Introduction
As one of earliest discovered oncogenes, v-myc avian
myelocytomatosis viral oncogene homolog (Myc) regulates cell
proliferation, differentiation and apoptosis (1). The N-Myc downstream-regulated gene
(NDRG) family is novel class of Myc-repressed genes. At present
four members (NDRG1–4) have been identified, which are expressed in
the brain, heart, skeletal muscle and kidneys (2,3).
Since it is cloned as a downregulated gene in glioblastoma, NDRG2
has different expression patterns in tumor and normal tissue
(4–6). As a novel p53-inducible gene, NDRG2
may be involved in the p53-mediated apoptosis pathway in response
to DNA damage; however, NDRG2 may also have a role in the
inhibition of proliferation that is independent of p53 (7).
Renal cell carcinoma (RCC), a major neoplasm arising
from the kidney, accounts for 3% of all malignant tumors in adults
(8). The incidence rates are
higher in Western countries than those in Asian countries. NDRG2
expression in two cancer cell lines, A-498 and 786-O, has been
found to be markedly decreased compared with that in two human
kidney proximal tubular cell lines, HK-2 and HKC. The expression of
NDRG2 is also downregulated in RCC tissues compared with that in
adjacent normal tissue, indicating that NDRG2 may have an important
role in carcinogenesis (9).
Furthermore, a high proportion of RCC cases exhibit reduced NDRG2
expression. According to a recent multivariate analysis, NDRG2 is
an independent poor prognostic factor predicting survival in RCC
(10).
In the present study, NDRG2 was overexpressed in
human renal cancer OS-RC-2 cells in order to determine whether
NDRG2 is a potential therapeutic target in RCC.
Materials and methods
Cell culture
The human renal cancer cell line, OS-RC-2 (Shanghai
Institute of Cell Biology, Chinese Academy of Science, Shanghai,
China), was cultured in RPMI-1640 medium supplemented with 10%
heat-inactivated fetal bovine serum, 100 U/ml penicillin and 100
mg/l streptomycin in a humidified atmosphere containing 5%
CO2, which was maintained at 37°C. The present study was
approved by the Ethics Committee of Guangdong Medical College
(Guangdong, China).
Constructs and infection
The recombinant adenoviruses
pAV.EX1d-NDRG2/IRES/eGFP and pAV.EX1d-eGFP were constructed as
previously described (11). A
multiplicity of infection of 40 was determined experimentally for
the OS-RC-2 cells. Cells were seeded in six-well plates and
incubated to reach ~80% confluence. The growth medium was removed
and adenoviruses expressing NDRG2 (OS-RC-2-NDRG2 group) or the
control gene green fluorescent protein (GFP) (OS-RC-2-GFP group)
were added to OS-RC-2 cells with serum-free medium, and the cells
were then incubated for 16 h. The medium was subsequently replaced
with growth medium and the cells were incubated for different
periods of time. Uninfected cells served as another control
(OS-RC-2 group).
Western blot analysis
Protein from lysed cells was measured using the
bicinchoninic acid protein assay (Pierce Biotechnology, Inc.,
Rockford, IL, USA). A total of 100 μg lysate was loaded per lane
onto SDS polyacrylamide gels for separation by electrophoresis and
transfer onto Hybond™ nitrocellulose membranes (GE Healthcare,
Piscataway, NJ, USA). Following transfer, the membranes were
incubated with 5% fat-free milk in Tris-buffered saline containing
0.05% Tween-20 for 1 h at 37°C. Anti-NDRG2 (Abnova, Taiwan, China)
was then added and the membranes were incubated overnight at 4°C.
The membranes were subsequently washed three times with
phosphate-buffered saline (PBS) prior to incubation with the
secondary antibody for 1 h at room temperature. The blots were then
developed using chemiluminescence substrate solution (Pierce
Biotechnology, Inc.) and exposed to X-ray film for
visualization.
Cell growth assays
The cell growth was monitored using the MTT assay.
Briefly, cells were seeded into 96-well plates at an initial
density of 2,000 cells/well in triplicate. At each time-point, the
cells were washed and incubated with tetrazolium salt (100 mg/ml;
Sigma, St. Louis, MO, USA) at 37°C for 4 h. The supernatant was
removed, and 150 μl dimethyl sulfoxide was added to each well. The
absorbance (optical density) of the reaction solution at 570 nm was
recorded.
Colony formation assay
OS-RC-2 cells, which were stably infected with
either the control virus (pAV.EX1d-eGFP) or the virus carrying
NDRG2 (pAV.EX1d-NDRG2/IRES/eGFP), were seeded into 100-mm dishes at
a density of 500 cells/dish. The cells were grown for two weeks in
culturing medium. The colonies were then fixed and stained with
Coomassie Brilliant Blue.
Cell cycle analysis
Cells were seeded overnight on 60-mm-diameter plates
in a complete medium, placed in a serum-free medium for 48 h to
synchronize the cells and then maintained again in the complete
medium. After 24 h, the cells were recovered and washed with
ice-cold PBS, prior to being suspended in ~0.5 ml 70% alcohol and
maintained at 4°C for 30 min. The suspension was filtered through a
50-mm nylon mesh, and the DNA content of the stained nuclei was
analyzed using a flow cytometer (Epics XL; Beckman Coulter, Miami,
FL, USA). The cell cycle was analyzed using Multicycle-DNA cell
cycle analysis software (FACScan™; Becton-Dickinson, San Jose, CA,
USA). The proliferation index (PI) was calculated using the
following formula: PI = (S + G2)/(S + G2 +
G1).
NDRG2 subcellular localization
analysis
The cells were grown on glass coverslips. At
different time-points subsequent to transfection, the cells were
incubated for 30 min at 37°C with either 5 nm Rhodamine 123 (a
cell-permeable mitochondria-selective dye; Molecular
Probes®, Invitrogen Life Technologies, Carlsbad, CA,
USA), Bodipy (4,4-difluoro-4-bora-3a,4a-diaza-s-indacene), a
cell-permeable Golgi apparatus-selective dye, or Lucifer Yellow (a
cell-permeable lysosome-selective dye) obtained from Sigma. The
cells were then rinsed with PBS three times and fixed in freshly
prepared cold 4% paraformaldehyde in PBS for 30 min at 4°C. The
glass coverslips were then placed on the glass slides, and mounting
medium was used to hold the specimens in place. Slides were
observed under a confocal laser fluorescence microscope.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analyses were performed using the SPSS software package
(SPSS, Inc., Chicago, IL, USA). A one-way analysis of variance
followed by the t-test for independent groups was used. The
χ2-test or Fisher exact test was used to assess
dichotomous variables. P<0.05 was considered to indicate a
statistically significant difference.
Results
Upregulation of NDRG2 in OS-RC-2
cells
To confirm that the cells had been successfully
transfected, the cell morphology was observed using light and
fluorescence microscopes. Almost all the cells were found to
express GFP in the GFP virus-infected group, compared with only 60%
of cells in the GFP-NDRG2 virus-infected group (Fig. 1).
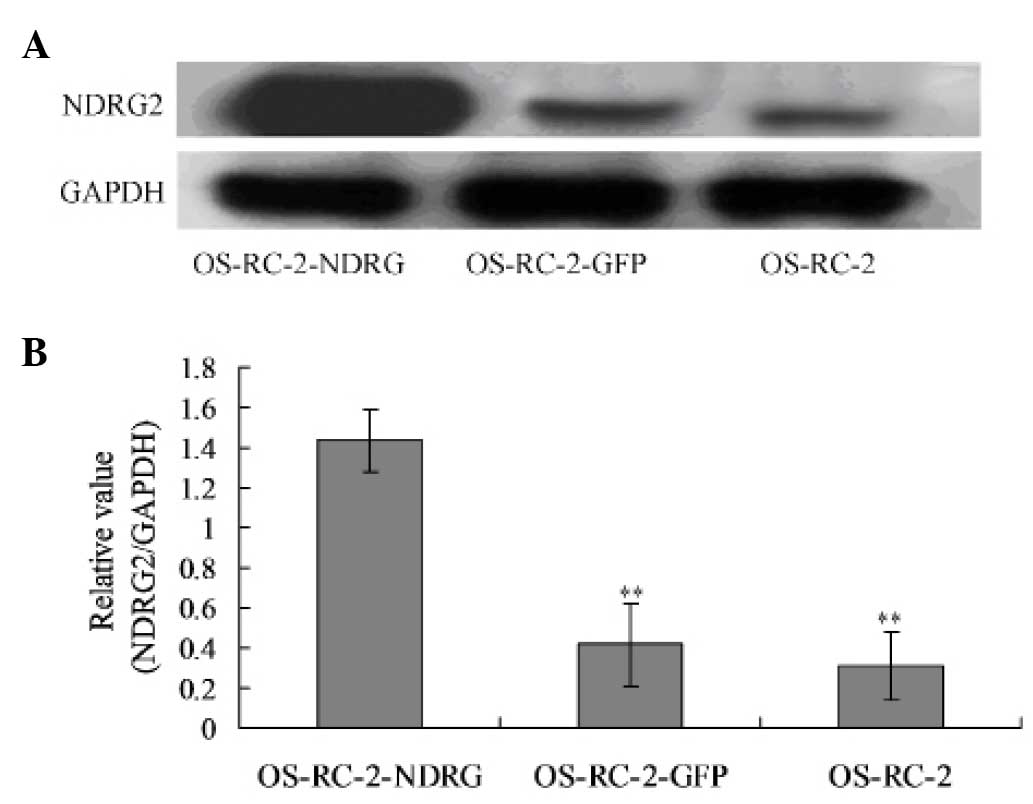
The protein expression levels of NDRG2 were then
analyzed using western blotting. In the normal cultured OS-RC-2
cells, little NDRG2 expression was detected. However, following
infection with the recombinant GFP-NDRG2 adenovirus, the NDRG2
levels were significantly elevated, while the NDRG2 expression
remained low in the cells infected with the control GFP vector
(Fig. 2).
Localization of NDRG2 in OS-RC-2
cells
To evaluate the subcellular localization of NDRG2,
organelle-specific dye was used. Following GFP-NDRG2 infection,
NDRG2 was found to be mainly expressed in the mitochondria, but not
in the Golgi apparatus or lysosome. The expression of NDRG2 in the
mitochondria increased between 6 and 72 h after transfection
(Fig. 3).
NDRG2 inhibits OS-RC-2 cell
proliferation
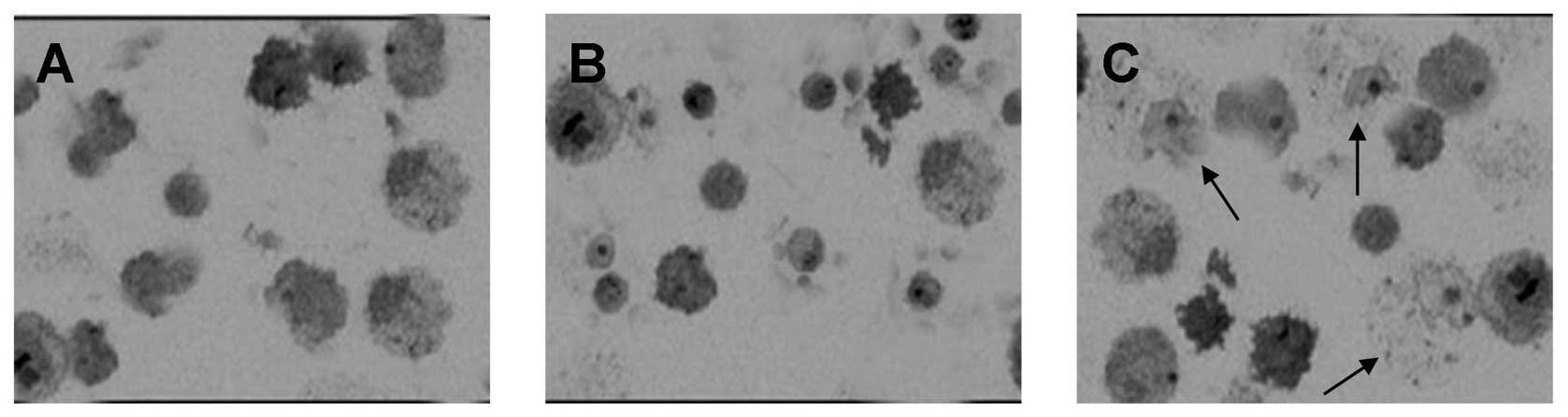
The results from the colony formation assay
demonstrated that NDRG2 inhibited the colony formation of OS-RC-2
cells at two weeks (OS-RC-2-NDRG2 cloning efficiency, 24.8%, vs.
OS-RC-2-GFP cloning efficiency, 51.6% and OS-RC-2 cloning
efficiency, 76.4%; Fig. 4A,
Table I)
 | Table ICloning efficiency of the three groups
of cells. |
Table I
Cloning efficiency of the three groups
of cells.
| Cells | Number of cloning
cells | Cloning efficiency
(%) |
|---|
| OS-RC-2 | 382 | 76.40 |
| OS-RC-2-GFP | 258 | 51.60 |
| OS-RC-2-NDRG2 | 124 | 24.80 |
Cell proliferation was then observed over time. The
results demonstrated that uninfected OS-RC-2 cells continued
proliferating up until day 7. However, the growth curve for the
cells infected with GFP-NDRG2 was significantly lower than that for
the control cells infected with GFP (P<0.05; Fig. 4B). This suggested that NDRG2 had
the potential to inhibit the proliferation of OS-RC-2 cells.
NDRG2 induces cell cycle arrest in
OS-RC-2 cells
To further investigate the mechanism by which NDRG2
inhibits OS-RC-2 cell growth, the effect of NDRG2 expression on the
cell cycle was analyzed using fluorescence-activated cell sorting
analysis. The results demonstrated that 84.3% of the
GFP-NDRG2-transfected cells were in S phase compared with 58.7% of
untransfected cells. Furthermore, 11.8% of the
GFP-NDRG2-transfected cells were in G1 phase compared
with 22.4% of untransfected cells (P<0.05; Fig. 5).
NDRG2 induces OS-RC-2 cell oncosis
instead of apoptosis
Forty-eight hours after infection, a small number of
cells appeared to have shrunk and showed marked nuclear chromatin
condensation, which are markers of apoptosis. By contrast, a
greater number of cells showed swelling and nuclear chromatin
clumping, which are markers of oncosis, in the GFP-NDRG2 infection
group compared with the GFP infection (Fig. 6).
Discussion
Several studies have previously shown that the
downregulation of NDRG2 in RCC is correlated with aggressive
clinicopathological features in patients with RCC (8–10).
The mechanism underlying this effect may involve p53-dependent
apoptosis and the p53-independent pathway. In the present study it
was demonstrated that NDRG2 is not only a biomarker of RCC, but
also a therapeutic target for the treatment of RCC.
It was demonstrated in this study that the
upregulation of NDRG2 by infection with NDRG2 recombinant
adenovirus inhibits OS-RC-2 cell proliferation. Additionally, it
was found that NDRG2 tumor suppressor activity is mediated by the
inhibition of cell cycle progression with an increased accumulation
of cancer cells in S phase. It has been previously reported that
NDRG2 suppresses activator protein-1 activity and regulates cyclin
D1 expression via the phosphorylation pathway in human colon
carcinoma cells (12). The results
from the present study are in accordance with those of Ma et
al (13), which also showed
that NDRG2 inhibits cancer cell proliferation. However, Ma et
al found an increase in the accumulation of cancer cells in the
G1 phase and a reduction in the number of cells in the S
phase of the cell cycle (13). The
different cell lines and adenovirus vectors used may account for
the different findings.
Another difference between the findings of the
present study and previous studies is that, in the present study,
NDRG2 induced oncosis instead of apoptosis (9,10).
Oncosis is a type of accidental cell death that is characterized by
cellular and organelle swelling, blebbing and an increase in
membrane permeability. These alterations in membrane permeability
are a result of the failure of the ion pumps in the plasma membrane
(14). A rapid decrease in
intracellular adenosine triphosphate (ATP) is an important
biochemical event leading to oncosis, as opposed to apoptosis
(15). Interference with ATP
synthesis rapidly leads to deactivation of the
Na+/K+-ATPase in the cell membrane, resulting
in an increase in the intracellular concentrations of
Na+ and Cl− accompanied by water influx,
cellular swelling and a rapid increase in the intracellular
Ca2+ concentration (16). A number of chemotherapeutic agents,
including arsenic trioxide, cisplatin and etoposide, have also been
found to induce both oncosis and apoptosis (17–19).
The interrelation between apoptosis and oncosis is increasingly
being recognized, including in models characterized by the initial
activation of apoptosis followed by oncosis and, conversely,
oncosis followed by apoptosis (20).
The differences between the findings of the present
study and previous reports may also be due to the localization of
NDRG2. In cells in a normal state, NDRG2 protein is primarily
located in the cytoplasm, although it is also associated with the
cell membrane and adherens junctions (21). Following damage to the DNA, NDRG2
can be translocated from the cytoplasm to the nucleus. Wang et
al (22) demonstrated that the
NDRG2 fragment comprising amino acid residues 101 to 178 is
responsible for the nuclear translocation. It has been proposed
that nuclear NDRG2 expression may affect cancer biology. However,
in the present study, NDRG2 was primarily expressed in the
mitochondria and its expression increased in a time-dependent
manner. Mitochondrial function is impaired in cancer cells. The
metabolism of proliferative tumor cells utilizes mitochondria as
functional biosynthetic organelles. In proliferating cells, the use
of mitochondrial enzymes in the synthesis of anabolic precursors
takes priority over the oxidative phosphorylation-dependent
production of ATP. It has been reported that NDRG2 overexpression
in malignant breast cancer cells specifically inhibits Akt
phosphorylation and induces the phosphorylation of p38
mitogen-activated protein kinase and stress-activated protein
kinase/ c-Jun NH2-terminal kinase, which drives anabolic
metabolism and tumorigenesis by reprogramming the mitochondria
(23,24). It has also been shown that a modest
increase in uncoupling protein 2 expression results in a reduction
in intracellular ATP levels and a marked, rapid fall in
mitochondrial membrane potential, which causes morphological
oncosis (25). However, whether
NDRG2 regulates renal cancer cell proliferation and oncosis through
mitochondrial reprogramming requires further investigation.
In conclusion, it was found in the present study
that NDRG2 is expressed in the mitochondria of OS-RC-2 cells, and
that NDRG2 expression arrests cells in the S phase, decreases renal
cancer cell proliferation and induces oncosis. These results
further expand the present understanding of NDRG2 and its role in
tumor cells, and may have important implications for targeting
NDRG2 for cancer therapy.
Acknowledgements
This study was supported by the Project of Science
and Technology Plan of Guangdong Province, P.R. China (no.
10B06090008063041).
References
|
1
|
Vervoorts J, Lüscher-Firzlaff J and
Lüscher B: The ins and outs of MYC regulation by posttranslational
mechanisms. J Biol Chem. 281:34725–34729. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou RH, Kokame K, Tsukamoto Y, Yutani C,
Kato H and Miyata T: Characterization of the human NDRG gene
family: a newly identified member, NDRG4, is specifically expressed
in brain and heart. Genomics. 73:86–97. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qu X, Zhai Y, Wei H, Zhang C, Xing G, Yu Y
and He F: Characterization and expression of three novel
differentiation-related genes belong to the human NDRG gene family.
Mol Cell Biochem. 229:35–44. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lusis EA, Watson MA, Chicoine MR, Lyman M,
Roerig P, Reifenberger G, Gutmann DH and Perry A: Integrative
genomic analysis identifies NDRG2 as a candidate tumor suppressor
gene frequently inactivated in clinically aggressive meningioma.
Cancer Res. 65:7121–7126. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lorentzen A, Vogel LK, Lewinsky RH, et al:
Expression of NDRG2 is down-regulated in high-risk adenomas and
colorectal carcinoma. BMC Cancer. 7:1922007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu N, Wang L, Liu X, et al: Promoter
methylation, mutation, and genomic deletion are involved in the
decreased NDRG2 expression levels in several cancer cell lines.
Biochem Biophys Res Commun. 358:164–169. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu N, Wang L, Li X, et al: N-Myc
downstream-regulated gene 2 is involved in p53-mediated apoptosis.
Nucleic Acids Res. 36:5335–5349. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma J, Jin H, Wang H, Yuan J, Bao T, Jiang
X, Zhang W, Zhao H and Yao L: Expression of NDRG2 in clear cell
renal cell carcinoma. Biol Pharm Bull. 31:1316–1320. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liang ZL, Kang K, Yoon S, Huang SM, Lim
JS, Kim JM, Lim JS and Lee HJ: NDRG2 is involved in the oncogenic
properties of renal cell carcinoma and its loss is a novel
independent poor prognostic factor after nephrectomy. Ann Surg
Oncol. 19:2763–2012. PubMed/NCBI
|
|
11
|
Zheng J, Li Y, Yang J, et al: NDRG2
inhibits hepatocellular carcinoma adhesion, migration and invasion
by regulating CD24 expression. BMC Cancer. 251:1–9. 2011.
|
|
12
|
Kim YJ, Yoon SY, Kim JT, Choi SC, Lim JS,
Kim JH, Song EY, Lee HG, Choi I and Kim JW: NDRG2 suppresses cell
proliferation through down-regulation of AP-1 activity in human
colon carcinoma cells. Int J Cancer. 124:7–15. 2009. View Article : Google Scholar
|
|
13
|
Ma JJ, Liao CG, Jiang X, Zhao HD, Yao LB
and Bao TY: NDRG2 suppresses the proliferation of clear cell renal
cell carcinoma cell A-498. J Exp Clin Cancer Res. 29:1032010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Majno G and Joris I: Apoptosis, oncosis,
and necrosis. An overview of cell death. Am J Pathol. 146:3–15.
1995.PubMed/NCBI
|
|
15
|
Trump BF, Berezesky IK, Chang SH and
Phelps PC: The pathways of cell death: oncosis, apoptosis, and
necrosis. Toxicol Pathol. 25:82–88. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Z, Gong L, Li X, Ye L, Wang B, Liu J,
Qiu J, Jiao H, Zhang W, Chen J and Wang J: Infrasound increases
intracellular calcium concentration and induces apoptosis in
hipocampi of adult rats. Mol Med Rep. 5:73–77. 2012.
|
|
17
|
Chanan-Khan A, Srinivasan S and Czuczman
MS: Prevention and management of cardiotoxicity from antineoplastic
therapy. J Support Oncol. 2:251–256; discussion 259–261, 264–266.
2004.PubMed/NCBI
|
|
18
|
Duan J, Lang Y, Song C, Xiong J, Wang Y
and Yan Y: siRNA targeting of PRDX3 enhances cisplatin-induced
apoptosis in ovarian cancer cells through the suppression of the
NF-κb signaling pathway. Mol Med Rep. 7:1688–1694. 2013.PubMed/NCBI
|
|
19
|
Zhu J, Okumura H, Ohtake S, Nakamura S and
Nakao S: The molecular mechanism of arsenic trioxide-induced
apoptosis and oncosis in leukemia/lymphoma cell lines. Acta
Haematol. 110:1–10. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Whelan RS, Kaplinskiy V and Kitsis RN:
Cell death in the pathogenesis of heart disease: mechanisms and
significance. Annu Rev Physiol. 72:19–44. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Boulkroun S, Fay M, Zennaro MC, Escoubet
B, Jaisser F, Blot-Chabaud M, Farman N and Courtois-Coutry N:
Characterization of rat NDRG2 (N-Myc downstream regulated gene 2),
a novel early mineralocorticoid-specific induced gene. J Biol Chem.
277:31506–31515. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang L, Liu N, Yao L, et al: NDRG2 is a
new HIF-1 target gene necessary for hypoxia-induced apoptosis in
A549 cells. Cell Physiol Biochem. 21:239–250. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu D, Wang Q, An Y and Xu L: MiR-203
regulates the proliferation, apoptosis and cell cycle progression
of pancreatic cancer cells by targeting Survivin. Mol Med Rep.
8:379–384. 2013.PubMed/NCBI
|
|
24
|
Park Y, Shon SK, Kim A, Kim KI, Yang Y,
Cho DH, Lee MS and Lim JS: SOCS1 induced by NDRG2 expression
negatively regulates STAT3 activation in breast cancer cells.
Biochem Biophys Res Commun. 363:361–367. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mills EM, Xu D, Fergusson MM, Combs CA, Xu
Y and Finkel T: Regulation of cellular oncosis by uncoupling
protein 2. J Biol Chem. 277:27385–27392. 2002. View Article : Google Scholar : PubMed/NCBI
|