Introduction
Major surgery-evoked ischemia has been demonstrated
to induce gastric mucosal injury and gastrointestinal dysmotility
(1,2). Previous studies (3–5) have
shown that the hypothalamic paraventricular nucleus and the lateral
hypothalamic area (LHA) are two specific hypothalamic nuclei that
modulate gastric activity and gastric mucosal injury. The
microinjection of GABAA receptor blocker in the LHA enhances GI-R
injury. However, little is known regarding GABAA receptor
expression and the protective effects of GABAAR
overexpression in the LHA against gastric ischemia-reperfusion
(GI-R) injury in rats. As determined by a previous study (6), cerebellar-hypothalamic circuits
regulate the gastric mucosal injury induced by
ischemia-reperfusion. However, the detailed GABAA
receptor (GABAAR)-mediated regulative mechanism in the
LHA upon GI-R injury is not clear. In the present study, the
effects of GABAAR overexpression induced by recombinant
adenovirus vectors in the LHA following GI-R injury in rats were
investigated. The aim of this study was to investigate the
potential mechanisms of the GABAA receptor in GI-R
injury.
Materials and methods
Animals
Adult male Sprague Dawley (SD) rats were obtained
from the Animal Resource Centre (Fudan University, Shanghai,
China). All experimental procedures used in this study were
performed in accordance with the Experimental Animal Care and Use
Committee of Fudan University and conformed to the guidelines set
out by the National Health and Medical Research Council of China.
All rats were housed under controlled conditions (12 h light
initiated at 20:00; 22–24°C) and provided access to water ad
libitum for the duration of the study. The animals were fasted
for 24 h prior to the experiment and were then allocated to the
different groups. Following the experiments, the animals were
deeply anesthetized with 10% chloral hydrate (solvent, 0.9% normal
saline) and euthanized by cervical dislocation followed by
decapitation.
Viral microinjection
Rats were anesthetized and placed in a stereotaxic
frame (51600; Stoelting, Chicago, IL, USA). The recombinant
adenoviral vectors overexpressing GABAAR
(Ad-GABAAR; Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA ) or control adenoviral vectors (Ad-Con) were bilaterally
microinjected into the LHA (15 μl for each side). The stereotaxic
coordinates of LHA:LHA:AP 2.8 mm, LR: 1.5 mm, H: 8.3–8.5 mm, which
was in accordance with Paxinos & Watson’s rat atlas (http://www.callisto-science.org/NSI/Neuroscience_Image_Database/Rat_Brain_Atlas.html).
GI-R injury model
GI-R was performed following the microinjection of
recombinant adenoviral vectors into the LHA according to previously
reported methods (7). In brief,
the abdominal cavity was cut open and the celiac artery was
carefully isolated from the adjacent tissues. The celiac artery was
clamped with a small vascular clip for 30 min and then reperfusion
was established by removal of the clip for 1 h.
Control animals underwent an identical surgical
procedure with the exception of clamping the celiac artery. At the
end of the experiments, the rats were sacrificed as described. The
stomachs were rapidly removed and were cut open along the greater
curvature, then the gastric mucosa was carefully assessed for
ulcers.
Assessment of gastric mucosal injury
index (GMII)
Gastric mucosal injury was measured as previously
described (6). The stomach was
incised along the greater curvature and washed with
phosphate-buffered saline. The GMII was determined by a
cumulative-length scale, in which an individual lesion limited by
the mucosal epithelium, including the pinpoint erosions, ulcers and
hemorrhagic spots, was scored according to length. The scores were
calculated using the following criteria: 1, lesions ≤1 mm; 2,
lesions >1 mm and ≤2 mm; 3, lesions >2 mm and ≤3 mm. For
lesions of width >1 mm, the lesion score was doubled. The sum of
the scores indicated the GMII. To prevent researcher bias, the GMII
was measured by a researcher who was blind to the treatments.
Measurement of greater splanchnic nerve
(GSN) activity
The rat was anesthetized with chloral hydrate (400
mg/kg intraperitoneally) and mounted onto a stereotaxic apparatus.
The LHA coordinates were as described. Ad-GABAAR (15 μl
for each side) was microinjected via a cannula connected to a
microsyringe with a polyethylene tube. The injection lasted 2 min
and the injection cannula was left for an additional 10 min to
prevent backflow. The central end of the GSN was placed on thin
bipolar platinum electrodes. The nerve-electrode junction was fixed
and was electrically insulated from the surrounding tissues with a
Vinyl Polysioxane Impression Material-auto-mixture (Sigma-Aldrich,
St. Louis, MO, USA). The rectified output from the amplifier was
monitored using the PowerLab® system (ADInstruments,
Dunedin, New Zealand) to record the raw nerve discharge. The basal
nerve activity (baseline) was determined by analysis of the
efferent GSN activity at the beginning of the experiment and
background noise was measured by the nerve activity recorded at the
end of the experiment. The nerve activity during the experiment was
calculated by subtracting the background noise from the recorded
value. The GSN activity response to the Ad-GABAAR
treatment was expressed as the percentage change from the basal
value.
Gastric mucosal blood flow (GMBF)
measurement
The GMBF was analyzed with a laser-Doppler flowmeter
(LDF-2; Nankai University, Tianjin, China). In brief, the rats were
anesthetized with chloral hydrate (400 mg/kg intraperitoneally),
the abdomen was opened, the stomach was exposed and transected, and
the gastric contents were marginally evacuated to the exterior
through the 5 mm incision in the stomach. Subsequently, the laser
probe was placed 0.5 mm above and perpendicular to the mucosal
surface to monitor GMBF, with measurements expressed in mV (value
of Doppler signal voltage) on the digital panel of the flowmeter.
When the GMBF was stable, four points for measurement were selected
(one point for 1 min), and the average value was calculated and
expressed as U/mV.
Western blot analysis of
GABAAR expression levels
Sample brain tissues were collected and homogenized
in RIPA lysis buffer. The concentration of total protein was
detected by bicinchoninic acid assay (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China). The proteins (40 μg)
were separated by 10% SDS-PAGE and transferred to nitrocellulose
membranes (Pall Corporation, Pensacola, FL, USA). The membranes
were probed with primary antibody to GABAAR (1:200;
Santa Cruz Biotechnology, Inc.) for 2 h. Next, the membranes were
washed three times with phosphate-buffered saline and incubated
with goat anti-rabbit IgG (1:1,000) secondary antibody for 2 h.
Following incubation with enhanced chemiluminescence solution
(Pierce Biotechnology, Inc., Rockford, IL, USA) and visualization
by exposure to BioMax films (Kodak, Rochester, NY, USA), the
membranes were stripped and probed with mouse monoclonal
anti-β-actin primary antibody (1:300; Santa Cruz Biotechnology,
Inc.) and rabbit anti-mouse secondary antibody for 2 h. The results
are expressed as the optical density of the experimental band
divided by that of the β-actin band of four replicate experiments.
The optical density was measured by a gel-pro analyzer (Shanghai
Furi Science & Technology Co., Ltd., Shanghai, China).
Measurement of plasma norepinephrine
(NE)
Blood samples were obtained from the carotid artery,
through a tube that contained ETDA. The sample was centrifuged and
mixed in an antioxidizing stabilizer sodium metabisulphite solution
(5.2 mM). The plasma NE level was determined by high-performance
liquid chromatography (HPLC) using a YWG-C18 column (250 mm × 4.6
mm × 5 μm) and electrochemical detection (Waters 2465; Waters
Corporation; Milford, MA, USA) as previously reported (8).
Measurement of plasma angiotensin (Ang)
II
Plasma Ang II levels were analyzed using commercial
ELISA kits (R&D systems, Minneapolis, MN, USA) according to the
manufacturer’s instructions. Briefly, following incubation of
96-well plates with antibody specific for rat Ang II (1:500), the
samples and standard diluent buffer were added to the wells, which
were subsequently incubated and washed. Horseradish
peroxidase-conjugated solution (1:100) was added and then washed
out. The reactions were terminated with stop solution and the final
solution was read at 450 nm using an ELISA plate reader (Shanghai
Utrao Medical Instrument Co., Ltd., Shanghai, China).
HPLC
The amino acids in the microdialysis sample were
separated by HPLC (LC-6A UQUID Chromatograph; Shimadzu Biotech
Corporation, Kyoto, Japan) using a reverse-phase column (C18;
Ultrasphere ODS; 4.6 mmx25 cm; 5 μm particles), and quantified by
o-phthaldialdehyde derivative and fluorescence detection (RF-10AXL
Shimadzu Fluorescence detector; 0.01 relative fluorescence units,
330 nm excitation wave-length and 450 nm emission wave-length). The
mobile phase consisted of 0.1 m 63% potassium phosphate (pH 6.00,
6.25), 35% methanol and 2% tetrahydrofuran, and the flow rate was 1
ml/min. The experiments were conducted at 19–23°C (9).
Histology
Following the experiments, the rats were euthanized
by an overdose injection of urethane followed by thoracotomy. The
brain was removed from the skull, fixed in 10% formalin for four
days or used to produce 40 mm coronal frozen sections and stained
with GABAAR antibody for immunohistochemical analysis,
as previously reported (6).
Statistical analysis
A student’s t-test and one-way analysis of variance
was used for data analysis, and the data are presented as the means
± standard error of the mean. GraphPad 5 software (GraphPad
Software, Inc., La Jolla, CA, USA) was used and a P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of Ad-GABAAR on GI-R
injury
It was first determined whether the overexpression
of GABAAR in the LHA exerted a protective effect upon
GI-R injury. Microinjection of Ad-GABAAR into the LHA
was found to attenuate GI-R injury. The GMII that followed
Ad-GABAAR microinjection into the LHA, was significantly
reduced as compared with the GMII subsequent to Ad-Con injection
(122.7±15.6 versus 57.0±6.67; P<0.05; Fig. 1).
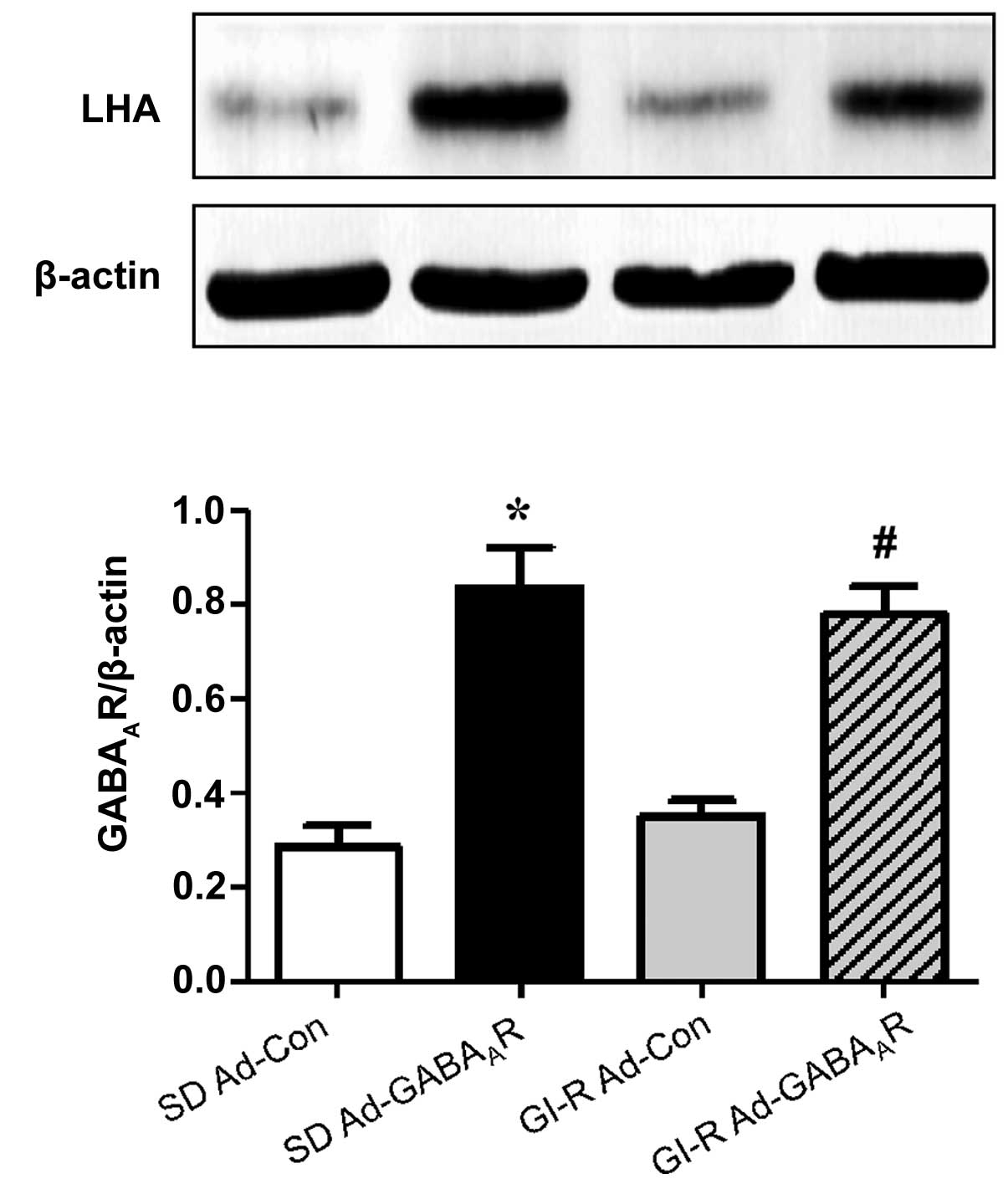
Effects of Ad-GABAAR
microinjection into LHAwon GABAAR expression
In order to detect the depressive effects of
Ad-GABAAR on the expression of GABAAR in the
LHA, GABAAR expression and cellular localisation were
detected by immunohistochemistry. The results revealed that
GABAAR expression was upregulated by microinjection of
Ad-GABAAR in the normal SD group and the GI-R group
(Fig. 2). The recombinant
adenoviral vectors encoding GABAAR significantly
increased the expression levels of GABAAR in the LHA at
two days after the viral microinjection in GI-R and normal SD rats
(P<0.05; Fig. 3).
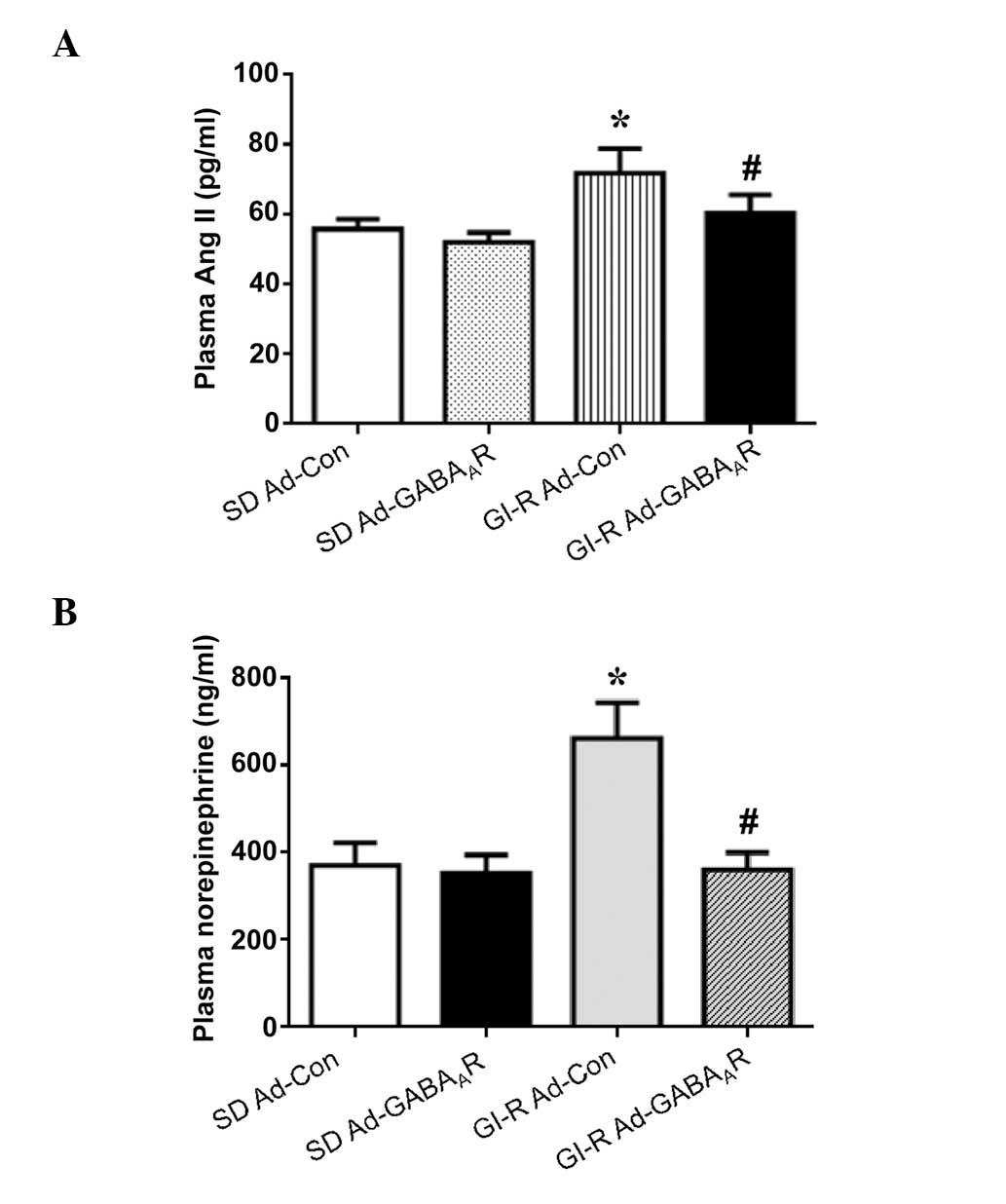
Plasma Ang II and NE levels
Plasma Ang II was significantly increased in the
GI-R injury rats, as compared with the SD control rats (P<0.05),
but Ad-GABAAR treatment significantly reduced the plasma
Ang II levels in the GI-R injury rats, as compared with Ad-Con
treatment (P<0.05; Fig. 4A).
The plasma NE levels, an indication of sympathetic activity
(10), were significantly
increased in the GI-R rats, as compared with the normal SD rats
(P<0.05), although this effect was significantly normalized by
Ad-GABAAR treatment (P<0.05; Fig. 4B).
Effects of Ad-GABAAR
expression on amino acid release
To investigate whether GI-R expression may result in
amino acid change in the LHA, Ad-GABAAR was
microinjected into the LHA in the GI-R and SD control rats, and
amino acid release was assessed. The amino acids examined in the
LHA included excitatory [glutamate (Glu) and aspartate (Asp)] as
well as inhibitory [taurine (Tau) and glycine (Gly)] amino acids.
The baseline release of excitatory amino acid neurotransmitters
(Glu and Asp) was significantly increased but that of the
inhibitory amino acids (Tau and Gly) was significantly reduced in
the GI-R injury rats, as compared with the normal SD rats (all
P<0.05). Microinjection of Ad-GABAAR into the LHA
significantly increased Tau and Gly release, and significantly
reduced Glu and Asp release, as compared with Ad-Con microinjection
(P<0.05; Fig. 5).
Effects of GABAAR on GSN
activity and GMBF
In order to determine whether the central
GABAAR mediated GSN activity, the GSN fire frequency was
analyzed. The GSN mediates GMBF, which regulates gastric activity.
As shown in Fig. 6, microinjection
of Ad-GABAAR into the LHA following GI-R, significantly
reduced GSN activity (P<0.05) and increased GMBF (P<0.05), as
compared with Ad-Con treatment.
Discussion
The present study provides evidence that
GABAAR overexpression in the LHA results in a profound
protection against GI-R injury in rats. In addition to reducing
plasma Ang II and NE levels, the LHA-targeted adenovirus reduces
the degree of gastric mucosal injury. These results suggest the
importance of the GABAAR in the LHA in the neural
gastrointestinal control and support the hypothesis that
GABAAR in the LHA is predominantly involved in the
pathophysiological process of GI-R injury (5,11).
The present study also demonstrated that an adenovirus, targeting
GABAAR in the LHA in GI-R rats, effectively inhibited
the GSN activity that contributes to the elevated GMBF (12).
An important finding from the present study was that
GABAAR gene overexpression in the LHA reduced the plasma
NE accompanied with improved GI-R injury. One limitation of the
present study is that the complex association between over-enhanced
GSN activity and the degree of gastric mucosal injury is difficult
to clarify. Nevertheless, GSN overdrive has been well-established
as a causative factor of GI-R injury, and a close association
between GSN activity and GMBF has been identified. Central enhanced
GABA signals have been shown to reduce plasma Ang II levels in
exercise-training rats (13). In
the present study, the plasma Ang II levels were increased in GI-R
rats. The adenovirus-induced GABAAR overexpression in
the LHA normalized plasma Ang II levels in GI-R rats, which may be
beneficial for the attenuation of GI-R injury. Furthermore,
adenoviruses allow the efficient delivery of relatively large
transgenes to the brain, and these viruses infect glial and
neuronal cells (14,15). The viruses used in the present
study were considered to be specific for GABAAR and not
for GABABR or GABACR.
The data from the present study support the
hypothesis that the GABAAR signaling pathway mediates
regulative effects on GI-R injury via an increase in excitatory and
a reduction in inhibitory amino acid release. Excitatory amino
acids (Glu and Asp) induce a GSN tension effect, whereas inhibitory
amino acids (Gly and Tau) cause a GSN relaxed response (16,17).
In the present study, the release of excitatory amino acids (Glu
and Asp) was higher and inhibitory amino acids (Gly and Tau) were
lower in the GI-R than in the normal SD control group. Therefore,
the GABAARs in the LHA may regulate gastric activity via
the modulation of amino acid release.
In conclusion, in the present study, a
GABAAR signaling pathway in the LHA during the
development of GI-R injury was investigated. Furthermore, the
protective effects of GABAAR overexpression in the LHA
against GI-R injury in rats were analyzed and an increased
inhibitory and suppressed excitatory amino acid release was
identified.
Acknowledgements
This study was sponsored by the National Natural
Science Foundation of China (grant nos. 31100838 and 31172147).
References
|
1
|
Tsukamoto T, Antonic V, El Hajj II, et al:
Novel model of peripheral tissue trauma-induced inflammation and
gastrointestinal dysmotility. Neurogastroenterol Motil. 23:379–386.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lucas CE, Sugawa C, Riddle J, et al:
Natural history and surgical dilemma of “stress” gastric bleeding.
Arch Surg. 102:266–273. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang JF, Zhang YM, Yan CD and Zhou XP:
Neuroregulative mechanism of hypothalamic paraventricular nucleus
on gastric ischemia-reperfusion injury in rats. Life Sci.
71:1501–1510. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang YM, Wei EQ, Li L, et al:
Extracellular signal-regulated kinase pathways may mediate the
protective effect of electrical stimulation of the paraventricular
nucleus against ischaemia-reperfusion injury of the gastric mucosa.
Clin Exp Pharmacol Physiol. 34:742–752. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu SP, Fei SJ, Zhang JF, et al: Lateral
hypothalamic area mediated the protective effects of microinjection
of glutamate into interpositus nucleus on gastric
ischemia-reperfusion injury in rats. Neurosci Lett. 1:39–43. 2012.
View Article : Google Scholar
|
|
6
|
Du DS, Zhu T, Ren ST, et al:
γ-aminobutyric acid-mediated neurotransmission in
cerebellar-hypothalamic circuit attenuates gastric mucosal injury
induced by ischemia-reperfusion. Neurogastroenterol Motil.
4:313–e249. 2013. View Article : Google Scholar
|
|
7
|
Du DS, Ma XB, Zhang JF, et al: The
protective effect of capsaicin receptor-mediated genistein
postconditioning on gastric ischemia-reperfusion injury in rats.
Dig Dis Sci. 55:3070–3077. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shi Z, Chen AD, Xu Y, et al: Long-term
administration of tempol attenuates postinfarct ventricular
dysfunction and sympathetic activity in rats. Pflugers Arch.
458:247–257. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang J, Peng YJ, Zhu DN, et al: Amino
acids mediate the hypotensive effect of angiotensin-(1-7) at the
caudal ventrolateral medulla in rats. Regul Pept. 129:1–7. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meng L, Ye XJ, Ding WH, et al: Plasma
catecholamine release-inhibitory peptide catestatin in patients
with essential hypertension. J Cardiovasc Med (Hagerstown).
12:643–647. 2011. View Article : Google Scholar
|
|
11
|
Zhu JZ, Fei SJ, Zhang JF, et al: Lateral
hypothalamic area mediated the aggravated effect of microinjection
of Baclofen into cerebellar fastigial nucleus on stress gastric
mucosal damage in rats. Neurosci Lett. 509:125–129. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao L, Fei S, Qiao W, et al: Protective
effect of chemical stimulation of cerebellar fastigial nucleus on
stress gastric mucosal injury in rats. Life Sci. 88:871–878. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rossi NF, Chen H and Maliszewska-Scislo M:
Paraventricular nucleus control of blood pressure in two-kidney,
one-clip rats: effects of exercise training and resting blood
pressure. Am J Physiol Regul Integr Comp Physiol. 305:R1390–R1400.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Davidson BL, Allen ED, Kozarsky KF, et al:
A model system for in vivo gene transfer into the central nervous
system using an adenoviral vector. Nat Genet. 3:219–223. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Toscano MG, Romero Z, Muñoz P, et al:
Physiological and tissue-specific vectors for treatment of
inherited diseases. Gene Ther. 18:117–127. 2011. View Article : Google Scholar
|
|
16
|
Koganezawa T, Shimomura Y and Terui N: The
viscerosympathetic response in rabbits is mediated by GABAergic and
glutamatergic inputs into the sympathetic premotor neurons of the
rostral ventrolateral medulla. Exp Physiol. 95:1061–1070. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kawabe T, Kawabe K and Sapru HN: Tonic
γ-aminobutyric acid-ergic activity in the hypothalamic arcuate
nucleus is attenuated in the spontaneously hypertensive rat.
Hypertension. 62:281–287. 2013. View Article : Google Scholar : PubMed/NCBI
|