suppresses the
expression levels of MMP-2 and MMP-9 proteins in a
concentration-dependent manner (Fig.
6). These effects may lead to the inhibition of the invasive
ability of SW620 and LoVo cells following exposure to PS VII.
PS VII suppresses the activity of MMP-2
and MMP-9
Although the protein expression of MMP-2 and MMP-9
was downregulated by PS VII, the activity of these two enzymes
during treatment with PS VII required further investigation.
Gelatinolytic activity at molecular masses of 72 and 92 kDa (which
correspond to the molecular mass of MMP-2 and MMP-9, respectively)
was detected in the conditioned medium of cells treated with PS
VII. The activity of MMP-2 and MMP-9 in the two cell lines was
markedly repressed by PS VII in a concentration-dependent manner
(Fig. 7).
Discussion
Saponins are natural glycosides which possess a wide
range of pharmacological properties, including cytotoxic activity.
In a previous study, it was determined that PS VII, a saponin
compound isolated from T. tschonoskii Maxim., possesses a
growth-inhibitory effect in vitro and in vivo.
Furthermore, non-apoptotic processes have also been revealed to be
involved in saponin cytotoxic activity, including cell cycle arrest
(18), autophagic cell death
stimulation (19), inhibition of
metastasis (20) and cytoskeleton
disintegration (21).
The suppression of cancer metastasis is an urgent
therapeutic requirement in the treatment of CRC. As resection of
the primary tumors is the treatment of choice, 30% of patients with
stage III CRC develop local recurrence or distant metastasis within
5 years of curative resection (22). However, the majority of existing
therapies only inhibit cancer cell proliferation and little has
been accomplished in terms of treating cancer metastasis. Cancer
cells are released from their primary locations early in
carcinogenesis, hence anti-metastasis drugs would be most effective
if they could assist in preventing the spread of cancer cells in
addition to suppressing existing tumor colonies. It has previously
been demonstrated that PS VII exerts a growth-inhibitory effect on
CRC cells. In the present study, the anti-metastatic activity of PS
VII was observed in two metastatic CRC cell lines, SW620 and
LoVo.
Metastasis is a complex, multistep process that
consists of a cascade of interrelated sequential steps, including
migration, invasion, adhesion, infiltration, colonization at a
distant site and the subsequent formation of new microvessels
(23). To successfully
metastasize, cancer cells must acquire the ability to migrate. The
migration of cells is based on cycles of lamellipodial extension,
attachment, cell body translocation, and retraction of the cell
(24). In a non-cytotoxic dose,
the present study evaluated the anti-migratory activity of PS VII
via a wound-healing assay which is a classic in vitro assay
of cell migration. The results revealed that PS VII reduces cell
migration in a concentration-dependent manner.
In the present study, the invasive ability of cells
was evaluated via a Transwell invasion assay. It was demonstrated
that PS VII inhibits the invasion rate of cells in a
concentration-dependent manner. Cell invasion requires proteolysis
of ECM components and transmigration to penetrate it. In these
steps, the expression of proteolytic enzymes, such as MMPs, is
crucial for ECM degradation (25).
Two members of the MMP family, the 72-kDa type IV collagenase MMP-2
(gelatinase A) and the 92-kD type IV collagenase MMP-9 (gelatinase
B), have been shown to be highly expressed and to be important
mediators in the pathogenesis of CRC metastasis (26). Increased MMP-2 and MMP-9 protein
expression levels are associated with a poor prognosis in patients
with CRC (27). Therefore, the
present study investigated whether the inhibitory effect of PS VII
on cell invasion occurred via the suppression of MMP-2 and MMP-9
expression. The results of gelatin zymography and western blot
assays verified that PS VII suppressed the activation and
expression of MMP-2 and MMP-9.
In the process of metastasis, the first step of
migration involves attachment of cells to the ECM and the process
of invading and exiting blood vessels requires attachment of cells
to the vascular endothelium. Therefore, the ability of cells adhere
to the ECM and vascular endothelial cells was assessed in this
study. Matrigel was used as an ECM in the migration and invasion
assays. The results demonstrated that cells which were pretreated
with PS VII exhibited reduced cell attachment to Matrigel. In the
cell-cell attachment assays, PS VII pretreatment reduced the
ability of cells to adhere to vascular endothelial cells. These
results indicate that PS VII also inhibits metastasis by impairing
the ability of cells to adhere.
In conclusion, the results of the current study
indicate that PS VII obtained from T. tschonoskii Maxim.
inhibits the migration, invasion and adhesion of human metastatic
CRC cells through inhibition of the activity and expression of
MMP-2 and MMP-9. Hence, PS VII may be a promising agent for
therapeutic and preventive purposes in the treatment of CRC, by not
only suppressing the proliferation of cancer cells but also by
inhibiting metastasis-associated events.
Acknowledgements
The authors of the present study would like to thank
the Department of Forestry of Shaanxi Province and the Taibaishan
Natural Preserve of Shaanxi Province for helping us collect the
Trillium tschonoskii Maxim. The present study was supported
by grants from the Taibaishan Natural Preserve of Shaanxi Province,
the National Nature Science Foundation of China (no. 81302787), and
the Postdoctoral Science Foundation of China (nos. 2012M512102 and
2013T60964).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2
|
Gupta GP and Massagué J: Cancer
metastasis: building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Steeg PS: Tumor metastasis: mechanistic
insights and clinical challenges. Nat Med. 12:895–904. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sanoff HK, Sargent DJ, Campbell ME, et al:
Five-year data and prognostic factor analysis of oxaliplatin and
irinotecan combinations for advanced colorectal cancer: N9741. J
Clin Oncol. 26:5721–5727. 2008.PubMed/NCBI
|
|
5
|
Prat A, Casado E and Cortés J: New
approaches in angiogenic targeting for colorectal cancer. World J
Gastroenterol. 13:5857–5866. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sarkar FH, Li Y, Wang Z and Kong D:
Cellular signaling perturbation by natural products. Cell Signal.
21:1541–1547. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cragg GM and Newman DJ: Natural products:
a continuing source of novel drug leads. Biochim Biophys Acta.
1830:3670–3695. 2013.PubMed/NCBI
|
|
8
|
Reddy L, Odhav B and Bhoola KD: Natural
products for cancer prevention: a global perspective. Pharmacol
Ther. 99:1–13. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Q, Xiao M, Guo L, et al: Genetic
diversity and genetic structure of an endangered species,
Trillium tschonoskii. Biochem Genet. 43:445–458. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yokosuka A and Mimaki Y: Steroidal
glycosides from the underground parts of Trillium erectum
and their cytotoxic activity. Phytochemistry. 69:2724–2730. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hayes PY, Lehmann R, Penman K, Kitching W
and De Voss JJ: Steroidal saponins from the roots of Trillium
erectum (Beth root). Phytochemistry. 70:105–113. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ono M, Sugita F, Shigematsu S, et al:
Three new steroid glycosides from the underground parts of
Trillium kamtschaticum. Chem Pharm Bull (Tokyo).
55:1093–1096. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Smicun Y, Gil O, Devine K and Fishman DA:
S1P and LPA have an attachment-dependent regulatory effect on
invasion of epithelial ovarian cancer cells. Gynecol Oncol.
107:298–309. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gamble JR and Vadas MA: A new assay for
the measurement of the attachment of neutrophils and other cell
types to endothelial cells. J Immunol Methods. 109:175–184. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hsu SC, Kuo CL, Lin JP, et al: Crude
extracts of Euchresta formosana radix inhibit invasion and
migration of human hepatocellular carcinoma cells. Anticancer Res.
27:2377–2384. 2007.
|
|
16
|
Huang YT, Hwang JJ, Lee LT, et al:
Inhibitory effects of a luteinizing hormone-releasing hormone
agonist on basal and epidermal growth factor-induced cell
proliferation and metastasis-associated properties in human
epidermoid carcinoma A431 cells. Int J Cancer. 99:505–513. 2002.
View Article : Google Scholar
|
|
17
|
Surgucheva IG, Sivak JM, Fini ME, Palazzo
RE and Surguchov AP: Effect of gamma-synuclein overexpression on
matrix metalloproteinases in retinoblastoma Y79 cells. Arch Biochem
Biophys. 410:167–176. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qin H, Du X, Zhang Y and Wang R:
Platycodin D, a triterpenoid saponin from Platycodon
grandiflorum, induces G2/M arrest and apoptosis in human
hepatoma HepG2 cells by modulating the PI3K/Akt pathway. Tumour
Biol. 35:1267–1274. 2014.PubMed/NCBI
|
|
19
|
Xu MY, Lee DH, Joo EJ, Son KH and Kim YS:
Akebia saponin PA induces autophagic and apoptotic cell death in
AGS human gastric cancer cells. Food Chem Toxicol. 59:703–708.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoon JH, Choi YJ and Lee SG: Ginsenoside
Rh1 suppresses matrix metalloproteinase-1 expression through
inhibition of activator protein-1 and mitogen-activated protein
kinase signaling pathway in human hepatocellular carcinoma cells.
Eur J Pharmacol. 679:24–33. 2012. View Article : Google Scholar
|
|
21
|
Ha TS, Lee JS, Choi JY and Park HY:
Ginseng total saponin modulates podocyte p130Cas in diabetic
condition. J Ginseng Res. 37:94–99. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kobayashi H, Mochizuki H, Sugihara K, et
al: Characteristics of recurrence and surveillance tools after
curative resection for colorectal cancer: a multicenter study.
Surgery. 141:67–75. 2007. View Article : Google Scholar
|
|
23
|
Weng CJ and Yen GC: Chemopreventive
effects of dietary phytochemicals against cancer invasion and
metastasis: phenolic acids, monophenol, polyphenol, and their
derivatives. Cancer Treat Rev. 38:76–87. 2012. View Article : Google Scholar
|
|
24
|
Berrier AL and Yamada KM: Cell-matrix
adhesion. J Cell Physiol. 213:565–573. 2007. View Article : Google Scholar
|
|
25
|
Friedl P and Wolf K: Tumour-cell invasion
and migration: diversity and escape mechanisms. Nat Rev Cancer.
3:362–374. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liska V, Sutnar A, Jr LH, et al: Matrix
metalloproteinases and their inhibitors in correlation to
proliferative and classical tumour markers during surgical therapy
of colorectal liver metastases. Bratisl Lek Listy. 113:108–113.
2012.
|
|
27
|
Langers AM, Verspaget HW, Hawinkels LJ, et
al: MMP-2 and MMP-9 in normal mucosa are independently associated
with outcome of colorectal cancer patients. Br J Cancer.
106:1495–1498. 2012. View Article : Google Scholar : PubMed/NCBI
|