Introduction
Glioblastoma accounts for ~10% of intracranial
tumors and ~22.3% of neuroepithelial tumors and is the second most
common intracranial tumor (1,2),
mainly occurring in individuals aged 30–50 years. The ratio of male
to female incidence is ~2.5:1 (1,3).
Glioblastoma located under the cortex displays infiltrative growth
and frequently encroaches upon multiple brain lobes, has a deep
structure and spreads to the contralateral cerebral hemisphere via
the corpus callosum (4).
Glioblastoma is highly malignant, grows rapidly and has a short
disease progression (5). Symptom
appearance is commonly within three months, and within six months
in ~70–80% of patients (6).
The rapid growth of glioblastoma is associated with
extensive cerebral edema and marked intracranial hypertension
(7,8). The majority of patients suffer from
headaches, vomiting and papilledema. Computed tomography revealed
lesions with inhomogeneous density, unclear boundaries and apparent
brain edema surrounding the lesions (9). Enhancement scanning further revealed
inhomogeneous enhancement or rim enhancement (10). At least one third of all
glioblastoma mortalities are a result of brain edema and herniation
(11). The hyperosmotic
dehydrants, diuretics and hormones currently used for the
symptomatic treatment of glioblastoma have poor outcomes (12); therefore, it is important to
elucidate the molecular mechanisms underlying the development of
glioblastoma-induced brain edema in order to aid in the development
of more effective treatment plans.
Cell-cell communication is the basis of molecular
transmission and transport among connecting cells (13–15).
Connexin is composed of six polygonal subunits arranged in a
circular shape to form a hydrophilic channel (16). This channel allows the transport of
ions, sugars, nucleotides, fatty acids and low-molecular-weight
polypeptides, as well as certain drugs and carcinogens whose
molecular weight is below 1,000–1,600 Da (17–21).
Connexin 43 (Cx43) is expressed in the brain with a high
specificity and forms the structural basis for substance transfer
among neurons and accessory cells, in particular, glial cells
(22,23). Aquaporins are a family of water
channel proteins that have critical roles in regulating the water
content of cells under pathological and physiological conditions
(24–26). Aquaporin-4 (AQP4) is specifically
expressed in the brain and spinal cord, where it has an important
role in maintaining the correct water balance in brain tissues
(27,28).
Previous studies have mainly focused on the role of
single genes in glioma-induced brain edema formation and have
rarely analyzed the interaction between genes (29). The present study aimed to evaluate
differences in Cx43 and AQP4 expression levels in glioblastoma
tumors and the surrounding edema in vivo, in order to aid
elucidation of the mechanisms underlying edema formation.
To further investigate the interaction between Cx43
and AQP4 in glioblastoma-induced brain edema formation, hypotonic
medium was used to simulate the environment of brain edema in
vitro and determine the effects on AQP4 and Cx43 messenger RNA
(mRNA) expression levels in C6 glioma cells and normal glial cells.
The effects of silencing of AQP4 in C6 glioma cells and Cx43 in
glial cells under hypotonic conditions were also evaluated. The
results of the present study may present a novel perspective on the
mechanisms underlying glioma-induced brain edema formation and
therefore aid in the development of drugs targeting the interaction
between AQP4 and Cx43 for the treatment of glioma-induced brain
edema.
Materials and methods
Reagents and animals
Murine C6 glioma cells (Shanghai Cancer Institute,
Shanghai, China), fetal bovine serum (Hangzhou Sijiqing Biological
Engineering Materials Co., Ltd., Huzhou, China), Dulbecco’s
modified Eagle’s medium and trypsin (Gibco-BRL, Invitrogen Life
Technologies, Carlsbad, CA, USA), Lipofectamine® 2000
(Invitrogen Life Technologies), short interfering RNA (siRNA)
targeting AQP4 and Cx43 (synthesized by Wuhan Genesil Biotechnology
Co., Ltd., Wuhan, China), reverse transcription quantitative
polymerase chain reaction (RT-qPCR) kit (Beijing Zhongshan Co.,
Beijing, China), PCR primers (synthesized by Beijing Sunbiotech
Company, Beijing, China), rabbit anti-rat Cx43 polyclonal antibody
(Chemicon, Billerica, MA, USA) and rabbit anti-rat AQP4 polyclonal
antibody (Santa Cruz Biotechnology Inc., Dallas, TX, USA) were used
in the present study. A total of 20 male Sprague-Dawley rats aged
7–8 weeks and weighing 200±10 g were purchased from the Animal
Center of Academy of Military Medical Sciences (Beijing, China) and
housed in the Experimental Animal Center (Tianjin Medical
University, Tianjin, China) under controlled temperature (22~24°C)
conditions with a 12 h light/dark cycle. The lights were on from
8:00 to 20:00. Food and water were made available ad libitum.
Experiments were performed during the light phase of the cycle. All
experimental procedures were carried out according to the
regulations and internal biosafety and bioethics guidelines of
Tianjin Medical University and the Tianjin Municipal Science and
Technology Commission
Establishment of intracranial glioma
model and magnetic resonance imaging (MRI)
C6 glioma cells in the logarithmic phase were
digested into a single cell suspension. Rats were anesthetized by
intraperitoneal injection of ketamine/xylazine (100 g/kg ketamine,
Parke-Davis, New Jersey, USA; 5 mg/kg xylazine, Bayer AG,
Leverkusen, Germany), and then were fixed on benches with a
stereotactic injection device and shaved. A C6 glioma cell
suspension (1×106 cells in 25 μl) was injected into the
brain in the right caudate nucleus. The duration of injection was
>5 min, following which the needle was maintained for at least
three minutes and the incision was subsequently sutured. Cranial
MRI was conducted at 3, 6, 9 and 12 days post-injection to observe
tumor growth and edema.
Immunofluorescence to analyze the spatial
expression of Cx43 and AQP4 within and surrounding the glioma
Following the final MRI examination (12 days
post-injection), the rats were sacrificed by cervical dislocation.
Brain tissues containing the glioma were embedded in paraffin
(Zhongshan Biocorp., Beijing, China), sliced into sections and
analyzed using immunofluorescence. Briefly, sections were boiled
for 5 min in Tris (10 mM)/ethylene diamine tetra acetic acid (1 mM,
Zhongshan Biocorp.; pH 9.0) buffer for antigen retrieval, treated
by Triton X-100 (Zhongshan Biocorp.), and were blocked with serum
(Hangzhou Sijiqing Biological Materials Co., Ltd., Hangzhou, China)
for one hour, treated with rabbit anti-rat Cx43 polyclonal antibody
(1:500) and rabbit anti-rat AQP4 polyclonal antibody (1:1,000) at
4°C overnight. The sections were subsequently incubated with
fluorescein isothiocyanate (FITC)- or tetramethylrhodamine
isothiocyanate (TRITC)-labeled secondary antibodies (FITC or TRITC
goat anti-rabbit IgG; Santa Cruz Biotechnology Inc.), followed by
mounting and observation under a fluorescence microscope(Olympus DP
70, Tokyo, Japan).
Western blot analysis for quantitative
detection of Cx43 and AQP4 expression levels within and surrounding
the glioma
The remaining brain tissues containing gliomas were
dissected. Tumor tissue and 5 mm of the surrounding tissue were
stored in liquid nitrogen prior to extraction of total proteins.
Total protein was extracted using standard lysis buffer (Sigma, St.
Louis, MO, USA) for 10 min The proteins were quantified using the
Bradford method (30), separated
by 10% SDS-PAGE and transferred onto polyvinylidene difluoride
membranes. The membranes were blocked with skimmed milk (Zhongshan
Biocorp.) for one hour, washed with phosphate-buffered saline
(Zhongshan Biocorp.) and incubated with primary antibodies
(anti-Cx43, 1:500; AQP4, 1:1,000) at 4°C with horizontal shaking
overnight. The following day, the membranes were treated with
horseradish peroxidase-labeled secondary antibodies (1:500) at 37°C
for two hours and visualized using a Chemiluminescence Detection
Kit (Weifang Kanghua Biotech Co., Ltd., Shandong, China). Bio-Rad
imaging system (Bio-Rad Laboratories, Hercules, CA, USA) was used
to measure absorbance values at a wavelength of 570 nm. Quantity
One software version 4.4.1 (Bio-Rad Laboratories) was used to
analyze the data. The dilution of the control β-actin antibody was
1:200.
RT-qPCR for the characterization of AQP4
and Cx43 expression in a hypotonic environment
Subcultured C6 glioma cells and primary cultured
normal glioma cells were separately incubated in hypotonic medium
at 250–260 mOsm/kg. AQP4 and Cx43 mRNA expression was determined in
C6 cells and normal glial cells at 0, 12, 24, 36, 48, 60 and 72
hours. The primers used for analysis were as follows: AQP4 forward,
5′-CCA GCT GTG ATT CCA AAA CGG AC-3′ and reverse, 5′-TCT AGT CAT
ACT GAA GAC AAT ACC TC-3′ (product size, 305 bp); Cx43 forward,
5′-TGT AAC ACT CAA CAA CCT GGC-3′ and reverse, 5′-GAT CTT GAT CTT
CAT GGT GCT AGG-3′ (product size, 764 bp). Reaction conditions were
as follows: 32 cycles of denaturation at 94°C for 30 sec, annealing
at 56°C for 30 min and extension at 72°C for 1 min, followed by
extension at 72°C for 10 min. Time x represents an arbitrary
time-point. Time 0 represents a 1-fold target gene expression
following β-actin correction. ΔΔCT was calculated using the formula
(CT.Target−CT.U6)Time
x−(CT.Target−CT.U6)Time
0.
AQP4 and Cx43 gene silencing
AQP4 and Cx43 full-length mRNAs were retrieved from
the NCBI gene database (http://www.ncbi.nlm.nih.gov/gene). siRNA was designed
with gene silencing design software in the coding regions. The
oligonucleotide sequence targeting AQP4 was 5′-AGA TCA GCA TCG CCA
AGT C-3′, and that targeting Cx43 was 5′-UUG AAG UUA UGU AUC CUC
CUU-3′. C6 glioma cells and glial cells that were growing
successfully were selected for transfection and their media was
replaced with fresh media on the day prior to transfection. For
transfection, 1 μg plasmid DNA and 100 μl LipoVec™ (Santa Cruz
Biotechnology, Inc.) were mixed for 20 min. The mixture was added
to the medium for 4 h and subsequently the medium was replaced by
medium containing 20% serum. Following 48 h of transfection, fresh
medium containing 200 ng/l geneticin (Zhongshan Biocorp.) was used
for selection. The selective medium was replaced every three days.
Following 14 days of culture, cell colonies were visible in
successfully transfected groups, whereas all non-transfected cells
had died. When the number of positive cells reached a certain
number, cell clones were transferred into 24-well plates for
further passaging. These cells were incubated in hypotonic
conditioned medium (Zhongshan Biocorp.) at 250–260 mOsm/kg. AQP4
and Cx43 mRNA expression levels were detected at 0, 12, 24, 36, 48,
60 and 72 h using the method described above.
Statistical analysis
Experiments were repeated three times. Data were
processed using SPSS 13.0 software (SPSS Inc., Chicago, IL, USA).
Values are expressed as the mean ± standard deviation. To evaluate
differences amongst the groups, χ2 analysis was
performed. A value of P<0.05 was considered to indicate a
statistically significant difference between values.
Results
Establishment of intracranial glioma
models and MRI examination
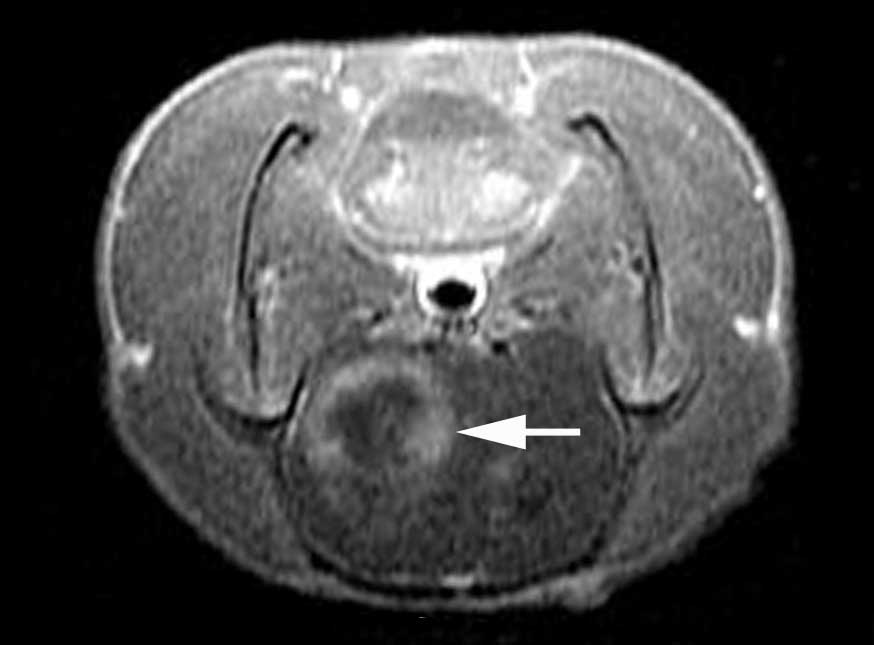
As displayed in Fig.
1, which was captured on the 10th day post-C6 glioma cell
injection, the examination revealed circular tumor shadows in the
brain of Sprague-Dawley rats. Cystic degeneration or necrosis was
observed in the center of certain tumors. Edema (>5 mm; Fig. 1, red arrow) was observed on the
periphery of the majority of tumors evaluated.
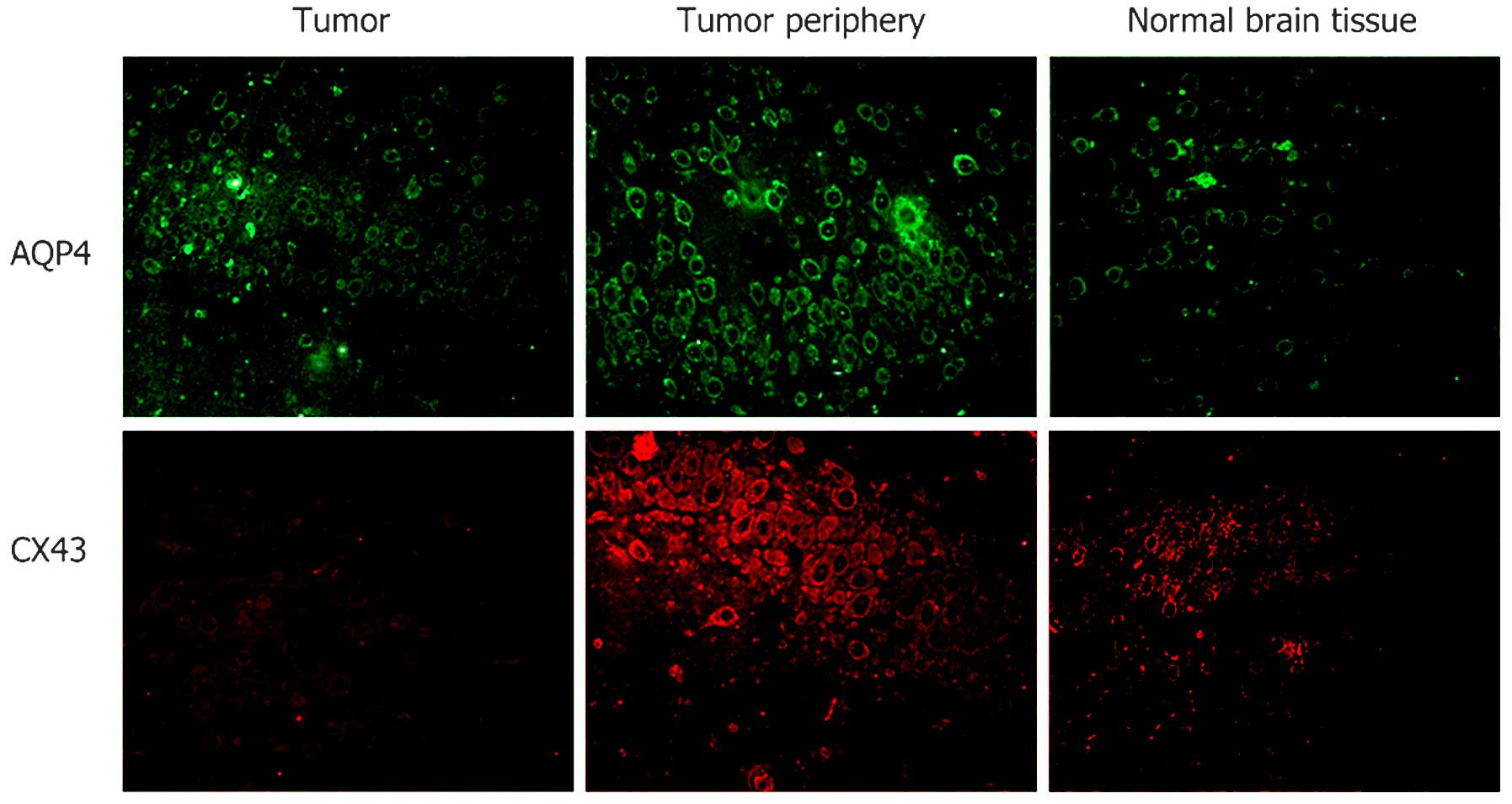
AQP4 and Cx43 expression levels are
higher in the tumor periphery than those in the center
In Fig. 2, green
fluorescence indicates FITC-labeled AQP4, and red fluorescence
indicates TRITC-labeled Cx43. AQP4 and Cx43 were expressed at low
levels in normal brain tissues. In glioma cells, AQP4 was expressed
at low levels and Cx43 was expressed at low to non-detectable
levels. In the 5-mm area of tissue surrounding the glioma,
significantly higher expression levels of AQP4 and Cx43 were
detected.
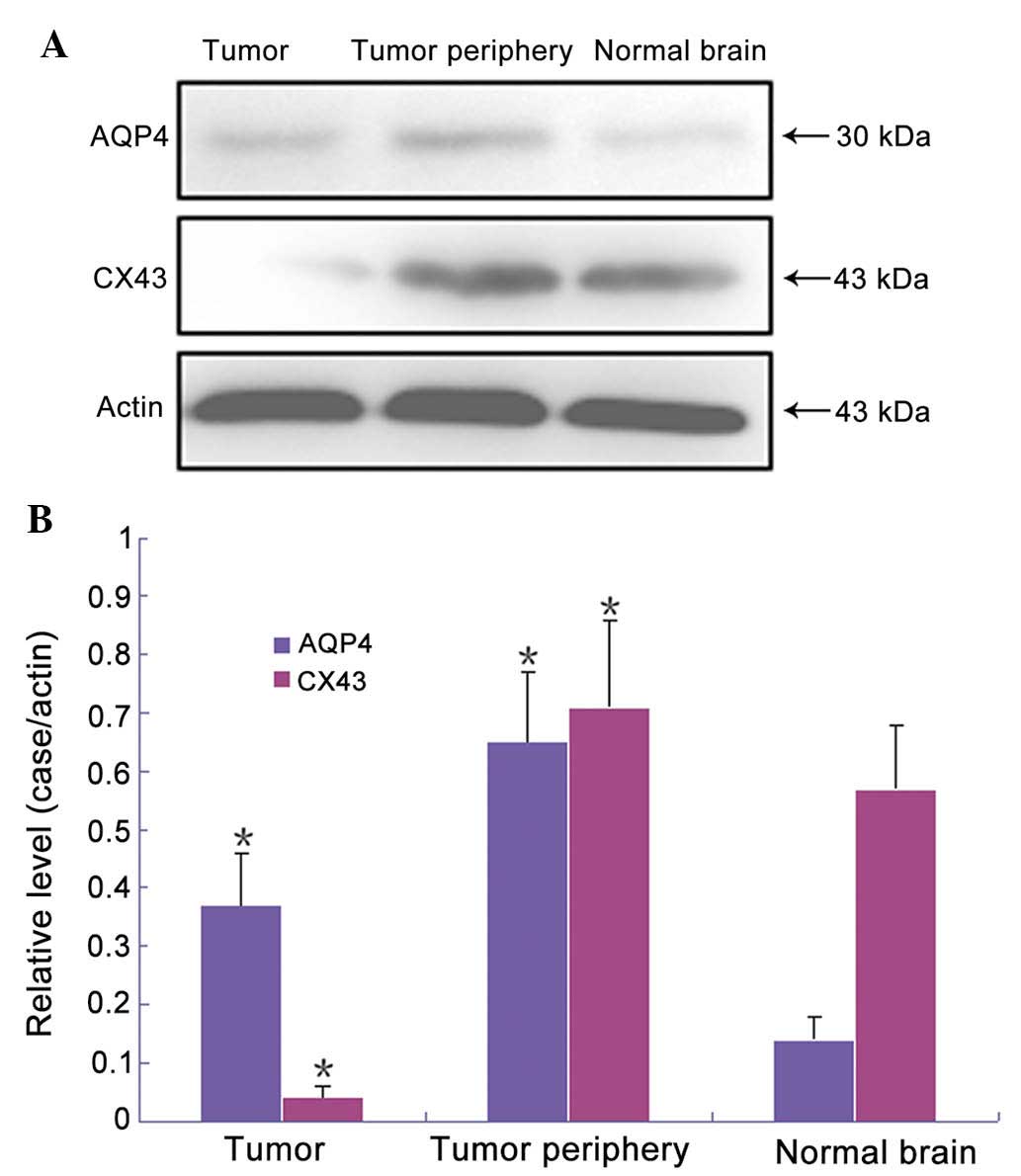
AQP4 and Cx43 expression levels are
altered in glioblastoma tissues
As exhibited in Fig.
3, relative expression levels of AQP4 were significantly higher
in tumor tissues than those in normal brain tissues (0.37±0.09 vs.
0.14±0.04), whereas Cx43 expression levels were significantly lower
in tumor tissues than those in normal brain tissues (0.04±0.02 vs.
0.57±0.11). In the edema tissue surrounding the tumor, the relative
expression levels of AQP4 were significantly higher than those in
normal brain tissues (0.65±0.12). Expression levels of Cx43 were
higher than those in normal brain tissues, while there was no
significant difference (0.71±0.15). Significant differences were
detected between the experimental groups and the control group, as
determined by paired comparisons (P<0.05).
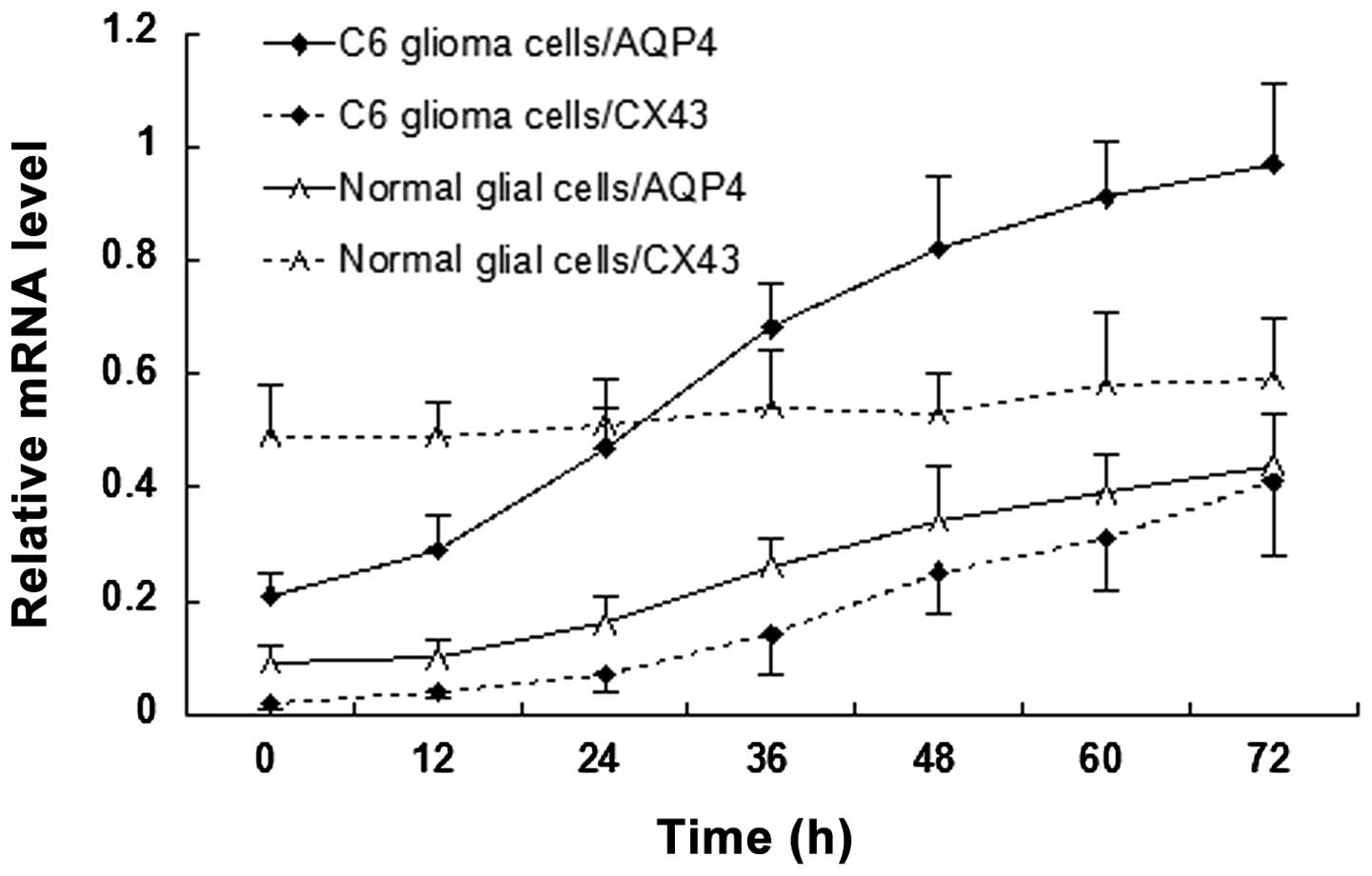
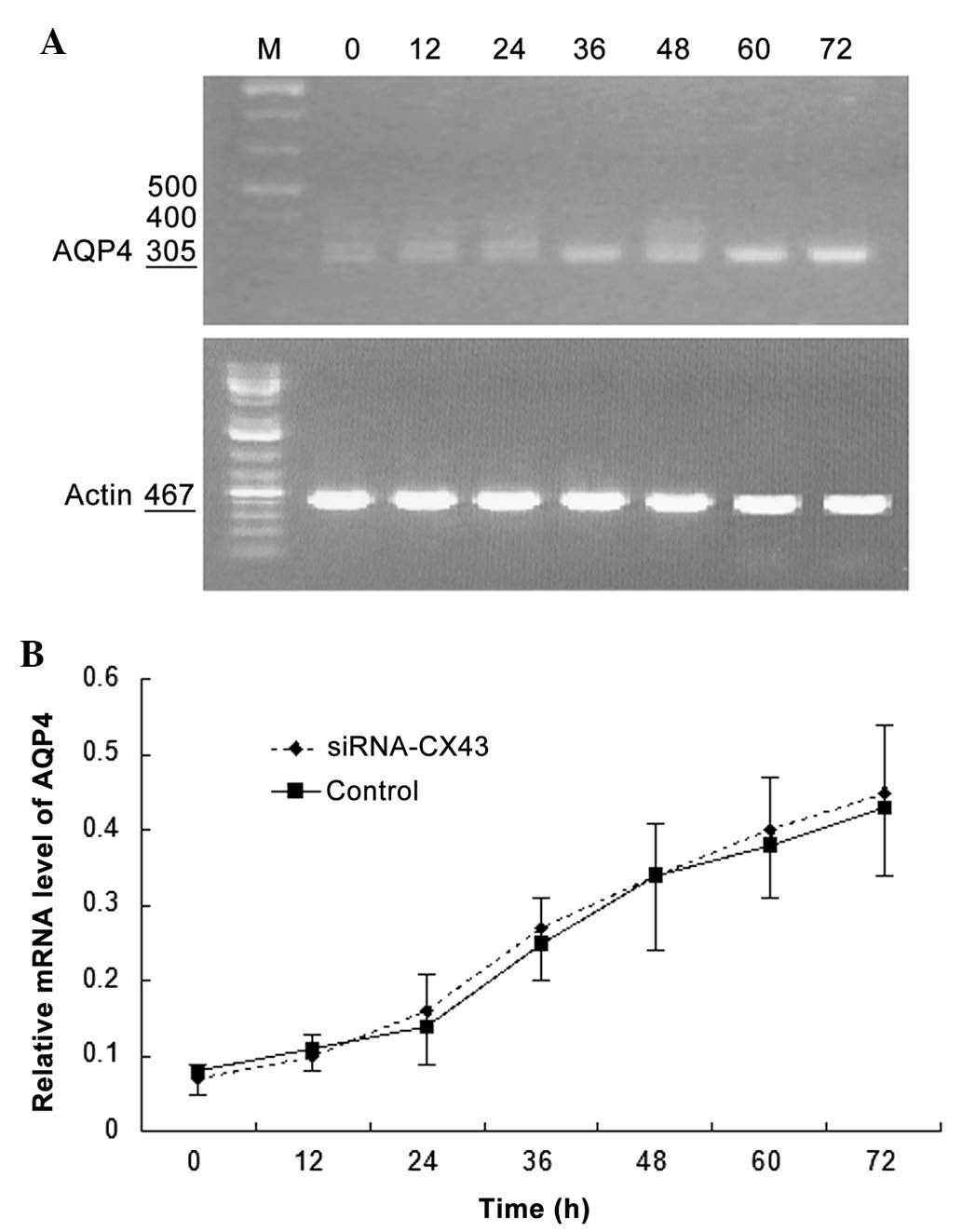
AQP4 and Cx43 mRNA expression levels
change with time under hypotonic conditions
The mRNA expression levels of AQP4 and Cx43 in C6
glioma cells and normal glial cells are revealed in Fig. 4. AQP4 mRNA expression was detected
in C6 glioma cells (0.21±0.04) and rapidly increased with time
under hypotonic conditions. AQP4 mRNA expression levels were lower
in normal glial cells (0.09±0.03) than those in C6 glioma cells and
slowly increased with time under hypotonic conditions. Cx43 mRNA
expression levels in C6 glioma cells were initially lower than
those in normal glial cells (0.02±0.01 vs. 0.49±0.09). Although
Cx43 mRNA expression levels in C6 glioma cells increased with time
under hypotonic conditions, the expression levels remained lower
than those of Cx43 and AQP4 in C6 glioma cells. An inflection point
appeared 24 h following hypotonic cultivation, which was later than
that of C6 glioma cells (12 h). No significant increase in Cx43
mRNA expression was detected in normal glial cells with prolonged
time under hypotonic conditions.
AQP4 silencing attenuates the increase in
Cx43 expression characteristic of C6 glioma cells
Fig. 5A reveals
Cx43 mRNA expression following selective AQP4 silencing in C6
glioma cells. Cx43 mRNA expression levels increased with prolonged
time in C6 glioma cells under hypotonic conditions and the
proportion of increase was significantly higher than that of the
control group (P<0.05; Fig.
5B).
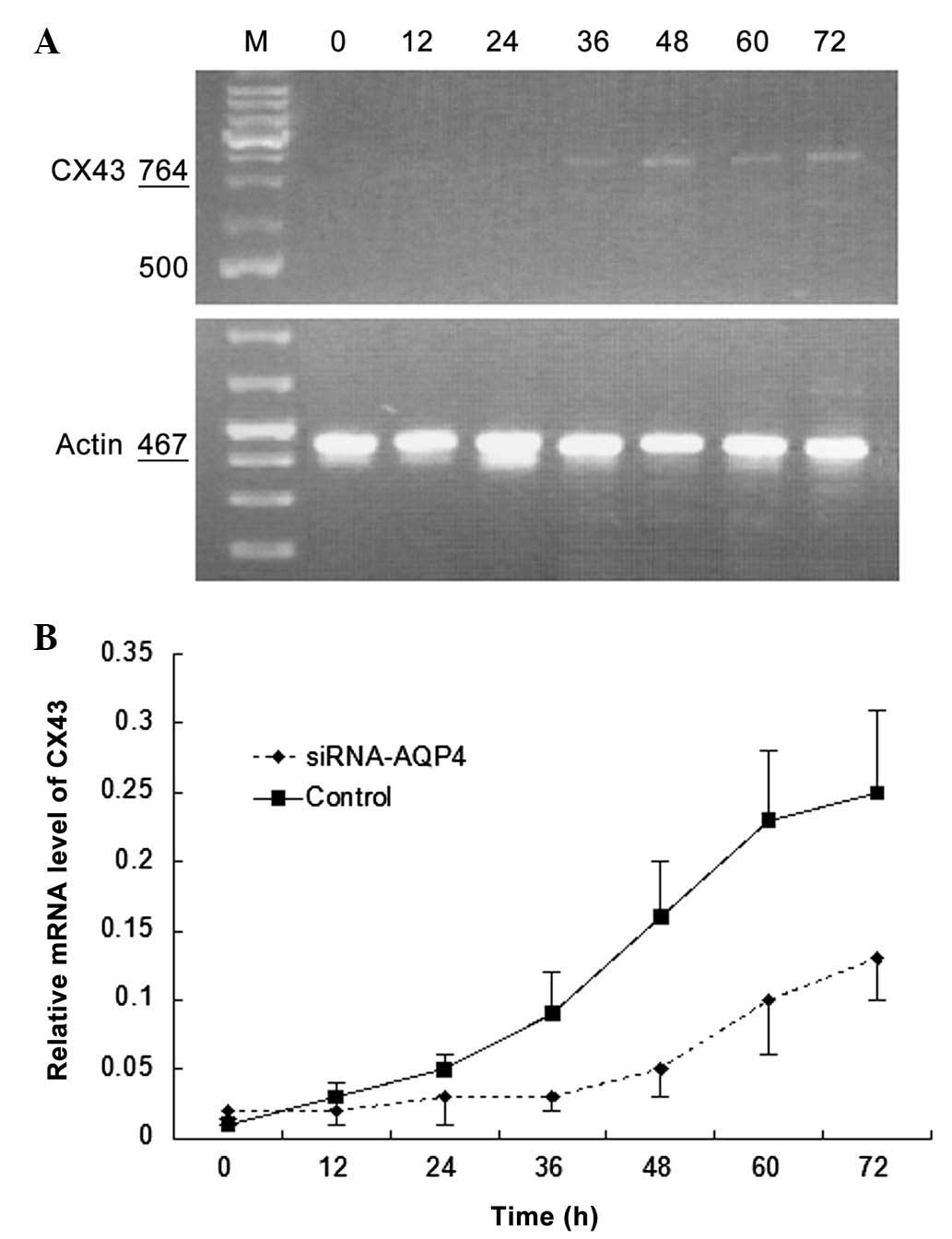 | Figure 5Effects of AQP4 silencing on CX43 mRNA
expression in C6 glioma cells cultured under hypotonic conditions.
(A) Representative image of polymerase chain reaction gel. M,
marker, 0, 12, 24, 36, 48, 60 and 72 h culture under hypotonic
conditions. (B) Comparison of relative mRNA expression level curves
between the small interfering RNA-AQP4 and control groups. Values
are expressed as the mean ± standard deviation. AQP4, aquaporin-4;
CX43, connexin 43, mRNA, messenger RNA. |
Cx43 silencing has no significant effect
on AQP4 expression in normal glial cells
Fig. 6A shows AQP4
mRNA expression in normal glial cells following selective Cx43
silencing. AQP4 mRNA expression gradually increased over time in
normal glial cells under hypotonic conditions and the increase was
similar to that of the control group (P>0.05; Fig. 6B).
Discussion
Brain edema is characterized by an excessive
accumulation of fluid in the intracellular or extracellular spaces
of the brain (31). Various
intracranial pathological changes, including hypoxia, poisoning,
metabolic disturbance, craniocerebral injury, tumors, abscesses,
blood vessels and inflammation, may induce increased fluid content
in the brain parenchyma, resulting in intracranial hypertension,
which may induce alterations in cerebral metabolism, blood supply
and aggravated brain edema (10).
When glioblastoma initially develops, edema in neoplastic cells and
the tumor nidus is not apparent (32). Following increased tumor nidus and
tumor blood vessel growth, the blood-brain barrier in the focal
zone is damaged, capillary permeability increases and plasma
components and water are released, triggering angioedema (33). Endothelial cells are subsequently
destroyed and tight connections in the vascular endothelial cell
membrane are opened. Water and partially ionic compositions
overflow and enter the tumor periphery (34). Normal cells at the tumor periphery
are subjected to hypotonic conditions for extended time-periods;
therefore, the dynamic configuration of the membrane is damaged,
resulting in dysfunction of the membrane transport system for water
and electrolytes; Ca2+ release from the mitochondria and
endoplasmic reticulum, which increases the intracellular
Ca2+ concentration; activation of phospholipase A and C
and induced phospholipase degradation, resulting in arachidonic
acid release, increased cell permeability and cellular dysfunction
(34,35). Therefore, the mitochondria,
lysosomes, microsomal membranes and subcellular organelles are
destroyed, resulting in dysfunction of neurons and accessory cells
and aggravation of brain edema (36). It was hypothesized that
glioblastoma-induced brain edema is a combination of angioedema and
cytotoxic brain edema (37). In
the present study, angioedema was mainly detected in the tumor
nidus and cytotoxic edema was mainly detected in the tumor
periphery.
In the present study, a Sprague-Dawley rat model of
intracranial glioma was established. MRI examination revealed tumor
formation and edema at the tumor periphery. Immunofluorescence
analysis and western blot assay verified marked differences in AQP4
and CX43 expression in the tumor bed and the tumor periphery. In
cells of the tumor bed, AQP4 expression levels increased, whereas
Cx43 expression decreased or was absent in comparison to those of
normal brain tissues. However, AQP4 expression levels were
significantly increased and Cx43 expression levels were higher in
the edema region at the tumor periphery compared with those of
normal brain tissues.
Aside from a small number of infiltrative glioma
cells, abundant normal glial cells were visible at the tumor
periphery. Normal glial cells also expressed AQP4 and Cx43 to a
certain extent. The present study hypothesized that edema formation
in the tumor bed was associated with glioma cells, whereas edema at
the tumor periphery was associated with normal glial cells.
Therefore, C6 glioma cells and normal glial cells were separately
incubated under hypotonic conditions to simulate edema, in order to
characterize AQP4 and Cx43 expression in both cell types. The
results demonstrated that AQP4 mRNA expression rapidly increased
with time under hypotonic conditions in C6 glioma cells, whereas it
increased slowly in normal glial cells. Simultaneously, Cx43 mRNA
expression levels were low in C6 glioma cells and increased with
prolonged time under hypotonic conditions; however, the increase in
expression levels was not so marked as that observed in that of
AQP4. An inflection point appeared at 24 h following hypotonic
cultivation, which was later than that observed in C6 glioma cells
(12 h). No increase in Cx43 mRNA expression levels was detected in
normal glial cells with time under hypotonic conditions.
To further identify the association between AQP4 and
Cx43, Cx43 expression under hypotonic conditions following AQP4
silencing in C6 glioma cells was observed. AQP4 expression levels
under hypotonic conditions following Cx43 silencing in normal glial
cells were also evaluated. The results confirmed that the increased
Cx43 expression observed in C6 glioma cells was significantly
attenuated following AQP4 silencing, while AQP4 expression was not
altered following Cx43 silencing in normal glial cells. Based on
these results, it was concluded that AQP4 and Cx43 within and
surrounding the glioma had separate mechanisms underlying brain
edema and that Cx43 may be a downstream effector of AQP4. Due to
the impairment of glioblastoma cell-cell junctions and
communication, numerous micromolecular substances and ions are
restricted in single tumor cells (38). The block in communication results
in disordered transport of water molecules, ions and micromolecular
substances (39). Although water
transport is not a major function of cell-to-cell junctions and
communication, intracellular and extracellular osmotic pressure
gradients induced by the disordered transport of ions and
micromolecular substances affect intracellular and extracellular
water distribution, resulting in impaired intracellular water
excretion and the formation of glioblastoma-induced brain edema
(40).
A previous study confirmed that a hypotonic
environment caused AQP4 expression levels to increase in glial
cells (41). Li et al
(25) found that a hypotonic
environment led to astrocyte edema and a reduction in viability,
with increased AQP4 mRNA and protein expression levels. Yang et
al (27) suggested that when
glial cells in the edema region at the tumor periphery were in a
hypotonic environment, increased AQP4 expression promoted the
entrance of extracellular water to cells. Intracellular water
overloading would therefore induce the occurrence of cytotoxic
edema. These studies did not clearly explain the mechanism
underlying glioma-induced brain edema within and around the edge of
the tumor. The results of the present study enhanced the available
knowledge in this field and provided a novel insight into the roles
of AQP4 and Cx43 in brain edema. However, further study is required
in order to fully elucidate this complex pathway.
References
|
1
|
Bonavia R, Inda MM, Cavenee WK and Furnari
FB: Heterogeneity maintenance in glioblastoma: a social network.
Cancer Res. 71:4055–4060. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Raizer JJ, Grimm S, Chamberlain MC, et al:
A phase 2 trial of single-agent bevacizumab given in an
every-3-week schedule for patients with recurrent high-grade
gliomas. Cancer. 116:5297–5305. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ivliev AE, ‘t Hoen PA and Sergeeva MG:
Coexpression network analysis identifies transcriptional modules
related to proastrocytic differentiation and sprouty signaling in
glioma. Cancer Res. 70:10060–10070. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gerstner ER, Chen PJ, Wen PY, Jain RK,
Batchelor TT and Sorensen G: Infiltrative patterns of glioblastoma
spread detected via diffusion MRI after treatment with cediranib.
Neuro Oncol. 12:466–472. 2010.PubMed/NCBI
|
|
5
|
Bleeker FE, Molenaar RJ and Leenstra S:
Recent advances in the molecular understanding of glioblastoma. J
Neurooncol. 108:11–27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Masica DL and Karchin R: Correlation of
somatic mutation and expression identifies genes important in human
glioblastoma progression and survival. Cancer Res. 71:4550–4561.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tabunoki H, Saito N, Suwanborirux K,
Charupant K and Satoh J: Molecular network profiling of U373MG
human glioblastoma cells following induction of apoptosis by novel
marine-derived anti-cancer 1,2,3,4-tetrahydroisoquinoline
alkaloids. Cancer Cell Int. 12:142012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoo AS, Sun AX, Li L, et al:
MicroRNA-mediated conversion of human fibroblasts to neurons.
Nature. 476:228–231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang PH, Miraldi ER, Xu AM, et al:
Phosphotyrosine signaling analysis of site-specific mutations on
EGFRvIII identifies determinants governing glioblastoma cell
growth. Mol Biosyst. 6:1227–1237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Solan JL and Lampe PD: Connexin43
phosphorylation: structural changes and biological effects. Biochem
J. 419:261–272. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang Q, Liu XZ, Kang CS, Wang GX, Zhong Y
and Pu PY: The anti-glioma effect of suicide gene therapy using
BMSC expressing HSV/TK combined with overexpression of Cx43 in
glioma cells. Cancer Gene Ther. 17:192–202. 2010. View Article : Google Scholar
|
|
12
|
Li W and Graeber MB: The molecular profile
of microglia under the influence of glioma. Neuro Oncol.
14:958–978. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ribot EJ, Miraux S, Konsman JP, et al: In
vivo MR tracking of therapeutic microglia to a human glioma model.
NMR Biomed. 24:1361–1368. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Salvati M, D’Elia A, Formichella AI and
Frati A: Insights into pharmacotherapy of malignant glioma in
adults. Expert Opin Pharmacother. 10:2279–2290. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiong Z and Ohlfest JR: Topical imiquimod
has therapeutic and immunomodulatory effects against intracranial
tumors. J Immunother. 34:264–269. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brisset AC, Isakson BE and Kwak BR:
Connexins in Vascular Physiology and Pathology. Antioxid Redox
Signal. 11:267–282. 2009. View Article : Google Scholar
|
|
17
|
Jain RK, di Tomaso E, Duda DG, Loeffler
JS, Sorensen AG and Batchelor TT: Angiogenesis in brain tumours.
Nat Rev Neurosci. 8:610–622. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Papadopoulos MC, Saadoun S, Davies DC and
Bell BA: Emerging molecular mechanisms of brain tumour oedema. Br J
Neurosurg. 15:101–108. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pavlisa G, Rados M, Pavlisa G, Pavic L,
Potocki K and Mayer D: The differences of water diffusion between
brain tissue infiltrated by tumor and peritumoral vasogenic edema.
Clin Imaging. 33:96–101. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Uchida S, Sasaki S, Fushimi K and Marumo
F: Isolation of human aquaporin-CD gene. J Biol Chem.
269:23451–23455. 1994.PubMed/NCBI
|
|
21
|
Mou KJ, Mao Q, Chen MN, et al: AQP4
expression in the brains of patients with glioblastoma and its
association with brain edema. Sichuan Da Xue Xue Bao Yi Xue Ban.
40:651–654. 2009.(In Chinese). PubMed/NCBI
|
|
22
|
Nico B, Mangieri D, Tamma R, et al:
Aquaporin-4 contributes to the resolution of peritumoural brain
oedema in human glioblastoma multiforme after combined chemotherapy
and radiotherapy. Eur J Cancer. 45:3315–3325. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Warth A, Mittelbronn M, Hülper P,
Erdlenbruch B and Wolburg H: Expression of the water channel
protein aquaporin-9 in malignant brain tumors. Appl Immunohistochem
Mol Morphol. 15:193–198. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nicchia GP, Srinivas M, Li W, Brosnan CF,
Frigeri A and Spray DC: New possible roles for aquaporin-4 in
astrocytes: cell cytoskeleton and functional relationship with
connexin43. FASEB J. 19:1674–1676. 2005.PubMed/NCBI
|
|
25
|
Li YH and Sun SQ: Effect of hypotonic
medium on expression of aquaporin-4 in astrocytes. Zhonghua Yi Xue
Za Zhi. 84:496–501. 2004.(In Chinese). PubMed/NCBI
|
|
26
|
Arima H, Yamamoto N, Sobue K, et al:
Hyperosmolar mannitol simulates expression of aquaporins 4 and 9
through a p38 mitogen-activated protein kinase-dependent pathway in
rat astrocytes. J Biol Chem. 278:44525–44534. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang B, Zador Z and Verkman AS: Glial cell
aquaporin-4 overexpression in transgenic mice accelerates cytotoxic
brain swelling. J Biol Chem. 283:15280–15286. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Figueroa XF and Duling BR: Gap junctions
in the control of vascular function. Antioxid Redox Signal.
11:251–266. 2009. View Article : Google Scholar
|
|
29
|
Criscuolo GR: The genesis of peritumoral
vasogenic brain edema and tumor cysts: a hypothetical role for
tumor-derived vascular permeability factor. Yale J Biol Med.
66:277–314. 1993.PubMed/NCBI
|
|
30
|
Bradford MM: Rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Simard JM, Kent TA, Chen M, Tarasov KV and
Gerzanich V: Brain oedema in focal ischaemia: molecular
pathophysiology and theoretical implications. Lancet Neurol.
6:258–268. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Walcott BP, Kahle KT and Simard JM: Novel
Treatment Targets for Cerebral Edema. Neurotherapeutics. 9:65–72.
2012. View Article : Google Scholar :
|
|
33
|
Cottin S, Ghani K and Caruso M: Bystander
effect in glioblastoma cells with a predominant cytoplasmic
localization of connexin43. Cancer Gene Ther. 15:823–831. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mennecier G, Derangeon M, Coronas V, Hervé
JC and Mesnil M: Aberrant expression and localization of connexin43
and connexin30 in a rat glioma cell line. Mol Carcinog. 47:391–401.
2008. View
Article : Google Scholar
|
|
35
|
Pu P, Xia Z, Yu S and Huang Q: Altered
expression of Cx43 in astrocytic tumors. Clin Neurol Neurosurg.
107:49–54. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Masato Y: Regulation, structure and
function of brain aquaporin. Rinsho Shinkeigaku. 49:786–788.
2009.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhu SM and Pan CF: Effect of propofol upon
ammonia-induced neocortical astrocyte swelling and aquaporin-4
expression. Zhonghua Yi Xue Za Zhi. 89:2077–2080. 2009.(In
Chinese). PubMed/NCBI
|
|
38
|
Chodobski A, Zink BJ and
Szmydynger-Chodobska J: Blood-brain barrier pathophysiology in
traumatic brain injury. Transl Stroke Res. 2:492–516. 2011.
View Article : Google Scholar
|
|
39
|
Ampawong S, Chaisri U, Viriyavejakul P,
Nontprasert A, Grau GE and Pongponratn E: Electron microscopic
features of brain edema in rodent cerebral malaria in relation to
glial fibrillary acidic protein expression. Int J Clin Exp Pathol.
7:2056–2067. 2014.PubMed/NCBI
|
|
40
|
Bailey DM, Bärtsch P, Knauth M and
Baumgartner RW: Emerging concepts in acute mountain sickness and
high-altitude cerebral edema: from the molecular to the
morphological. Cell Mol Life Sci. 66:3583–3594. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zador Z, Bloch O, Yao X and Manley GT:
Aquaporins: role in cerebral edema and brain water balance. Prog
Brain Res. 161:185–194. 2007. View Article : Google Scholar : PubMed/NCBI
|