Introduction
Dengue fever (DF), a mosquito-borne viral disease
caused by the dengue virus, is one of the most common infectious
diseases in tropical and subtropical regions. Patients infected
with dengue virus exhibit various clinical symptoms with different
levels of infection, from self-limited DF to life-threatening
dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS)
(1). Currently, there is no
effective antiviral therapy for patients with DF, and the lack of
effective mosquito control measures in dengue endemic areas
maintains a high incidence of dengue infection. Developing dengue
vaccines may provide a realistic approach for controlling dengue
infections.
Dengue viruses are members of the family
Flaviviridae and exist as four antigenically distinct
serotypes (DEN-1-4) (2). This has
been a major hindrance to vaccine development, as an individual who
has experienced a single dengue virus infection develops
neutralizing antibodies to that specific serotype, but remains
susceptible to the other three serotypes of dengue virus. The
cross-reactive, non-neutralizing antibody from the first dengue
infection may bind to other serotypes of dengue virus, enhancing
the uptake of virions into monocytic cell lines through interaction
with cell surface immunoglobulin (Ig) receptors. This is a
phenomenon known as antibody-dependent enhancement of infection
(ADE) (3). The mechanism of ADE is
considered as an important contributing factor to DHF and DSS
(4). Therefore, an effective
dengue vaccine must induce long-lasting and tetravalent
type-specific neutralizing antibodies against all four serotypes.
At present, there is no effective vaccine available, despite much
research into methods of immunization for dengue infection. Early
efforts to develop a dengue vaccine used the live attenuated virus
(5,6) and inactivated whole virion
vaccination methods (7). Other
approaches principally focused on recombinant strategies, including
the use of chimeric viruses encoding an antigen gene (8–10),
antigen-encoding plasmids (11–13)
and recombinant subunit antigens produced by heterologous
expression systems (14–18).
The envelope (E) protein is the major structural
protein of the dengue virus, and the majority of the recombinant
vaccine strategies of previous studies have focused on it, due to
its biological function of host cell surface receptor binding
(19). Previous studies have
demonstrated that the E protein is able to induce neutralizing
antibodies and protective immunity (14,20).
Further crystallographic studies have indicated that the E proteins
from dengue viruses and other flaviviruses contain three structural
domains (I, II and III) (21,22).
Domain III (DIII) is an independent IgG-like folding domain
(23) that contains multiple type-
and subtype-specific neutralizing epitopes and host cell receptor
recognition sites (19,24–27).
Furthermore, it has been reported that either recombinant fusion
proteins (15,17) containing an envelope or
plasmid-encoding DIII gene (11,12)
were able to induce neutralizing antibodies in mice. It has also
been demonstrated that the fusion DIII protein does not elicit
cross-reactive antibodies to heterologous dengue virus serotypes
(15).
In the present study, domain III of the E protein
(defined as EDIII) of the dengue type 1 and 2 viruses were spliced
with a flexible peptide linker (Gly-Gly-Ser-Gly-Ser)3.
The fusion gene was then subcloned into either a prokaryotic
expression plasmid pET30a (+) or a eukaryotic expression vector
pcDNA3.1 (+), then expressed in Escherichia (E.) coli and
mammalian BHK-21 cells. The three immunization strategies were
examined using the bivalent DNA vaccine, the recombinant bivalent
antigen, or a combination of the two to determine whether the level
of neutralizing antibodies is increased following immunization with
a combination of two vaccines compared with levels of antibodies
following a single vaccination.
Materials and methods
Materials
Escherichia coli host strains DH5α and BL21
(DE3) were purchased from Takara Biotechnology, Dalian, China. The
expression plasmid pET30a (+) was obtained from Novagen (Darmstadt,
Germany) and pcDNA3.1 (+) from Invitrogen Life Technologies
(Carlsbad, CA, USA). DEN-2 virus-specific monoclonal antibody (mAb;
3H5) (catalog no. MAB8702) was from Chemicon (Temecula, CA, USA).
The secondary antibody-enzyme conjugates (anti-mouse IgG-alkaline
phosphatase, anti-mouse IgG-fluorescein isothiocyanate conjugate)
were obtained from SouthernBiotech (Birmingham, AL, USA). The ion
exchange column (DEAE-5PW, TSK-GEL®) was purchased from
Tosoh Bioscience (Tokyo, Japan) and the high-performance liquid
chromatography (HPLC) system (Agilent 1100 Series HPLC Value
system) was from Agilent Technologies, Inc. (Santa Clara, CA,
USA).
The DEN-1 (Hawaii strain) and DEN-2 (NGC strain)
were gifts from Dr Hui-Ming Luo (Center for Disease Control and
Prevention, Guangdong, China). C6/36 mosquito cells and BHK-21
cells were obtained from Wuhan Culture Collection, (Wuhan, China).
C6/36 cells were maintained in RPMI-1640 medium (Gibco, Grand
Island, NY, USA) with 10% fetal bovine serum (FBS) in a humidified
incubator with 5% CO2 at 28°C. BHK-21 cells were
cultured in the same medium as C6/36 but incubated at 37°C in a
humidified 5% CO2 incubator. The QIAmp Viral RNA Mini
Kit, One-Step RT-PCR kit and the EndoFree Plasmid Maxi kit were
obtained from Qiagen (Hilden, Germany).
Construction of expression vectors
C6/36 cells were infected (MOI=0.01–0.1 PFU/cell)
with either DEN-1 or DEN-2 virus. After 4 days, tissue culture
supernatants underwent RNA extraction using the QIAmp Viral RNA
Mini Kit. The genome RNAs were used as templates to obtain DEN-1
and DEN-2 EDIII gene fragments (designated as D1EDIII and D2EDIII)
by one-step real-time polymerase chain reaction (RT-PCR). To
facilitate head-to-tail fusion of two fragments, a linker region
consisting of (Gly-Gly-Ser-Gly-Ser)3 was introduced,
taking advantage of the presence of a BamHI site in its
sequence. The sequence of the linker was
GGTGGCTCTGGATCTGGCGGTTCTGGATCC GGTGGCTCTGGATCT (the underlined
sequence is the BamHI restriction site). The primers used
for PCR amplification are presented in Table I. The PCR was performed as follows:
denaturing at 94°C for 2 min, 35 cycles (denaturation at 94°C for
30 sec, renaturation at 60°C for 30 sec and extension at 72°C for
30 sec), and final extension at 72°C for 7 min. Following PCR, 10
μl PCR products were separated using agarose gel electrophoresis.
The PCR generated two fragments that were ~400 bp in length,
predicted to code aa290–420 [encompassing the DEN-1 EDIII
(aa293–412) and the DEN-2 EDIII (aa298–400) (28)]. The fragment D1EDIII was purified
and digested with KpnI and BamHI, then ligated into
the corresponding KpnI and BamHI restriction sites of
the expression vector pET30a (+), generating a recombinant plasmid
that was labeled as pET30a-D1EDIII. To create the D1/D2EDIII
chimeric bivalent gene, fragment D2EDIII was digested with
BamHI and XhoI and inserted into the BamHI and
XhoI sites of pET30a-D1EDIII. The resulting pET30a-D1/2EDIII
recombinant plasmid was identified by restriction analysis and
sequencing.
 | Table IPrimers used in the present
study. |
Table I
Primers used in the present
study.
| Primer | Sequence
(5′–3′) |
|---|
| D1F |
5′-GTGGTACCATGGACAAACTGACCTTAAAAGGG-3′ |
| D1R |
5′-GTGGATCCAGAACCGCCAGATCCAGAGCCACCCCATGCGGTGTCTCCTAG-3′ |
| D2F |
5′-GTGGATCCGGTGGCTCTGGATCT
GACAAACTACAGCTCAAA-3′ |
| D2R |
5′-GATCTCGAGTCACCAGGCTGTGTCACCTAAAAT-3′ |
To construct the recombinant eukaryotic expression
vector, pET30a-D1/2EDIII was digested with KpnI and
XhoI to release the chimeric bivalent gene, D1/2EDIII. The
fragment D1/2EDIII was then ligated into the
KpnI/XhoI restriction sites of pcDNA3.1 to generate
pcDNA-D1/2EDIII. The recombinant clones were amplified in E.
coli DH5α and purified with the EndoFree Plasmid Maxi
purification kit (Takara Biotechnology, Dalian, China).
Expression and purification of
recombinant pro-D1/D2EDIII protein in E. coli
E. coli BL21 (DE3) were transformed with
plasmid pET30a-D1/2EDIII and grown in lysogeny broth (LB) medium
(Sigma-Aldrich, St Louis, MO, USA) containing 30 μg/ml kanamycin at
37°C until an optical density (OD) of 0.6 was reached. Then, 0.6 mM
isopropylthiogalactoside (IPTG) was added. After 4 h, 1 ml of cells
was harvested, lysed and analyzed by SDS-PAGE. The bacteria
transformed with expression vector pET30a (+) were included as
negative controls. To obtain large yields of recombinant protein,
bacteria transfected with pET30a-D1/2EDIII were used to inoculate
into 1 liter LB growth medium containing 30 μg/ml kanamycin
(Sigma-Aldrich). When the stationary growth phase was reached, 0.6
mM IPTG was added to induce expression for 4 h. The cell pellet was
obtained by centrifugation (4,000 × g for 10 min), resuspended in
lysis buffer [50 mM Tris-HCl (pH 7.2), 20 mM EDTA and 100 mM NaCl]
and then sonicated. Following centrifugation at 12,000 × g for 10
min, aliquots of the supernatant and the pellet were analyzed by
SDS-PAGE to determine whether the recombinant protein was
soluble.
The pellet containing the inclusion bodies was
resuspended in solubilization buffer (20 mM Tris-HCl (pH 8.0), 5 mM
EDTA, 100 mM NaCl and 8 M urea), dialyzed overnight in dialysis
buffer I [10 mM phosphate buffer (pH 6.0) and 4 M urea] and
filtered through 0.22-μm-pore filter units (EMD Millipore,
Billerica, MA, USA). The supernantant was applied to HPLC on a
cation exchange column (TSKgel DEAE-5PW, Tosoh Bioscience)
pre-equilibrated with buffer I. The column was washed with buffer I
for 30 min, then with a linear gradient from buffer I to buffer II
(1 M NaCl in buffer I) for 60 min and finally washed with buffer II
for 30 min. The elution fractions were collected and analyzed by
SDS-PAGE. The elution containing the recombinant protein
(designated as pro-D1/D2EDIII) was dialyzed against
phosphate-buffered saline (PBS) to remove urea, and then the total
protein from the E. coli transformants was analyzed by 12%
SDS-PAGE, followed by Coomassie blue staining (Sigma-Aldrich).
Western blot analysis
Purified pro-D1/D2EDIII protein was purified by 12%
SDS-PAGE and transferred electrophoretically to a nitrocellulose
membrane. The membrane was blocked overnight with blocking buffer
(5% non-fat dried milk in PBS; Sigma-Aldrich) and then incubated
with primary antibody (either a serum from mouse polyclonal
anti-DEN-1 EDIII or anti-DEN-2 EDIII antibody at 1:500 dilution in
blocking buffer) at room temperature for 1 h. The membrane was
washed with PBST (1× PBS containing 0.1% Tween 20) three times and
reacted with alkaline phosphatase-conjugated goat anti-mouse IgG at
1:5,000 in blocking buffer for 1 h at room temperature. Following
washing, the protein bands were detected with 5-bromo-4-chloro-3
indolyl phosphate toluidinium (BCIP) and nitroblue tetrazolium
chloride (NBT) (Sigma-Aldrich).
Cell transfection and immunoflourescence
assay (IFA)
BHK-21 cells were transfected with plasmid
pcDNA-D1/2EDIII using Lipofectamine 2000 reagent (Life
Technologies, Carlsbad, CA, USA). Cells were cultured in RPMI-1640
medium supplemented with 10% FBS without antibiotic at 37°C and 5%
CO2. One day prior to transfection, cells were
trypsinized and plated at a density of 1×105 cells/well
on six-well plates, so that they were 40–50% confluent on the day
of transfection. A total of 2 μg pcDNA-D1/2 EDIII or pcDNA3.1 (+)
(as negative control) was used and transfection was performed
according to the manufacturer’s instructions. Cells were analyzed
48 h post-transfection by indirect IFA using either a mouse
polyclonal antibody prepared against purified bacterial expressed
pro-D1/D2EDIII protein or mAb 3H5. Briefly, cells were washed once
with PBS, air dried, fixed with ice-cold acetone and incubated for
45 min at 37°C with either polyclonal anti-pro-D1/D2EDIII antibody
(at 1:40 dilution in 0.01 M PBS) or 3H5 mAb (at 1:200 dilution in
0.01 M PBS). Cells were washed with PBS three times, incubated for
30 min with fluorescein-conjugated goat anti-mouse IgG (at 1:50
dilution in 0.01 M PBS), and detected under a fluorescent
microscope.
Mouse immunization and antibody
detection
Groups of BALB/c mice (4–6 weeks old; Huafukang
Biotechnology Ltd., Beijing, China) were immunized
intraperitoneally with 50 μg recombinant pro-D1/D2EDIII protein
emulsified with Freund’s complete adjuvant (PRO group), and/or 100
μg pcDNA-D1/2EDIII (DNA group or PRO/DNA group) on day 0, 14 and
28. The mice were maintained under a light/dark cycle, at 25°C.
Negative control mice received PBS at similar time-points. Blood
was obtained from the mice 10 days after the final injection and
sera were collected and stored at −70°C until use. The mice were
sacrificed by cervical dislocation 28 days after the final
injection. Animal experiments were reviewed and approved by the
Fujian Medical Research Center of Animal Care and adhered to the
guidelines of the Government of China.
The antibody titers of mouse sera were evaluated by
an indirect ELISA approach. Each of the monovalent DEN-1 and DEN-2
EDIII proteins (pro-D1EDIII, pro-D2EDIII) were used as a capture
antigen to assess serotype specificity of antisera. Following
coating for 2 h at 37°C with pro-D1EDIII or pro-D2EDIII (100 ng in
100 μl/well), 96-well microplates were blocked with 1% bovine serum
albumin in PBS-Tween 20 for 2 h at 37°C, washed three times with
PBS-Tween 20 and incubated with serial two-fold dilutions of mouse
sera for 30 min at 37°C. The wells were then washed a further three
times and incubated with peroxidase-conjugated goat anti-mouse IgG
(1:5,000 dilution in blocking buffer) at 37°C for 30 min. Following
three washes, 3,3′-5,5′-tetramethly benzidine substrate (100
μl/well) was added and the samples were incubated at 37°C for 15
min. The reaction was stopped by adding 50 μl of 1 M
H2SO4 and the absorbance of each well was
read at 450 nm using a microplate reader (GelDox XR; Bio-Rad
Laboratories, Hercules, CA, USA).
Neutralizing antibody titers were determined by
plaque reduction neutralization test (PRNT) using either DEN-1 or
DEN-2 virus with BHK-21 cells, as previously described (29). The neutralization titer was defined
as the maximal dilution yielding a 50% reduction in the number of
plaque-forming units, expressed as the PRNT50 titer.
Statistical analysis
Logarithmic transformations of the reciprocal
PRNT50 titers of the mice in each immunization group
were made, and the mean log titers were compared by analysis of
variance. The mean log titers were converted to genometric mean
(GM) titers for presentation. P-values were calculated using SAS
version 8.2 statistical software (SAS Inc., Cary, NC, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Creation of recombinant expression
vectors
The construction of the recombinant pET30a-D1/2EDIII
is illustrated in Fig. 1A. This
construct was predicted to encode a 335-aa recombinant protein with
a molecular weight of 37 kDa, and verified to be correct by
restriction and sequencing (data not shown).
The D1/2EDIII chimeric bivalent gene was obtained
from pET30a-D1/2EDIII by restriction digestion with
KpnI/XhoI and cloned into the eukaryotic expression
vector pcDNA3.1 (+) under the control of the cytomegalovirus
promoter. A His tag was used to construct pcDNA-D1/2EDIII (Fig. 1B). The recombinant plasmid was
correctly expressed in transfected BHK-21 cells, as demonstrated by
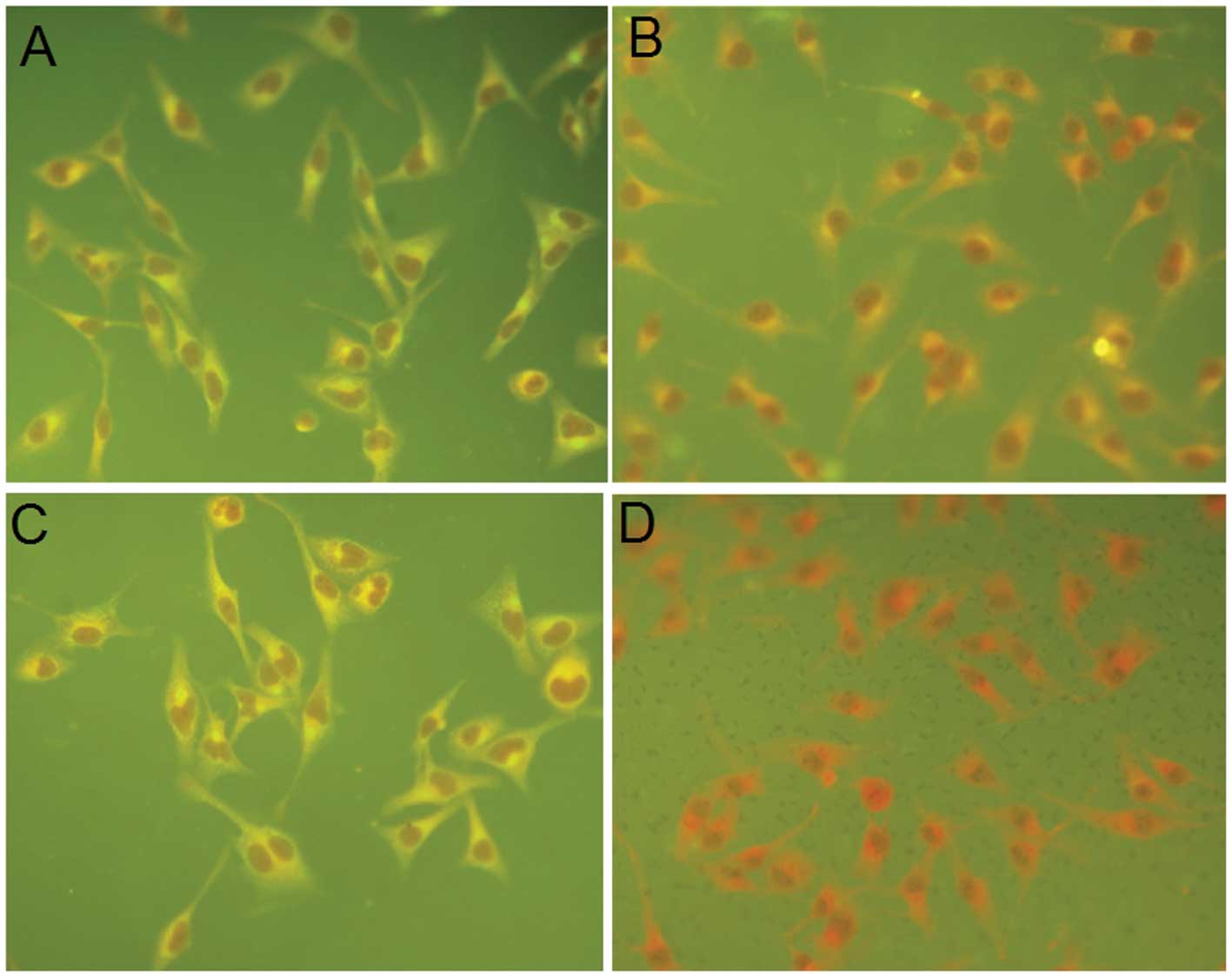
IFA results (Fig. 2). The
pcDNA-D1/2EDIII-transfected cells manifested a strong fluorescent
signal when detected with either polyclonal anti-pro-D1/D2EDIII
antibody or 3H5 mAb (Fig. 2A and
C), compared with cells transfected with pcDNA3.1 (+)(Fig. 2B and D).
Expression and purification of
recombinant pro-D1/D2EDIII bivalent protein in E. coli
The recombinant plasmid pET30a-D1/2EDIII was
transformed into E. coli BL21 (DE3), and expression was
induced by IPTG for ~4 h. Total protein from E. coli
transformants was analyzed by 12% SDS-PAGE followed by Coomassie
blue staining. As displayed in Fig.
3A, a dominant band corresponding to the predicted size was
detected in the pellet from bacteria carrying pET30a-D1/2EDIII
(lane 2) but not from cells transformed with pET30a alone (lane 3).
To examine the relative distribution of expressed recombinant
protein in the soluble or insoluble fraction, 1 liter culture of
bacteria transformed with pET30a-D1/2EDIII was prepared. The cell
pellets were suspended in lysis buffer and sonicated. Following
centrifugation, the supernatant and the pellet fractions were
analyzed by SDS-PAGE. No dominant band was detected in the
supernatant of the cell lysates (Fig.
3A, lane 4), but the majority of the expressed recombinant
protein was associated with the pellet fraction (Fig. 3A, lane 5). Hence, the recombinant
protein was present in the form of inclusion bodies.
The insoluble inclusion bodies were lysed in 8 M
urea, dialyzed in buffer I and applied to an HPLC cation exchange
column. The fractions, eluted with varying concentrations of NaCl
for different time periods, were colleted and analyzed by SDS-PAGE
(data not shown), which indicated that the 37 kDa pro-D1/2EDIII
protein was eluted at 44–46 min. The 44–46 min fractions were
pooled and dialyzed to eliminate urea, and thus, purified D1/2EDIII
protein was obtained. As demonstrated in Fig. 3B, lane 2, a single band of 37 kDa
was obtained following the purification steps, the final
concentration of the purified protein was 1.05 mg/ml. Thus, ~20 mg
of purified protein was obtained from a liter culture of induced
cells.
In order to demonstrate that the recombinant
bivalent protein preserves the antigenicity of DEN-1 and DEN-2
EDIII, purified pro-D1/D2EDIII was detected by western blot assay
using sera from mouse polyclonal anti-DEN-1 EDIII or anti-DEN-2
EDIII antibodies. The results are presented in Fig. 3C. The anti-DEN-1 EDIII or
anti-DEN-2 EDIII antibody was able to recognize the chimeric
pro-D1/2EDIII protein (lanes 2 and 3).
Serum antibody response in mice
All of the mice inoculated with recombinant protein
or/and DNA demonstrated an IgG antibody response against either
pro-D1EDIII (Fig. 4A) or
pro-D2EDIII (Fig. 4B). The OD
value of the PRO/DNA group was the highest and the D group was the
lowest, while the PRO group exhibited an intermediate response.
PBS-immunized sera did not display significant ELISA reactivity
against pro-D1EDIII or pro-D2EDIII.
To evaluate the ability of antibodies to neutralize
the DEN-1 and DEN-2 viruses, PRNT was performed. The results are
summarized in Table II. The GM
PRNT50 titers of the PRO/DNA group against either DEN-1
or DEN-2 were significantly higher than those of the PRO group
(P<0.05), which were significantly higher than those of the DNA
group (P<0.05).
 | Table IIVirus-neutralizing antibody titers in
sera of immunized mice. |
Table II
Virus-neutralizing antibody titers in
sera of immunized mice.
| GM
PRNT50 titers |
|---|
|
|
|---|
| Immunogen | DEN-1 | DEN-2 |
|---|
| PRO (n=8) | 34.9 | 45.3 |
| DNA (n=8) | 24.7 | 26.9 |
| PRO/DNA (n=8) | 64.0 | 76.1 |
| PBS (n=8) | ND | ND |
Discussion
The development of a dengue vaccine continues to be
a challenge, as the vaccine must protect against four serotypes of
dengue virus without eliciting the ADE reaction in order to be
effective. Previous approaches have focused on live attenuated
dengue virus, and development of a ‘tetravalent’ formulation by
mixture of four monovalent dengue serotypes (5,30–34).
However, simple physical mixture may lead to a predominant immune
response towards a single serotype of dengue virus, which has been
attributed to viral interference (8,33,35,36).
Recently, increasing attention is being focused on the recombinant
strategies, mainly based on subunit proteins or DNA plasmids.
The EDIII of the DEN-2 virus has been successfully
expressed in E. coli with or without fusion protein
(15,17,37),
and these investigations demonstrated that the recombinant DEN-2
EDIII protein has the biological function of blocking the virus
from binding to host cells (37)
and the ability to elicit a neutralizing antibody reaction
(15,17). A tetravalent dengue vaccine,
created by physically mixing four monovalent components into a
single formulation based on domain III, has also been studied
(12,16). However, a single tetravalent
vaccine has a clear advantage over a mixture of four ‘monovalent’
components, as it provides a significant cost benefit and has the
potential to eliminate non-uniform immune responses.
In the present study, a bivalent dengue subunit
vaccine and DNA vaccine were constructed using a prokaryotic
expression plasmid or eukaryotic expression vector containing a
bivalent gene, obtained by splicing EDIIIs of dengue virus
serotypes 1 and 2. A recombinant plasmid pET30a-D1/2EDIII was
created by splicing the EDIII genes of DEN-1 and 2. A linker
(Gly-Gly-Ser-Gly-Ser)3 was introduced to maintain
structure integrity and biological function of the two
serotype-specific EDIII proteins, taking advantage of glycine’s
lack of a β carbon and serine’s propensity of hydrogen bonding
(38). The recombinant bivalent
antigen was successfully expressed in E. coli and purified
by HPLC with a yield of ~20 mg/liter. To verify whether the
purified pro-D1/D2EDIII protein still retained the antigenicity of
the monovalent EDIIIs it was derived from, a western blot analysis
was performed using mouse polyclonal anti-DEN-1 or DEN-2 EDIII
antibodies. The results demonstrated that the recombinant bivalent
protein was recognized by the anti-DEN-1 or DEN-2 EDIII antibodies.
Furthermore, the result of the ELISA indicated that the antibodies
from mice immunized with the recombinant protein may react with
monovalent pro-D1EDIII and D2EDIII at high levels. It is possible
that the recombinant bivalent protein preserved the antigenic
integrity of its precursors.
Furthermore, it was determined whether these
antibodies were able to neutralize the DEN-1 and DEN-2 viruses. The
PRNT data indicated that the GM PRNT50 titers of
antibody were 34.9 for DEN-1 and 45.3 for DEN-2. Neutralizing
antibody titers are widely accepted as markers of protective
immunity, with PRNT50 titers of 1:10 being considered
indicative of protective immunity (39,40).
The present study suggested that the recombinant bivalent protein
was able to produce protective antibodies against the DEN-1 and
DEN-2 viruses. These results are similar to those obtained by
Khanam et al (41), who
created a chimeric dengue antigen expressed in E. coli by
splicing EDIIIs of virus serotypes 2 and 4 that was able to elicit
neutralizing antibodies against dengue virus serotypes 2 and 4. The
titers of neutralizing antibodies have been demonstrated to be
lower than those induced by EDIII-MBP proteins (16). The contrast is theorized to be
attributed to the differences of the experimental parameters of the
PRNT assays and the host cells that were used. Furthermore, the
uncertainty of the carrier activity and safety of MBP in humans
causes an obstacle in the application of these fusion proteins in a
vaccine.
Promising DNA vaccines have been developed against
the dengue virus previously: Mota et al (12) developed a tetravalent DNA vaccine
consisting of a mixture of four plasmids, each encoding EDIII genes
specific to one DEN serotype. However, extrapolation from the study
by Mota et al (12) was
limited as only the level of DEN-2 neutralizing antibodies was
examined and the other three serotypes were not evaluated. In the
present study, a pcDNA-D1/2EDIII recombinant plasmid was generated,
which, as a bivalent gene, encoded the EDIIIs of DEN-1 and 2. To
develop an effective DNA vaccine, it is essential that the
recombinant protein, encoded by the DNA plasmid, is expressed
correctly (42). The result of IFA
indicated that cells transfected with pcDNA-D1/2EDIII were able to
react with anti-pro-D1/D2EDIII antibody (Fig. 2A) or the 3H5 mAb (Fig. 2C), which is specific to DEN-2 EDIII
(29,30). The results of the present study
suggested that the recombinant plasmid pcDNA-D1/2EDIII was capable
of being expressed adequately in mammalian cells. The GM
PRNT50 titers induced by DNA were 24.7 for DEN-1 and
26.9 for DEN-2, which is in contrast with the neutralizing antibody
level of ~1:10 that was reported by Mota et al (12).
It has previously been reported that the use of a
combination of DNA vaccine and recombinant protein with a
prime-boost strategy can induce higher antibody titers than the
individual vaccines alone (11,43,44).
A study by Simmons et al (11) indicated that the combination of a
DEN-2 DNA vaccine coding for premembrane and E proteins and a
recombinant fusion protein containing the domain III of the DEN-2 E
protein fused to the maltose-binding protein (MBP) induced higher
neutralizing titers than the DNA or protein alone. In the current
study, various immunization strategies were tested to observe
whether it is possible to increase the level of neutralizing
antibodies against DEN-1 and DEN-2 induced by a combination of DNA
and protein, compared with when the vaccines were used alone. The
results indicated that when mice were immunized with a combination
of DNA and protein, significantly higher titers of neutralizing
antibodies against either DEN-1 or DEN-2 (64.0 or 76.1,
respectively) were observed, compared with titers following
vaccination with the DNA or the recombinant protein alone. Similar
antibody responses were observed using ELISA, and an association
between total antibody levels measured by ELISA and neutralizing
antibody titers was indicated. This result is in contrast with the
study by Simmons et al (11), which noted a lack of correlation
between total antibody levels and PRNT50 titers. This
contrast may be explained by the fact that Simmon et al
(11) had a larger number of total
epitopes available in the DNA vaccine coding for the premenbrane
and E proteins, whereas the present study used only domain IIIs for
the construction of a DNA vaccine.
In conclusion, a recombinant prokaryotic/eukaryotic
expression plasmid containing fusion EDIIIs of DEN-1 and DEN-2 was
successfully constructed in the present study, using a flexible
peptide linker. The recombinant protein and DNA vaccines were able
to induce neutralizing antibodies against DEN serotypes 1 and 2.
The current strategies used to construct fusion bivalent genes,
expression and one-step purification are valuable in developing an
inexpensive scale-up tetravalent dengue subunit vaccine or DNA
vaccine. However, the combination of bivalent DNA and the
recombinant protein resulted in higher levels of neutralizing
antibodies, and may have the potential to induce improved
cell-mediated immune responses in addition to humoral immune
responses, compared with DNA or protein subunit vaccines alone.
Further studies are required to assess the ability to release
cytokines and the duration of neutralizing antibodies in response
to the three immunization strategies (DNA vaccine, recombinant
subunit vaccine, or a combination of the two).
References
|
1
|
Agarwal R, Kapoor S, Nagar R, et al: A
clinical study of the patients with dengue hemorrhagic fever during
the epidemic of 1996 at Lucknow, India. Southeast Asian J Trop Med
Public Health. 30:735–740. 1999.
|
|
2
|
Rosen L: Comments on the epidemiology,
pathogenesis and control of dengue. Med Trop (Mars). 59:495–498.
1999.
|
|
3
|
Morens DM and Halstead SB: Measurement of
antibody-dependent infection enhancement of four dengue virus
serotypes by monoclonal and polyclonal antibodies. J Gen Virol.
71:2909–2914. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Anderson R, Wang S, Osiowy C and Issekutz
AC: Activation of endothelial cells via antibody-enhanced dengue
virus infection of peripheral blood monocytes. J Virol.
71:4226–4232. 1997.PubMed/NCBI
|
|
5
|
Bhamarapravati N and Sutee Y: Live
attenuated tetravalent dengue vaccine. Vaccine. 18(Suppl 2): 44–47.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eckles KH, Dubios DR, Putnak R, et al:
Modification of dengue virus strains by passage in primary dog
kidney cells: preparation of candidate vaccines and immunization of
monkeys. Am J Trop Med Hyg. 69(Suppl): 12–16. 2003.
|
|
7
|
Putnak R, Barvir DA, Burrous JM, et al:
Development of a purified, inactivated, dengue-2 virus vaccine
prototype in Vero cells: immunogenicity and protection in mice and
rhesus monkeys. J Infect Dis. 174:1176–1184. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guirakhoo F, Pugachev K, Zhang Z, et al:
Safety and efficacy of chimeric yellow fever-dengue virus
tetravalent vaccine formulations in nonhuman primates. J Virol.
78:4761–4775. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Van der Most RG, Murali-Krishna K, Ahmed R
and Strauss JH: Chimeric yellow fever/dengue virus as a candidate
dengue vaccine: Quantitation of the dengue virus-specific CD8
T-cell response. J Virol. 74:8094–8101. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guirakhoo F, Arroyol J, Pugachev KV, et
al: Construction, safety, and immunogenecity in nonhuman primates
of a chimeric yellow fever dengue virus tetravalent vaccine. J
Virol. 75:7290–7304. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Simmons M, Murphy GS, Kochel T, et al:
Characterization of antibody responses to combinations of a
dengue-2 DNA and dengue-2 recombinant subunit vaccine. Am J Trop
Med Hyg. 65:420–426. 2001.PubMed/NCBI
|
|
12
|
Mota J, Acosta M, Argotte R, et al:
Induction of protective antibodies against dengue virus by
tetravalent DNA immunization of mice with domain III of the
envelope protein. Vaccine. 23:3469–3476. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu SF, Liao CL, Lin YL, et al: Evaluation
of protective efficacy and immune mechanisms of using a
non-structural protein NS1 in DNA vaccine against dengue 2 virus in
mice. Vaccine. 21:3919–3929. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Men R, Wyatt L, Tokimatsu I, et al:
Immunization of rhesus monkeys with a recombinant of modified
vaccina virus Ankara expressing a truncated envelope glycoprotein
of dengue type 2 virus induced resistance to dengue type 2 virus
challenge. Vaccine. 18:3113–3122. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Simmons M, Nelson WM, Wu SJ and Hayes CG:
Evaluation of the protective efficacy of a recombinant dengue
envelope B domain fusion protein against dengue 2 virus infection
in mice. Am J Trop Med Hyg. 58:655–662. 1998.PubMed/NCBI
|
|
16
|
Simmons M, Murphy GS and Hayes CG: Short
report: Antibody response of mice immunized with a tetravalent
dengue recombinant protein subunit vaccine. Am J Trop Med Hyg.
65:159–161. 2001.PubMed/NCBI
|
|
17
|
Hermida L, Rodriguez R, Lazo L, et al: A
dengue-2 envelope fragment inserted within the structure of the
P64k meningococcal protein carrier enables a functional immune
response against the virus in mice. J Virol Methods. 115:41–49.
2004. View Article : Google Scholar
|
|
18
|
Kelly EP, Greene JJ, King AD and Innis BL:
Purified dengue 2 virus envelope glycoprotein aggregates produced
by baculovirus are immunogenic in mice. Vaccine. 18:2549–2559.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Y, Maguire T and Marks RM:
Demonstration of binding of dengue envelope protein to target
cells. J Virol. 70:8765–8772. 1996.PubMed/NCBI
|
|
20
|
Bray M and Lai CJ: Dengue virus
premembrane and membrane proteins elicit a protective immune
response. Virology. 185:505–508. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rey FA, HeinA FX, Mandl C, et al: The
envelope glycoprotein from tick-borne encephalitis virus at 2 A
resolution. Nature. 375:291–298. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roehrig JT, Volpe KE, Squires J, et al:
Contribution of disulfide bridging to epitope expression of the
dengue type 2 virus envelope glycoprotein. J Virol. 78:2648–2652.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bhardwaj S, Holbrook M, Shope RE, et al:
Biophysical characterization and vector-specific antagonist
activity of domain III of the tick-borne flavivirus envelope
protein. J Virol. 75:4002–4007. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Crill WD and Roehrig RT: Monoclonal
antibodies that bind to domain III of dengue virus E glycoprotein
are the most efficient blockers of virus E glycoprotein are most
efficient blockers of virus adsorption to Vero cells. J Virol.
75:7769–7773. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Megret F, Hugnot JP, Falconar MK, et al:
Use of recombinant fusion proteins and monoclonal antibodies to
define linear and discontinuous antigenic sites on the dengue
envelope glycoprotein. Virology. 187:480–491. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Roehrig JT, Johnson AJ, Hunt AR, et al:
Antibodies to dengue 2 virus E-glycoprotein synthetic peptides
identity antigenic conformation. Virology. 177:668–675. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hung JJ, Hsieh MT, Young MJ, et al: An
external loop region of domain III of dengue virus type 2 envelope
protein is involved in serotype-specific binding to mosquito but
not mammalian cells. J Virol. 78:378–388. 2004. View Article : Google Scholar :
|
|
28
|
Simmons M, Nelson WM, Wu SJ and Hayes CG:
Evaluation of the protective efficacy of a recombinant dengue
envelope B domain fusion protein against dengue 2 virus infection
in mice. Am J Trop Med Hyg. 58:655–662. 1998.PubMed/NCBI
|
|
29
|
Morens DM, Halstead SB, Repik PM, et al:
Simplified plaque reduction assay for dengue viruses by
semi-micromethods in BHK21 cells: comparison of the BHK suspension
test standard plaque reduction neutralization. J Clin Microbio.
22:250–254. 1985.
|
|
30
|
Trirawatanapong T, Chandran B, Putnak R
and Padmanabhan R: Mapping of a region of dengue virus type-2
glycoprotein required for binding by a neutralizing monoclonal
antibody. Gene. 116:139–150. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun W, Edelman R, Kanesa-Thasan N, et al:
Vaccination of human volunteers with monovalent and tetravalent
live-attenuated dengue vaccine candidates. Am J Trop Med Hyg.
69(Suppl): 24–31. 2003.
|
|
32
|
Edelman R, Wasserman SS, Bodison SA, et
al: Phase I trial of 16 formulations of a tetravalent
live-attenuated dengue vaccine. Am J Trop Med Hyg. 69(Suppl):
48–60. 2003.
|
|
33
|
Kitchener S, Nissen M, Nasveld P, et al:
Immunogenicity and safety of two live-attenuated tetravalent dengue
virus vaccine formulations in healthy Australian adults. Vaccine.
24:1238–1241. 2006. View Article : Google Scholar
|
|
34
|
Sabchareon A, Lang J, Chanthavanich P, et
al: Safety and immunogenicity of a three dose regimen of two
tetravalent live-attenuated dengue vaccines in five- to
twelve-year-old Thai children. Pediatr Infect Dis J. 23:99–109.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Swaminathan S and Khanna N: Viral vaccines
for dengue: the present and the future. WHO Dengue Bul. 27:181–191.
2003.
|
|
36
|
Kanesa-thasan N, Sun W, Kim-Ahn G, et al:
Safety and immunogenicity of attenuated dengue virus vaccines
(Aventis Pasteur) in human volunteers. Vaccine. 19:3179–3188. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jaiswal S, Khanna N and Swaminathan S:
High-level expression and one-step purification of recombinant
dengue virus type 2 envelope domain III protein in Escherichia
coli. Protein Expr Purif. 33:80–91. 2004. View Article : Google Scholar
|
|
38
|
Robinson CR and Sauer RT: Optimizing the
stability of single-chain proteins by linker length and composition
mutagenesis. Pro Natl Acad Sci USA. 95:5929–5934. 1998. View Article : Google Scholar
|
|
39
|
Putnak R, Feighny R, Burrous J, et al:
Dengue-1 virus envelope glycoprotien gene expressed in recombinant
baculovirus elicits virus-neutralizing antibody in mice and
protects them from virus challenge. Am J Trop Med Hyg. 45:159–167.
1991.PubMed/NCBI
|
|
40
|
Delenda C, Staropoli I, Frenkiel MP, et
al: Analysis of C-terminally truncated dengue 2 and dengue 3 virus
envelope glycoproteins: processing in insect cells and immunogenic
properties in mice. J Gen Virol. 75:1569–1578. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Khanam S, Etemad B, Khanna N and
Swaminathan S: Induction of neutralizing antibodies specific to
dengue virus serotypes 2 and 4 by a bivalent antigen composed of
linked envelope domains III of these two serotypes. Am J Trop Med
Hyg. 74:266–277. 2006.PubMed/NCBI
|
|
42
|
Chang GJ, Davis BS, Hunt AR, et al:
Flavivirus DNA vaccines: current status and potential. Ann N Y Acad
Sci. 951:272–285. 2001. View Article : Google Scholar
|
|
43
|
Sedegah M, Jones TR, Kaur M, et al:
Boosting with recombinant vaccinia increases immunogenicity and
protective efficacy of malaria DNA vaccine. Proc Natl Acad Sci USA.
95:7648–7653. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Barnett SW, Rajasekar S, Legg H, et al:
Vaccination with HIV-1 gp120 DNA induces immune responses that are
boosted by a recombinant gp 120 protein subunit. Vaccine.
15:869–873. 1997. View Article : Google Scholar : PubMed/NCBI
|