Introduction
The burden of breast cancer continues to increase
according to global cancer statistics (1). Breast cancer is the most frequently
diagnosed cancer and the leading cause of cancer-associated
mortality among females. Systemic chemotherapy is an important type
of complementary treatment for breast cancer. As the effectiveness
of chemotherapy significantly decreases, even leading to
resistance, it is crucial to identify definite predictors of
sensitivity to chemotherapy, including the widely accepted factors
programmed cell death protein 5, human epidermal growth factor
receptor 2 and topoisomerase IIα (2). Hepatocyte growth factor (HGF) belongs
to the family of plasminogen-related growth factors (PRGFs) and is
also called PRGF-1. As evidenced by previous studies, HGF is the
ligand of the c-Met receptor and is a multifunctional cytokine,
which is involved in tumor cell-cell interactions, matrix adhesion,
migration, invasion and angiogenesis (3–6).
Breast cancer cells can produce HGF that acts in a
paracrine as well as in an autocrine manner (7). HGF is able to induce CXC chemokine
receptor 4 expression and contributes to tumor cell invasiveness in
breast cancer and the c-Met-HGF axis can enhance the metastatic
behavior of breast cancer cells (8). Serum levels of HGF in breast cancer
patients were significantly increased when compared with controls
(6). It was reported that patients
with more advanced tumor-node-metastasis (TNM) staging have higher
serum soluble HGF levels (9).
Furthermore, HGF/Met signaling was reported to be involved in
breast cancer progression (10).
However, to the best of our knowledge, few studies have
demonstrated that HGF is expressed in breast cancer and the
possibility that HGF may be a potential predictor of the
effectiveness of chemotherapy remains to be elucidated (4,11).
The present study was designed to confirm the expression profile of
HGF in breast cancer tissues from 125 patients and in breast cancer
cells, and to elucidate the possible association between the
expression level of HGF, the effectiveness of chemotherapy and the
prognosis of patients.
Materials and methods
Clinical data
Samples were collected from 125 patients diagnosed
with breast cancer through breast biopsy prior to chemotherapy at
the Henan Tumor Hospital (Zhengzhou, China) between June 2008 and
June 2010. The pathological findings of the patients were analyzed
following surgery. Of the 125 patients, 62 received 1 week
presurgical chemotherapy among whom 41 patients adopted the
cyclophosphamide (CTX) epirubicin (EPI) and 5-fluorouracil (5-FU)
regimen, including intravenous injection of CTX at 800
mg/m2 on day 1 and 8, intravenous drip of EPI at 60
mg/m2 on day 2 and 3 and an intravenous drip of 5-FU at
500 mg/m2 during day 4 and 6. In total, 21 adopted the
cyclophosphamide (CTX) tetrahydrofuran (THF) and 5-FU regimen,
including intravenous injection of CTX at 800 mg/m2 on
day 1 and 8, intravenous drip of THF at 30 mg/m2 on day
2 and 3 and an intravenous drip of 5-FU at 500 mg/m2
during day 4 and 6. All the 125 patients were female and the
average age was 44.5±6.3 years. The histopathological types were as
follows: 92 patients had invasive ductal carcinoma, 18 invasive
lobular carcinoma, 3 tubular carcinoma, 2 papillary carcinoma, 4
mucous carcinoma and 6 typical medullary carcinoma. In addition, 52
patients had postoperative lymph node metastasis and 76 were
estrogen-receptor (ER) (+) and 71 were progesterone receptor (PR)
(+). Their histological grades were as follows: 94 were grade I and
II and 31 were grade III. Their TNM clinical stages were as
follows: 87 were stage I and II and 38 were stage III. Follow-up
data of 112 patients were completed. The present study was
conducted in accordance with the declaration of Helsinki and with
approval from the Ethics Committee of Henan Tumor Hospital. Written
informed consent was obtained from all participants.
Cell culture and transfection
The human breast cancer cell line, MCF-7, was
purchased from the Shanghai Cell Bank of the Chinese Academy of
Sciences (Shanghai, China). The MCF-7 cells were cultured in
Dulbecco’s modified Eagle’s medium (Gibco-BRL, Grand Island, NY,
USA) with 10% fetal bovine serum (Gibco-BRL), 100 U/ml penicillin
and 100 mg/ml streptomycin at 37°C in an incubator with 5%
CO2 and 95% relative humidity. MCF-7 cells were
trypsinized and passaged into 6-well or 96-well plates and were
transfected with small interfering (si)RNAs to HGF when the cell
density reached 30–50% confluence. Lipofectamine 2000 (Invitrogen
Life Technologies, Carlsbad, CA, USA) was used for transfection in
accordance with the manufacturer’s instructions.
Immunohistochemistry
Immunohistochemistry was performed using the
two-step EnVision procedure (Dako, Copenhagen, Denmark). Briefly,
each tissue section was deparaffinized, hydrated and then incubated
with fresh 3% hydrogen peroxide (H2O2) in
methanol for 15 min. Following rinsing with phosphate-buffered
saline (PBS), antigen retrieval was performed by microwave
treatment in 0.01 M sodium citrate buffer (pH 6.0) at 100°C for 15
min. Following this, tissue sections were incubated with primary
monoclonal mouse-anti-human HGF antibodies (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) diluted in PBS containing
0.2% Triton X-100 for 30 min at room temperature. Following rinsing
with PBS, slides were incubated with the ChemMate™
EnVision™+/horseradish peroxidase for 30 min at room temperature.
The reaction was visualized using ChemMate™ 3,3′-diaminobenzidine
(DAB). Negative controls were prepared by substituting primary
antibodies with PBS.
Immunohistochemical staining
Cells that generated a brown-colored polymeric
oxidation product in their cytoplasm were defined as HGF-positive
cells. Analysis of 10 discrete foci was performed in every section.
The positive cells were graded in a blinded manner according to the
following criteria: 0, positive cells ≤5% and 1, positive cells
>5%. The stain intensity was graded according to the following
criteria: 0, no apparent brown-colored polymeric oxidation product
and 1, clear brown-colored polymeric oxidation product. The final
histoscore grade was calculated as the aggregate of positive cell
grade plus the stain intensity grade: Negative, 0–1 or positive, 2.
As the estrogen receptor (ER) and progesterone receptor (PR) are
expressed deep inside the nucleus, the DAB staining for them must
be located within the nucleus. All experiments were repeated three
times. Histological interpretation was performed independently by
two pathologists blinded to the study conditions using an Olympus
CX41 microscope (magnification, ×40; Olympus Corporation, Tokyo,
Japan).
Reverse-transcription quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the MCF-7 cells 48 h
after transfection with siRNA-HGF using an RNeasy mini kit (Qiagen,
Valencia, CA, USA). RT-qPCR was performed and the primer sequences
used were as follows: HGF, forward 5′-CCACACGAACACAGCTATCGGGG-3′
and reverse 5′-TGGGAGCAGTAGCCAACTCGGA-3′; GAPDH, forward
5′-GTCAGTGGTGGACCTGACCT-3′ and reverse 5′-ACCTGGTGCRCAGTGRAGCC-3′.
RNA without reverse transcriptase was also amplified and used as a
negative control to rule out possible genomic DNA contamination.
The PCR products were electrophoresed using a 1.2% agarose gel. The
density of visualized bands was measured with a Tocan 240 imaging
analysis device (Tocan, Shanghai, China).
Western blot analysis
The MCF-7 cells transfected with siRNA-HGF were
lysed in lysis buffer. Following centrifugation at 15,000 × g for
15 min, the protein concentration was measured using a BCA protein
detection kit (Pierce Biotechnology, Inc., Rockford, IL, USA) and
adjusted for equal loading. Subsequently, cell lysates were
subjected to SDS-PAGE. Immunoreactivity was visualized by enhanced
chemiluminescence. Quantification of immunoreactive bands was
performed using Image Gauge software (Fuji Photo Film Co., Ltd.,
Tokyo, Japan).
MTT
The MCF-7 cells were passaged in 96-well plates and
transfected with siRNA-HGF in five duplicates. MTT (20 μl of 5
mg/ml) was added into the media 24, 48 or 72 h after transfection,
respectively. Dimethyl sulfoxide (150 μl) was added following
incubation in a culture hood for another 4 h and then agitated on
an orbital shaker for 10 min. The optical density value was
detected using a 650–60 spectrophotometer (Hitachi, Ltd., Tokyo,
Japan) at 490 nm. In each group, different concentrations of EPI
(0.5, 1, 2, 4 and 8 μg/μl) was added 72 h after transfection and
the absorbance reading was performed again following incubation for
another 24 h. The experiment was repeated three times.
Assessment of chemotherapy
effectiveness
The assessment of chemotherapy effectiveness was
based on the Response Evaluation Criteria In Solid Tumors
Guidelines established by the National Institutes of Health
(Bethesda, MA, USA) and confirmed at 4 weeks: Complete response
(CR), complete disappearance of all target lesions; partial
response (PR), at least a 30% decrease in the sum of the longest
diameter of target lesions; stable disease (SD), neither sufficient
shrinkage to qualify for partial response nor sufficient increase
to qualify for progressive disease; progressive disease (PD), at
least a 20% increase in the sum of the longest diameter of target
lesions. In the present study, ‘effective’ was defined as ‘CR + PR’
and ‘ineffective’ as ‘SD + PD’.
Statistical analysis
All data were analyzed using SPSS 13.0 statistical
software (SPSS, Inc., Chicago, IL, USA). Student’s paired t-test
and χ2 test were performed to analyze statistical
significance in continuous variables and categorical variables,
respectively. Survival rate was analyzed by a log-rank test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of HGF in human breast cancer
tissues
In order to analyze the association between the
expression of HGF and the clinical parameters in the present study,
the positive rate of HGF in breast cancer tissues was determined.
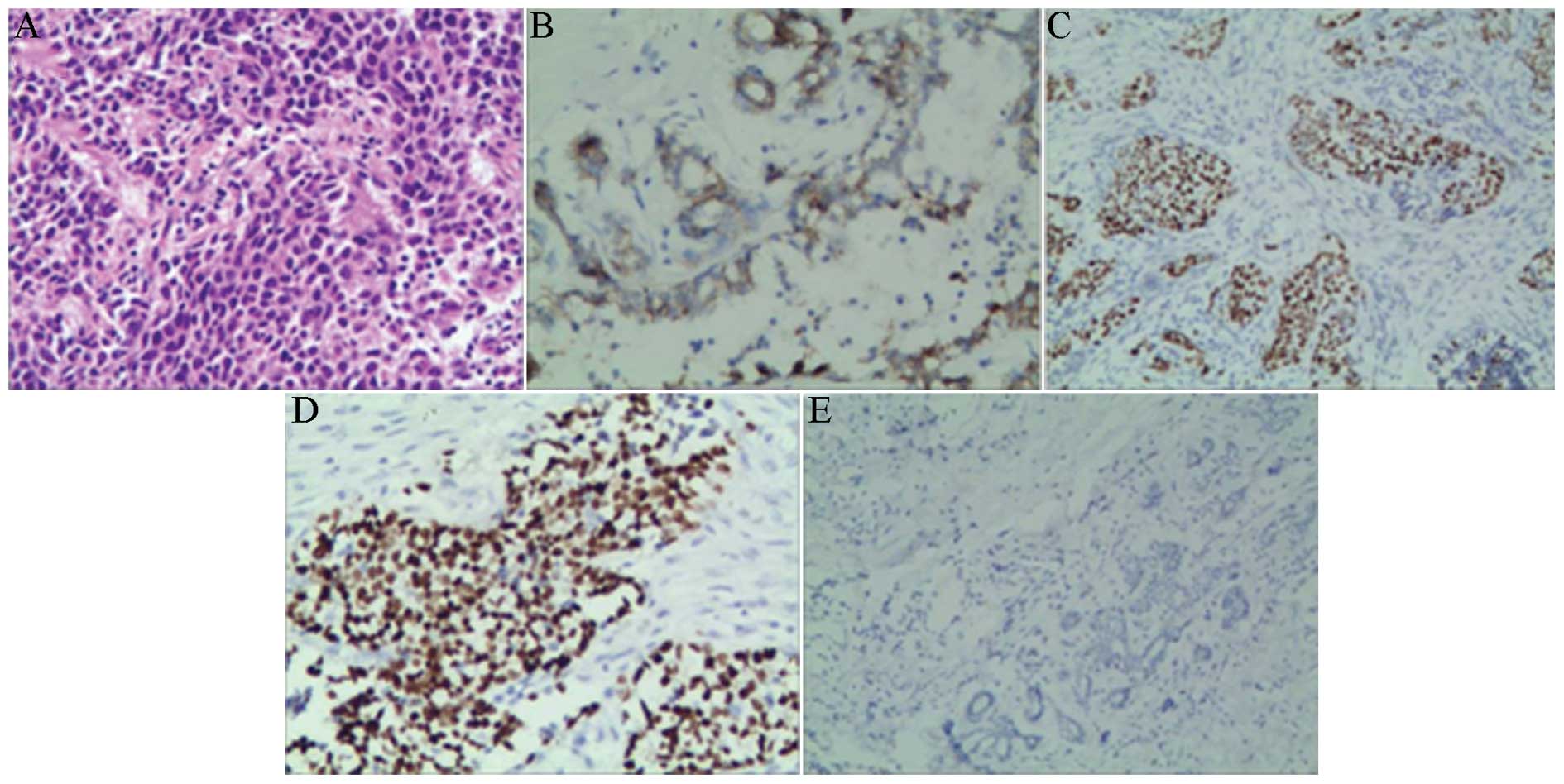
Breast cancer tissues were diagnosed by two pathologists and 92 of
the 125 patients were diagnosed as infiltrating ductal carcinoma
(Fig. 1A). The positive rate of
HGF in human breast cancer tissues was 52% and was associated with
TNM clinical stage, histological grade, lymph node metastasis
(P<0.05), however, HGF was not associated with patient age and
location, size and hormone receptor status of tumor (P>0.05;
Table I; Fig. 1B–E).
 | Table IExpression of HGF and its association
with patient characteristics. |
Table I
Expression of HGF and its association
with patient characteristics.
| Pathological
feature | HGF | X2/T | P-value |
|---|
|
|---|
| − | + |
|---|
| Age | 28 | 35 | 0.0529 | >0.05 |
| <44 | 32 | 30 | | |
| ≥44 | | | | |
| Location |
| Left | 27 | 36 | 1.3459 | >0.05 |
| Right | 33 | 29 | | |
| Tumor size (cm) |
| <5 | 37 | 50 | 3.4322 | >0.05 |
| ≥5 | 23 | 15 | | |
| Histological
grade |
| I–II | 50 | 44 | 5.4597 | <0.05 |
| III | 10 | 21 | | |
| TNM clinical
stage |
| I–II | 51 | 36 | 12.9332 | <0.01 |
| III | 9 | 29 | | |
| Lymph node
metastasis |
| + | 14 | 38 | 15.8475 | <0.01 |
| − | 46 | 27 | | |
| ER |
| + | 33 | 43 | 1.6286 | >0.05 |
| − | 27 | 22 | | |
| PR |
| + | 35 | 36 | 0.1106 | >0.05 |
| − | 25 | 29 | | |
Correlation between HGF level and patient
sensitivity to chemotherapy
In order to demonstrate the correlation between HGF
level and patient sensitivity to chemotherapy, the effectiveness of
chemotherapy in HGF positive or negative patients was compared. As
is shown in Table II, the
effeciency of chemotherapy in HGF negative patients was 90%, which
was significantly higher (P<0.05) than that in HGF positive
patients (68.75%).
 | Table IICorrelation between HGF and
chemotherapy sensitivity. |
Table II
Correlation between HGF and
chemotherapy sensitivity.
| Items | CR | PR | SD | PD | Efficiency (%) | P-value |
|---|
| HGF+ | 3 | 19 | 8 | 2 | 68.75 | <0.05 |
| HGF− | 7 | 20 | 2 | 1 | 90 | <0.05 |
mRNA expression of HGF following
transfection in MCF-7 cells
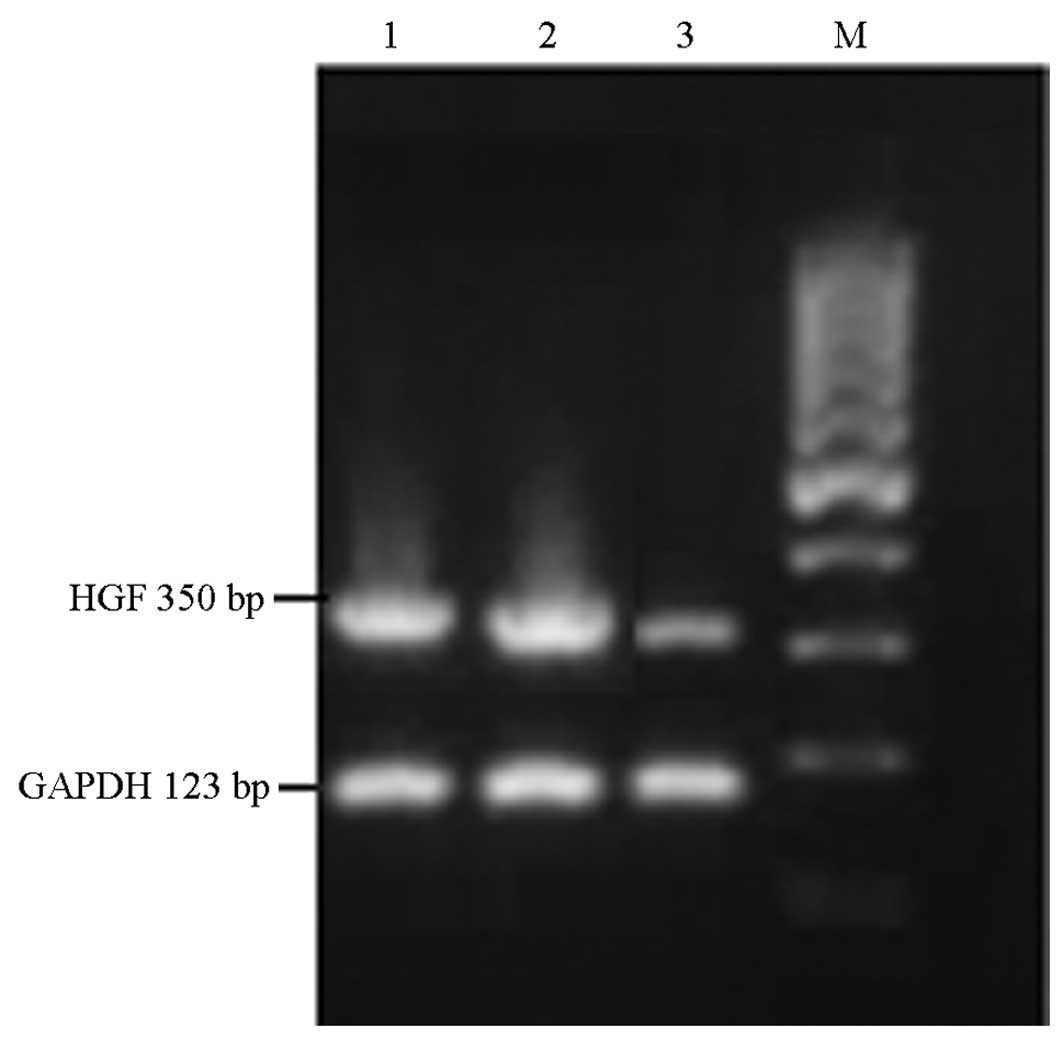
In order to confirm the inhibition of HGF mRNA
expression by transfection with HGF-siRNA, RT-qPCR was performed in
MCF-7 cells following transfection. As shown in Fig. 2, the mRNA expression of HGF was
expressed in non-transfected MCF-7 cells. A similar expression
level was found in the siRNA-GFP group. This was significantly
downregulated following siRNA-HGF transfection (55%), demonstrating
that the mRNA expression of HGF was successfully inhibited.
Protein expression of HGF following siRNA
transfection
To further confirm the inhibition of HGF at the
protein level, western blot analysis was performed on the three
groups. As shown in Fig. 3, HGF
protein was expressed in non-transfected MCF-7 cells and a similar
expression level was found in the siRNA-GFP group. This was
significantly downregulated following siRNA-HGF transfection,
indicating that the protein expression of HGF was successfully
inhibited.
Proliferation assay following HGF-siRNA
transfection and EPI co-culture
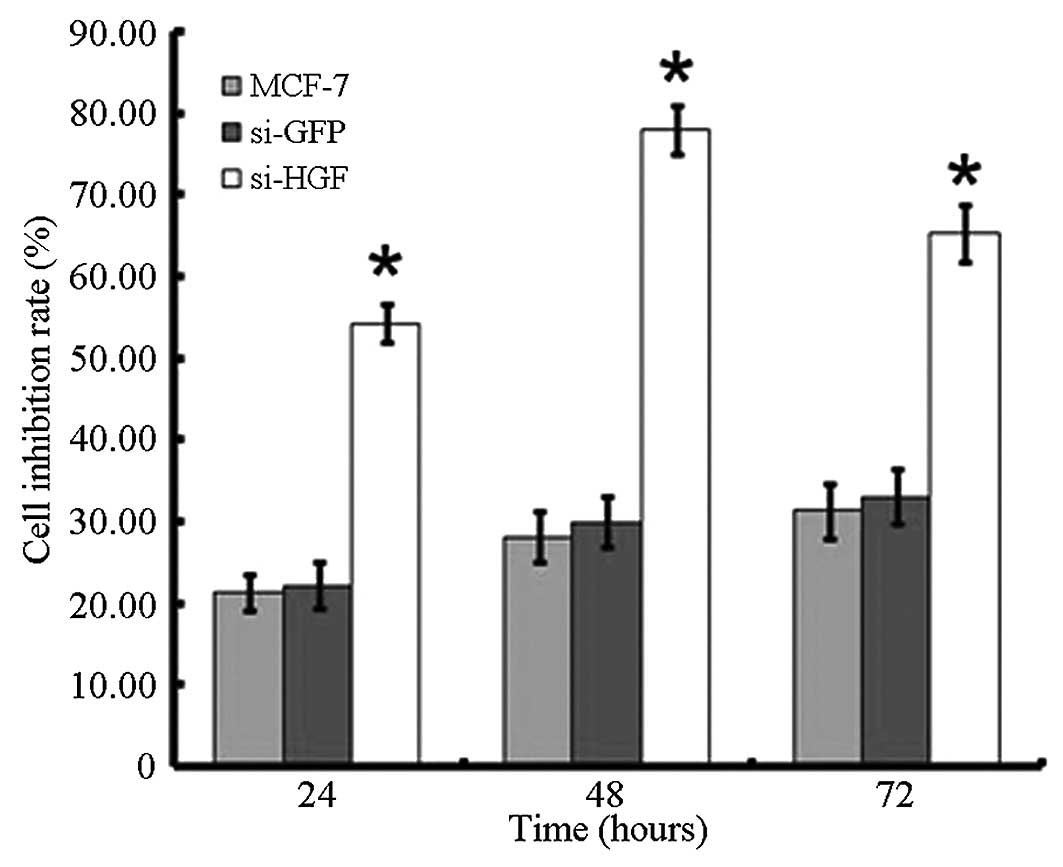
In order to determine whether HGF can affect cell
proliferation, an MTT assay was performed. As shown in Fig. 4, the cell inhibition rate increased
following the downregulation of HGF by HGF-siRNA transfection. The
inhibitory rate after 24, 48 and 72 h was 54.33, 78.09 and 65.33%,
respectively, and was significantly higher than that of
non-transfected and siRNA-GFP transfected groups (P<0.05;
Fig. 4).
As sensitivity to EPI in MCF-7 cells can represent
cell sensitivity to chemotherapy, the sensitivity to EPI in the
three groups was compared. The cell viability in the siRNA-HGF
transfected group was significantly lower than the non-transfected
and siRNA-GFP transfected groups, indicating that the expression of
HGF has a vital role in cell survival when co-cultured with EPI
(Fig. 5).
Correlation between HGF level and
prognosis of breast cancer patients
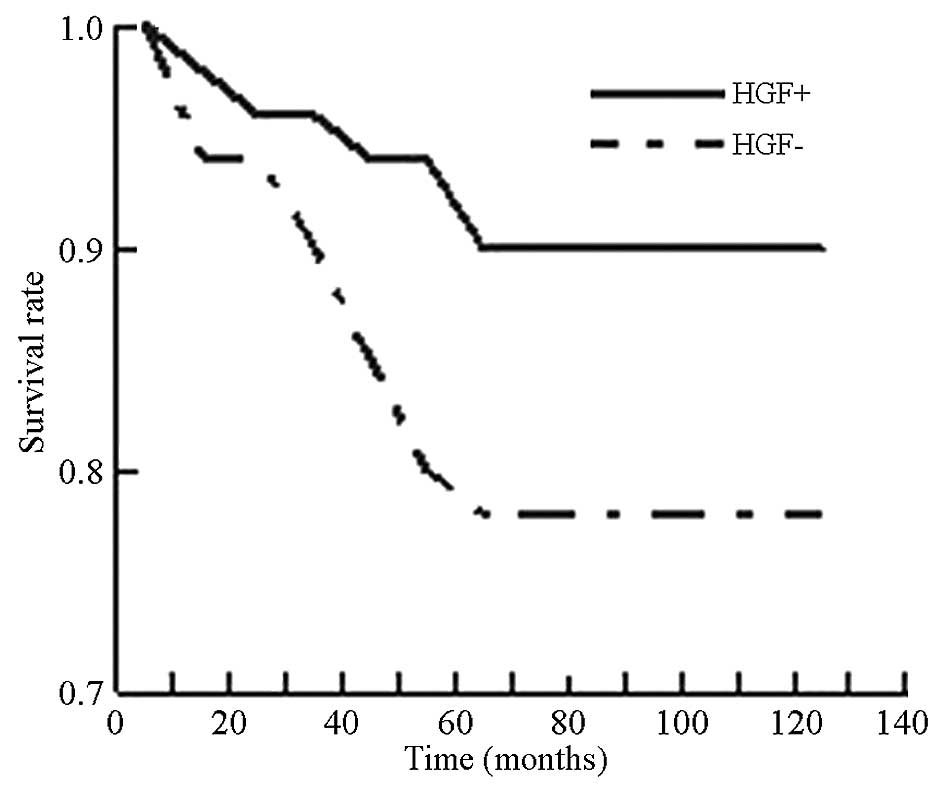
Correlation analysis was performed to characterize
the association between the expression of HGF and patient survival
time. As shown in Fig. 6, the
5-year survival rate of HGF positive patients was 78%, which was
significantly lower (P<0.05) than that of HGF negative patients
(90%). The correlation analysis suggested that HGF may be an
important factor in patient survival in breast cancer.
Discussion
Breast cancer is clinically characterized as having
a high morbidity, low chance of success through surgery alone, high
recurrence and metastasis rate, poor prognosis and resistance to
chemotherapy. It remains the most frequently diagnosed cancer and
the leading cause of cancer-associated mortality among females.
Thus, it is important to investigate the factors that can be used
to predict metastasis, prognosis and sensitivity to chemotherapy
post-surgically. HGF is a type of multifunctional peptide factor
that is secreted by epithelial cells. It promotes the process of
proliferation, migration, invasion and angiogenesis of various
types of tumor (3,4,12).
It has been widely reported that high expression of HGF is closely
associated with the prognosis of non-small cell lung cancer and
colon cancer patients (9,13). Yamashita et al (14) demonstrated that HGF was highly
expressed in breast cancer and the expression level was closely
associated with the prognosis of patients. Sheen-Chen et al
(15) demonstrated that HGF is
significantly correlated with the histological grade, clinical
stage, tumor size and lymph node metastasis while investigating the
association between the expression level of HGF and the
pathological parameters in breast cancer patients. Parr et
al (4) reported that breast
cancer specimens express a significantly higher level of HGF, which
indicates that the HGF regulatory system may be important in the
progression of breast cancer. In the present study, the expression
of HGF in breast cancer tissues of 125 patients was detected by
immunohistochemistry. The results indicated that HGF was highly
expressed in breast cancer tissues of patients and the positive
rate was 52%. The expression of HGF was not associated with patient
age and location, size and hormone receptor status of tumor,
however, HGF expression was associated with TNM clinical stage,
histological grade, lymph node metastasis and prognosis.
Furthermore, all 125 patients were followed up and their survival
time and survival rate were compared and evaluated. The 5-year
survival rate of HGF positive patients was significantly lower than
that of HGF negative patients. This result indicated that HGF may
be one of the essential predictors that contribute to the prognosis
of breast cancer patients, which was consistent with a study by
Eichbaum et al (16).
As it can improve the survival rate and surgical
outcomes of breast cancer patients, neoadjuvant chemotherapy (NACT)
has been used more and more widely in clinical practice.
Nevertheless, few studies investigating the effect of NACT on HGF
and HGF as a predictor for sensitivity to chemotherapy was
insufficiently characterized. In order to avoid the unnecessary
side effects of chemotherapy that patients may undergo and to
improve the sensitivity to chemotherapy, defined valuable
indicators are required to better understand NACT. Of the breast
cancer patients in the present study, 62 were in the presurgical
NACT group. Among them, the effective rate of chemotherapy in HGF
negative patients was ~90% and was significantly higher than that
in HGF positive patients (68.75%), which suggested that HGF is
closely associated with sensitivity to chemotherapy. Analogous
results were reported by Lengyel et al (17). They identified that the HGF
receptor, c-Met was closely associated with sensitivity to
chemotherapy and of all the patients in the study, five converted
to complete remission and the positive rate of c-Met was 20%.
Furthermore, in vitro studies were performed
to confirm that HGF was able to increase the sensitivity to
chemotherapy in breast cancer patients. The HGF expression profile
of MCF-7 cells was identified following the specific silence of HGF
by siRNA. As shown in the RT-qPCR and western blotting results, HGF
was significantly downregulated following siRNA transfection in
MCF-7 cells, whose proliferation rate significantly decreased
compared with the control groups. This confirmed that HGF had the
capacity to enhance cell proliferation. By contrast, when
co-cultured with EPI for 24 h, the survival rate of transfected
cells was significantly lower than that of the non-transfected
group. In this manner, HGF has been verified to be important in
resistance to EPI at the cellular level. The conclusion that HGF is
associated with sensitivity to chemotherapy in breast cancer
patients was also supported by other studies (18,19).
It was hypothesized that this function was associated with the
activation of intracellular AKT, which is linked to cellular
resistance to chemotherapeutic drugs (20). Future studies may determine the
concrete mechanisms by which HGF functions during this process.
In conclusion, HGF may be a promising, new
therapeutic target for breast cancer and may enable clinical
practitioners to better predict patient sensitivity to NACT and
prognosis through detecting patient HGF levels. Although the
present study demonstrated the possibility and availability of HGF
as a useful predictor, understanding the mechanisms underlying the
effect requires further investigation.
Acknowledgements
This study was supported by a grant from the Project
of National Natural Science Funds and Youth Funds, China (grant no.
81000914).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang L, Wang C, Su B, et al: Recombinant
human PDCD5 protein enhances chemosensitivity of breast cancer in
vitro and in vivo. Biochem Cell Biol. 91:526–531. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Singh-Kaw P, Zarnegar R and Siegfried JM:
Stimulatory effects of hepatocyte growth factor on normal and
neoplastic human bronchial epithelial cells. Am J Physiol.
268:L1012–L1020. 1995.PubMed/NCBI
|
|
4
|
Parr C, Watkins G, Mansel RE and Jiang WC:
The hepatocyte growth factor regulatory factors in human breast
cancer. Clin Cancer Res. 10:202–211. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang W, Hiscox S, Matsumoto K and
Nakamura T: Hepatocyte growth factor/scatter factor, its molecular,
cellular and clinical implications in cancer. Crit Rev Oncol
Hematol. 29:209–248. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
El-Attar HA and Sheta MI: Hepatocyte
growth factor profile with breast cancer. Indian J Pathol
Microbiol. 54:509–513. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gallego MI, Bierie B and Hennighausen L:
Targeted expression of HGF/SF in mouse mammary epithelium leads to
metastatic adenosquamous carcinomas through the activation of
multiple signal transduction pathways. Oncogene. 22:8498–8508.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang S, Ouyang N, Lin L, et al:
HGF-induced PKCζ activation increases functional CXCR4 expression
in human breast cancer cells. PLoS One. 7:e291242012. View Article : Google Scholar
|
|
9
|
Siegfried JM, Weissfeld LA, Luketich JD,
Weyant RL, Gubish CT and Landreneau RJ: The clinical significance
of hepatocyte growth factor for non-small cell lung cancer. Ann
Thorac Surg. 66:1915–1918. 1998. View Article : Google Scholar
|
|
10
|
Locatelli A, Lofgren KA, Daniel AR, Castro
NE and Lange CA: Mechanisms of HGF/Met signaling to Brk and Sam68
in breast cancer progression. Horm Cancer. 3:14–25. 2012.
View Article : Google Scholar
|
|
11
|
Woodbury RL, Varnum SM and Zangar RC:
Elevated HGF levels in sera from breast cancer patients detected
using a protein microarray ELISA. J Proteome Res. 1:233–237. 2002.
View Article : Google Scholar
|
|
12
|
Hirose Y, Kojima M, Sagoh M, Murakami H,
Yoshida K, Shimazaki K and Kawase T: Immunohistochemical
examination of c-Met protein expression in astrocytic tumors. Acta
Neuropathol. 95:345–351. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takeuchi H, Bilchik A, Saha S, et al:
c-MET expression level in primary colon cancer: a predictor of
tumor invasion and lymph node metastases. Clin Cancer Res.
9:1480–1488. 2003.PubMed/NCBI
|
|
14
|
Yamashita J, Ogawa M, Yamashita S, Nomura
K, Kuramoto M, Saishoji T and Shin S: Immunoreactive hepatocyte
growth factor is a strong and independent predictor of recurrence
and survival in human breast cancer. Cancer Res. 54:1630–1633.
1994.PubMed/NCBI
|
|
15
|
Sheen-Chen SM, Liu YW, Eng HL and Chou FF:
Serum levels of hepatocyte growth factor in patients with breast
cancer. Cancer Epidemiol Biomarkers Prev. 14:715–717. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eichbaum MH, de Rossi TM, Kaul S, Bruckner
T, Schneeweiss A and Sohn C: Serum levels of hepatocyte growth
factor/scatter factor in patients with liver metastases from breast
cancer. Tumour Biol. 28:36–44. 2007. View Article : Google Scholar
|
|
17
|
Lengyel E, Prechtel D, Resau JH, et al:
C-Met overexpression in node-positive breast cancer identifies
patients with poor clinical outcome independent of Her2/neu. Int J
Cancer. 113:678–682. 2005. View Article : Google Scholar
|
|
18
|
Minuti G, Cappuzzo F, Duchnowska R, et al:
Increased MET and HGF gene copy numbers are associated with
trastuzumab failure in HER2-positive metastatic breast cancer. Br J
Cancer. 107:793–799. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Parr C and Jiang WG: Hepatocyte growth
factor activation inhibitors (HAI-1 and HAI-2) regulate HGF-induced
invasion of human breast cancer cells. Int J Cancer. 119:1176–1183.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Knuefermann C, Lu Y, Liu B, et al:
HER2/PI-3K/Akt activation leads to a multidrug resistance in human
breast adenocarcinoma cells. Oncogene. 22:3205–3212. 2003.
View Article : Google Scholar : PubMed/NCBI
|