Introduction
Chronic kidney disease (CKD) is an intractable
disease, which continues to increase worldwide and has increased
the financial burden on health care systems (1). It is therefore necessary to elucidate
the underlying mechanism and to develop an additional treatment
strategy to slow the progression of CKD. Previous studies have
demonstrated that a persistent inflammatory response was a
characteristic of CKD in animal models and in patients, which
revealed the importance of inflammation in renal injury (2,3). In
addition, oxidative stress also accelerated the progression of CKD.
A review of previous studies found that reactive oxygen species
(ROS) increased in a graded manner as renal function deteriorated
and identified an inverse correlation between oxidative stress and
glomerular filtration rate (4).
Furthermore, inhibition of nicotinamide adenine dinucleotide
phosphate (NADPH) oxidase, which produces ROS, was beneficial to
renal injury (5,6), thus it is an effective treatment
strategy to suppress inflammation and oxidative stress in CKD.
Statins (3-hydroxy-3-methylglutaryl-coenzyme A
reductase inhibitors) are drugs with potent lipid-lowering effects.
In addition to their cholesterol-lowering properties, statins have
pleiotropic effects (7). Statins
suppress mesangial cell proliferation, prevent the decrease in
glomerular filtration and the deterioration of renal function in
patients with nephropathy (8).
Therefore, statins may provide a beneficial therapy in preventing
and delaying the progression of CKD. However, the exact mechanism
remains to be fully elucidated.
Mesangial cells are a major glomerular cell type and
are involved in inflammatory reactions and oxidative stress in the
kidney (9) leading to CKD
(10). In kidney injury, mesangial
cells are the major target of angiotensin II (Ang II), which
accelerates inflammation and other adverse actions. Thus, in the
present study, the effect of simvastatin on human mesangial cells
(HMCs) in the presence of Ang II was examined, and the hypothesis
that the mechanism underlying the protective effect of simvastatin
is through attenuating inflammation and oxidative stress was
assessed.
Materials and methods
Cell culture
HMCs were purchased from the American Type Cell
Collection (Manassas, VA, USA) and cultured in RPMI-1640 containing
10% fetal bovine serum. Mesangial cells were incubated at 37°C in a
humidified incubator containing 5% CO2 . The cells up to
the 4th passage were used and were plated in 6-well plates and
cells at 90% confluence were used for experiments The control group
was treated without additives and the other four groups were
pretreated with simvastatin at different concentrations (0, 0.1, 1
or 10 μM) for 1 h and then stimulated by Ang II (1 μM) for 24 h.
Following stimulation, all the cells were collected.
RNA extraction and quantitative
polymerase chain reaction (qPCR)
The cells were harvested and total RNA was extracted
using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA,
USA). cDNA was synthesized and qPCR was performed according to the
manufacturer’s instructions. Relative mRNA levels for target genes
were analyzed with the comparative CT method. Human GAPDH levels
served as an internal control. The primer sequences used were as
follows: monocyte chemoattractant protein-1 (MCP-1), forward
5′-TGTTGATGTGAAACATTATGCC-3′ and reverse
5′-AATGATTCTTGCAAAGACCCTC-3′; tumor necrosis factor-α (TNF-α),
forward 5′-TTCGAGAAGATGATCTGATGC-3′ and reverse
5′-TCAGCCTCTTCTCCTTCCT-3′; interleukin (IL)-1β, forward
5′-ATGGGATAACGAGGCTTATG-3′ and reverse 5′-CAAGGCCACAGGTATTTTGTC-3′;
IL-6, forward 5′-ATGAACTCCTTCTCCACAAGCGC-3′ and reverse
5′-GAAGAGCCCTCAGGCTGGACTG-3′; cyclooxygenase-2 (COX-2), forward
5′-TCCCTGAGCATCTACGGTTT-3′ and reverse 5′-TACTCTGTTGTGTTCCCGCA-3′
and GAPDH, forward 5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse
5′-ATGGTGGTGAAGACGCCAGT-3′. All experiments were repeated three
times and the mean values were derived.
Western blot analysis
The protein was extracted from the five groups using
radioimmunoprecipitation assay lysis buffer with a protease
inhibitor (phenylmethanesulfonyl fluroide; Beyotime, Beijing,
China) and protein concentrations were determined using a
bicinchoninic acid protein assay. Equal quantities of protein were
resolved by 10% SDS-PAGE and transferred onto nitrocellulose
membranes (Plano, TX, USA), which were inhibited with 5% skimmed
milk for 2 h. Subsequently, the membranes were incubated with
specific primary antibodies at 4°C overnight, and then horseradish
peroxidase-conjugated secondary antibodies (goat anti-mouse
polyclonal antibody to IgG or goat anti-rabbit polyclonal antibody
to IgG) against the primary antibody was added at room temperature
for 2 h. Finally, the immunoreactive bands were visualized using
enhanced chemiluminescence plus reagents (Millipore, Plano, TX,
USA). β-actin was used as an endogenous loading control. All
experiments were repeated three times and the mean values were
derived.
The primary antibodies used in the experiments were
as follows: rabbit polyclonal anti-NOX2 (1:1,000; Abcam, Cambridge,
UK), rabbit monoclonal anti-protein kinase Cα (PKCα; 1:1,000;
Abcam), rabbit monoclonal anti-PKCβ1 (1:1,000; Abcam), rabbit
polyclonal anti-COX-2 (1:1,000; Abcam), rabbit polyclonal
anti-peroxisome proliferator activated receptor γ (PPARγ; 1:1,000;
Abcam), rabbit polyclonal anti-nuclear factor-κB (NF-κB) p65
(1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA),
rabbit polyclonal anti-(phosphorylation-NF-κB p65; 1:1,000; Cell
Signaling Technology, Inc.), rabbit monoclonal anti-β-actin
(1:1,000; Cell Signaling Technology, Inc.) and rabbit polyclonal
anti-p47phox (1:1,000; Millipore).
Enzyme-linked immunosorbent assay
(ELISA)
The supernatant of mesangial cells among all groups
were collected and stored at −80°C. The levels of prostaglandin E2
(PGE2) were assessed with an ELISA kit according to the
manufacturer’s instructions (R&D Systems, Minneapolis, MN,
USA).
Statistical analysis
The values are expressed as the mean ± standard
error of the mean. The differences between any two groups were
analyzed by Student’s t-test and for more than two groups by
one-way analysis of variance. P<0.05 was considered to indicate
a statistically significant difference.
Results
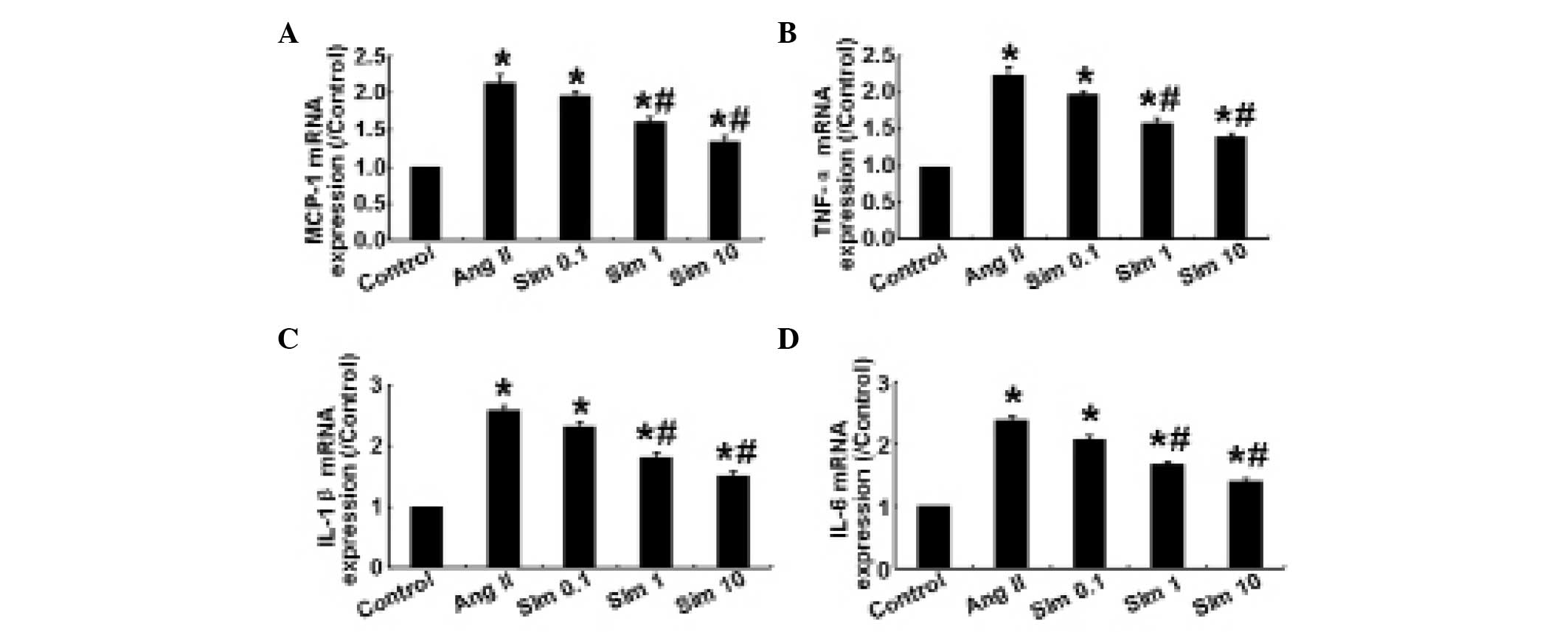
Simvastatin downregulates Ang II-induced
proinflammatory cytokine expression in HMCs
Ang II regulates the synthesis of proinflammatory
cytokines and chemokines, including MCP-1, TNF-α, IL-1β and IL-6
(11,12). In the present study, the mRNA
expression levels of MCP-1, TNF-α, IL-1β and IL-6 were determined
by qPCR. The results demonstrated that in HMCs, 1 and 10 μm
simvastatin significantly decreased the expression levels of
proinflammatory cytokines compared with the control group
(P<0.05; Fig. 1), whereas no
significant difference in the expression of these cytokines was
observed between 0.1 μm simvastatin and the control group
(P>0.05; Fig. 1), indicating
that simvastatin downregulated the inflammatory response in HMCs in
a dose-dependent manner.
Simvastatin suppresses Ang II-induced
oxidative stress in HMCs
The level of ROS in HMCs, as determined by
dihydroethidium staining, was increased significantly following Ang
II stimulation (P<0.05; Fig. 2A and
B), however, this effect was attenuated by simvastatin in a
dose-dependent manner (P<0.05; Fig.
2A and B). Increases in renal NADPH oxidase activity and
activation of the PKC system are important in oxidative stress and
kidney damage (13,14). Therefore, the present study also
examined the expression levels of NADPH oxidase subunits (NOX2 and
p47phox) and PKC isoforms (PKCα and PKCβ1) in cultured
mesangial cells. As shown in Fig. 2C
and D, Ang II significantly increased the expression levels of
the above proteins, while simvastatin reversed the effect of Ang II
in a dose-dependent manner. These results demonstrated that
simvastatin reduced oxidative stress by suppressing the NADPH
oxidase and PKC signaling pathways.
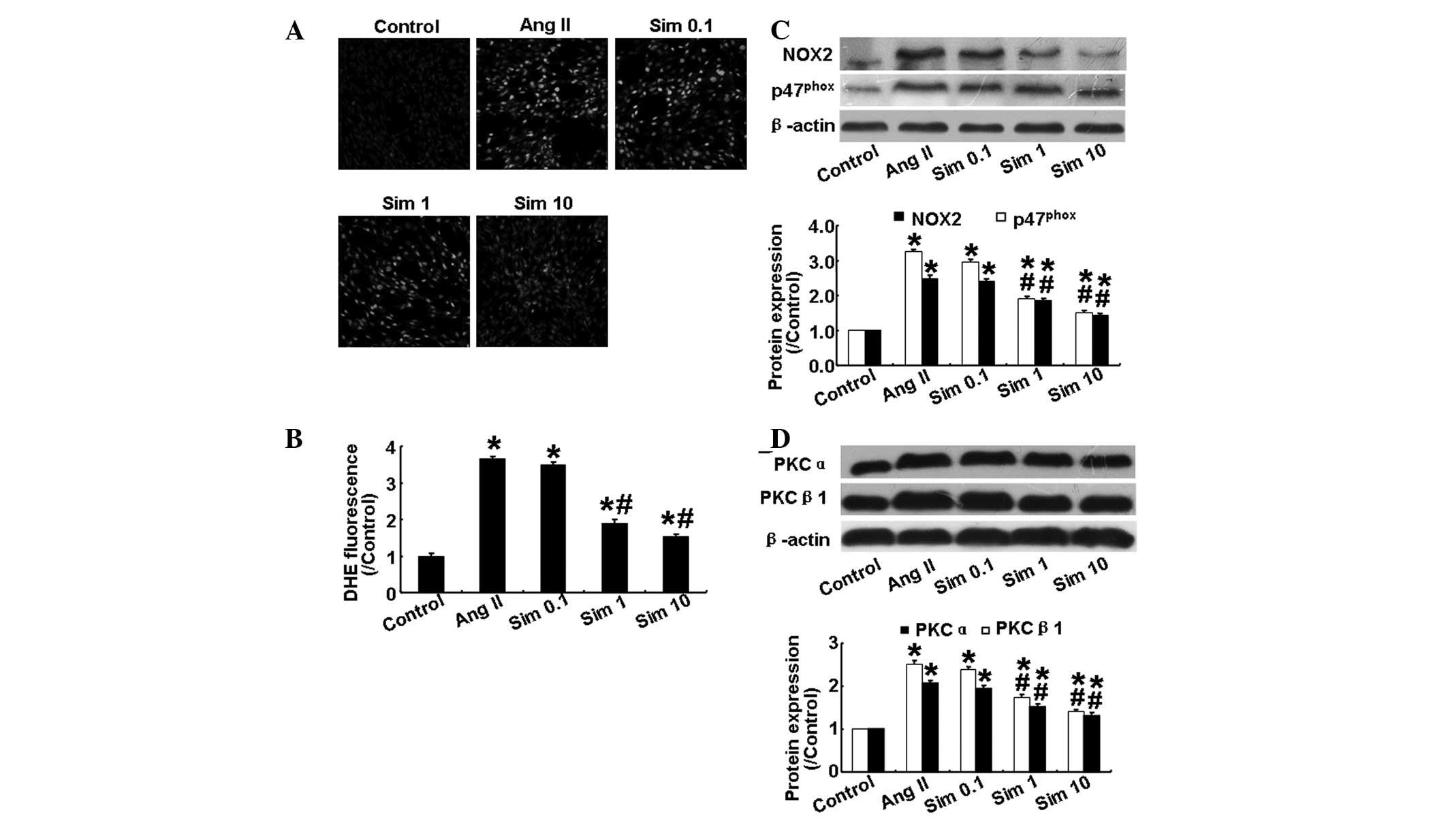 | Figure 2Effects of simvastatin on oxidative
stress and the expression of NADPH oxidase subunits and the PKC
isoforms in human mesangial cells. (A) Representative DHE staining
in the five groups. (B) Quantitative analysis of A. (C) Protein
expression of NOX2 and p47phox in the five groups by
western blotting and quantitative analysis. (D) Protein expression
of PKCα and PKCβ1 in the five groups by western blotting and
quantitative analysis. Sim 0.1, 0.1 μm simvastatin group; Sim 1, 1
μm simvastatin group; Sim 10, 10 μm simvastatin group.
*P<0.05, vs. control group; #P<0.05,
vs. Ang II group. NADPH, nicotinamide adenine dinucleotide
phosphate; PKC, protein kinase C; NOX2, NADPH oxidase; Ang II,
angiotensin II; Sim, simvostatin; DHE, dihydroethidium. |
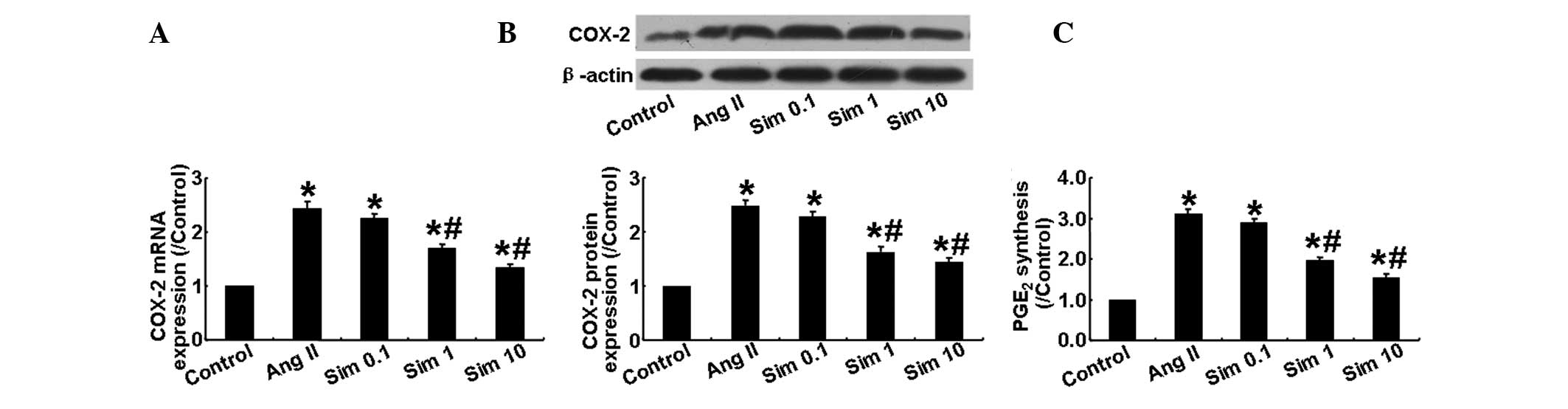
Simvastatin inhibits COX-2 expression in
HMCs
The present study also examined the mRNA and protein
expression of COX-2 in HMCs stimulated with Ang II and found that,
compared with the control group, the mRNA and protein expression of
COX-2 in the Ang II group was markedly increased. However, it was
significantly lower in the 1 and 10 μm simvastatin groups than in
the Ang II and 0.1 μm simvastatin groups (P<0.05; Fig. 3A and B). In addition to its effect
on COX-2 expression, Ang II significantly increased the production
of PGE2, while simvastatin markedly suppressed this
effect in a dose-dependent manner (P<0.05; Fig. 3C). These results demonstrated that
Ang II induced COX-2 expression in HMCs, which was partially
inhibited by simvastatin treatment.
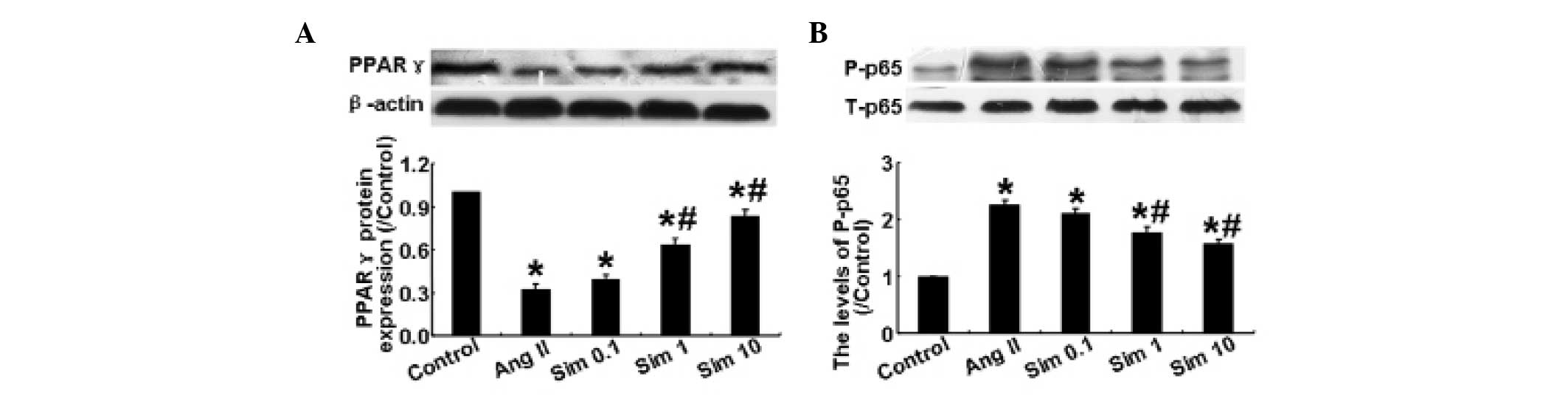
Simvastatin suppresses PPARγ expression
and NF- κB activation in HMCs
Ang II has been demonstrated to reduce the decrease
in expression of PPARγ, which can further augment inflammation
(15). As shown in Fig. 4A, Ang II significantly decreased
the expression of PPARγ compared with the control group, while
simvastatin reversed the Ang II-induced decrease in PPARγ
expression at doses of 1 and 10 μm. The phosphorylation level of
NF-κB p65 among the five groups was also examined by western blot
analysis. The results demonstrated that Ang II significantly
increased the levels of phosphorylation of NF-κB p65, while 1 and
10 μm simvastatin reversed this increase (P<0.05; Fig. 4B). However, a simvastatin
concentration of 0.1 μm did not have a significant effect. These
results demonstrated that simvastatin suppressed the effect of Ang
II on the expression of PPARγ and on the activation of NF-κB in
HMCs.
Discussion
CKD is an intractable disease worldwide and a
rational therapy is critical for improving public health. Statins
have been demonstrated to have organ-protective properties
independent of their cholesterol-lowering effects. In the present
study, the effect of simvastatin on HMCs stimulated by Ang II was
examined. The results demonstrated that simvastatin significantly
suppressed the inflammatory response by inhibiting COX-2 expression
and NF-κB activation, and reversing the expression of PPARγ
suppressed by Ang II. In addition, simvastatin alleviated oxidative
stress by decreasing the expression of NADPH oxidase and PKCs.
Therefore, simvastatin may have a beneficial effect in renal
injury.
The renin-angiotensin system (RAS) has been
demonstrated to be important in the pathogenesis of CKD. Ang II is
the major product of RAS and its principle target in the kidney is
the mesangial cells. Ang II affects the local hemodynamics in the
kidney and induces glomerular mesangial dysfunction, mesangial cell
hypertrophy and proliferation, or promotes extracellular matrix
deposition, which appears to be directly linked to inflammation and
oxidative stress (16). Therefore,
in the present study, HMCs were selected and stimulated with Ang II
as an in vitro model to examine the effect of simvastatin on
CKD and the corresponding mechanism.
Inflammation is important in numerous diseases,
including progressive renal disease, and CKD has been viewed as a
chronic inflammatory disease (17,18).
Persistent inflammation inside the kidney affects the local
hemodynamics and induces glomerular injury and renal fibrosis,
which promotes renal dysfunction and contributes to the progression
of kidney disease (19,20). Ang II, a known inflammatory
mediator, activates the expression of a diverse range of
proinflammatory factors in the kidney and contributes to renal
injury (21). In the present
study, Ang II significantly increased the expression of MCP-1,
TNF-α, IL-1β and IL-6 in HMCs, while simvastatin suppressed the
effect of Ang II on the expression of proinflammatory factors in a
dose-dependent manner, indicating that simvastatin had
anti-inflammatory effects in CKD.
Oxidative stress has emerged as an important
pathogenic factor in numerous renal diseases, including acute and
chronic renal failure (22). ROS
produce oxidative stress and overproduction or reduced elimination
of ROS has a toxic effect on cells and causes damage to tissues
(23). Previous studies
demonstrated that inhibition of ROS production was beneficial in
experimental and human kidney disease (23). There is increasing evidence that
Ang II directly promotes the production of ROS via NADPH oxidase in
mesangial cells, which is important in glomerular injury (24). In the present study, the effect of
simvastatin on the production of ROS in HMCs stimulated with Ang II
was examined. The results indicated that, compared with the control
group, the content of ROS was significantly increased in the Ang II
group and was suppressed in the simvastatin groups in a
dose-dependent manner. Among the three doses of simvastatin, 1 and
10 μm significantly decreased the content of ROS, suggesting that
simvastatin has an anti-oxidative stress effect in CKD.
The present study also detected the mechanism of
simvastatin on anti-inflammation and anti-oxidative stress in HMCs
stimulated with Ang II. PPARγ is a member of the nuclear receptor
superfamily of transcription factors, which ameliorates the
inflammatory response (25).
Inhibition of PPARγ activity, involved in the pathogenesis of
inflammation and its agonists, exerted an anti-inflammatory effect
by downregulating the transcription of NF-kB (26). In addition, it was confirmed that
PPARγ was present in mesangial cells and PPARγ agonists modulated
the proliferation and differentiation of mesangial cells and has
been effective in the therapy of renal dysfunction (27). NF-κB modulates the levels of genes
involved in inflammatory responses and the activation of NF-κB in
mesangial cells has a major pathogenic effect on inflammatory renal
disease (28,29). In addition, COX-2 catalyzes the
conversion process of arachidonic acids to prostaglandins and
upregulates the expression of proinflammatory chemokines in the
kidney (30). Therefore, the
present study examined the effects of simvastatin on the expression
of PPARγ and COX-2 and the phosphorylation level of NF-κB p65 in
HMCs. Ang II was found to significantly increase the expression of
COX-2 and the level of phosphorylation of NF-κB p65, and decrease
the expression of PPARγ. However, 1 and 10 μm simvastatin clearly
reversed the effects of Ang II. Simvastatin may therefore have an
anti-inflammatiory effect in CKD through regulating the expression
of PPARγ and COX-2 and the activation of NF-κB.
Oxidative stress is important in CKD and the major
producer of this is ROS. A previous study demonstrated that NADPH
oxidase was the predominant enzyme in ROS production and was
recognized as an important factor in cell proliferation and
extracellular matrix accumulation in renal disease (31). NADPH oxidase is composed of two
membrane-associated components, p22phox and NOX2 and
four cytosolic components (p47phox, p67phox,
p40phox and rac-1/2). The subunits NOX2 and
p47phox are mainly expressed in the kidney (32). Thus, the present study detected the
expression of NOX2 and p47phox in HMCs and found that
simvastatin reversed the Ang II-induced increase in NOX2 and
p47phox in a dose-dependent manner, reaching its maximum
effect at 10 μm simvastatin. In addition, activation of the PKC
system is known to be important in the pathophysiology of kidney
damage (33). There is evidence
indicating that PKC mediates the Ang II-induced activation of NADPH
oxidase and then increases the production of ROS (34). The PKC system has several isozymes,
including PKCα and PKCβ1, which have been identified in mesangial
cells (35). The present study
demonstrated that Ang II significantly increased the expression of
PKCα and PKCβ1, and simvastatin suppressed the effect of Ang II in
a dose-dependent manner. Therefore, simvastatin may inhibit
oxidative stress induced by Ang II through suppressing the
expression of NADPH oxidase and PKCs.
In conclusion, simvastatin ameliorated Ang
II-induced inflammation and oxidative stress in a dose-dependent
manner. The possible underlying mechanisms of the
simvastatin-mediated effects may involve the reduced expression of
COX-2, NADPH oxidase and PKCs, the activation of NF-κB and the
increased expression of PPARγ.
Acknowledgements
The present study was supported by a grant from the
Liaoning Province Natural Science Foundation (no. 2013022010).
References
|
1
|
Ruggenenti P, Cravedi P and Remuzzi G:
Mechanisms and treatment of CKD. J Am Soc Nephrol. 23:1917–1928.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stenvinkel P, Ketteler M, Johnson RJ, et
al: IL-10, IL-6, and TNF-alpha: central factors in the altered
cytokine network of uremia - the good, the bad, and the ugly.
Kidney Int. 67:1216–1233. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abbate M, Zoja C and Remuzzi G: How does
proteinuria cause progressive renal damage? J Am Soc Nephrol.
17:2974–2984. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cachofeiro V, Goicochea M, de Vinuesa SG,
Oubina P, Lahera V and Luno J: Oxidative stress and inflammation, a
link between chronic kidney disease and cardiovascular disease.
Kidney Int Suppl. S4–S9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yi F, Xia M, Li N, Zhang C, Tang L and Li
PL: Contribution of guanine nucleotide exchange factor Vav2 to
hyperhomocysteinemic glomerulosclerosis in rats. Hypertension.
53:90–96. 2009. View Article : Google Scholar :
|
|
6
|
Tian N, Thrasher KD, Gundy PD, Hughson MD
and Manning RD Jr: Antioxidant treatment prevents renal damage and
dysfunction and reduces arterial pressure in salt-sensitive
hypertension. Hypertension. 45:934–939. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meng X, Zhang K, Li J, et al: Statins
induce the accumulation of regulatory T cells in atherosclerotic
plaque. Mol Med. 18:598–605. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Athyros VG, Papageorgiou AA, Elisaf M and
Mikhailidis DP: Statins and renal function in patients with
diabetes mellitus. Curr Med Res Opin. 19:615–617. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Imaizumi T, Aizawa-Yashiro T, Tsuruga K,
et al: Melanoma differentiation-associated gene 5 regulates the
expression of a chemokine CXCL10 in human mesangial cells:
implications for chronic inflammatory renal diseases. Tohoku J Exp
Med. 228:17–26. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schlondorff D and Banas B: The mesangial
cell revisited: no cell is an island. J Am Soc Nephrol.
20:1179–1187. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ruiz-Ortega M, Ruperez M, Lorenzo O, et
al: Angiotensin II regulates the synthesis of proinflammatory
cytokines and chemokines in the kidney. Kidney Int. 62:S12–S22.
2002. View Article : Google Scholar
|
|
12
|
Zhong J, Guo D, Chen CB, et al: Prevention
of angiotensin II-mediated renal oxidative stress, inflammation,
and fibrosis by angiotensin-converting enzyme 2. Hypertension.
57:314–322. 2011. View Article : Google Scholar
|
|
13
|
Thallas-Bonke V, Thorpe SR, Coughlan MT,
et al: Inhibition of NADPH oxidase prevents advanced glycation end
product-mediated damage in diabetic nephropathy through a protein
kinase C-alpha-dependent pathway. Diabetes. 57:460–469. 2008.
View Article : Google Scholar
|
|
14
|
Kitada M, Koya D, Sugimoto T, et al:
Translocation of glomerular p47phox and p67phox by protein kinase
C-beta activation is required for oxidative stress in diabetic
nephropathy. Diabetes. 52:2603–2614. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kintscher U, Lyon CJ and Law RE:
Angiotensin II, PPAR-gamma and atherosclerosis. Front Biosci.
9:359–369. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pearse DD, Tian RX, Nigro J, Iorgulescu
JB, Puzis L and Jaimes EA: Angiotensin II increases the expression
of the transcription factor ETS-1 in mesangial cells. Am J Physiol
Renal Physiol. 294:F1094–F1100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Varghese Z, Fernando R, Moorhead JF, Powis
SH and Ruan XZ: Effects of sirolimus on mesangial cell cholesterol
homeostasis: a novel mechanism for its action against
lipid-mediated injury in renal allografts. Am J Physiol Renal
Physiol. 289:F43–F48. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stenvinkel P, Heimburger O, Paultre F, et
al: Strong association between malnutrition, inflammation, and
atherosclerosis in chronic renal failure. Kidney Int. 55:1899–1911.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pawluczyk IZ, Tan EK and Harris KP: Rat
mesangial cells exhibit sex-specific profibrotic and
proinflammatory phenotypes. Nephrol Dial Transplant. 24:1753–1758.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang M, Gao X, Wu J, et al: Oxidized
high-density lipoprotein enhances inflammatory activity in rat
mesangial cells. Diabetes Metab Res Rev. 26:455–463. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Theuer J, Dechend R, Muller DN, et al:
Angiotensin II induced inflammation in the kidney and in the heart
of double transgenic rats. BMC Cardiovasc Disord. 2:32002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Presti RL, Carollo C and Caimi G: Wine
consumption and renal diseases: new perspectives. Nutrition.
23:598–602. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li JM and Shah AM: ROS generation by
nonphagocytic NADPH oxidase: potential relevance in diabetic
nephropathy. J Am Soc Nephrol. 14:S221–S226. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jaimes EA, Galceran JM and Raij L:
Angiotensin II induces superoxide anion production by mesangial
cells. Kidney Int. 54:775–784. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang C, Ting AT and Seed B: PPAR-gamma
agonists inhibit production of monocyte inflammatory cytokines.
Nature. 391:82–86. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu X and Li L: Rosiglitazone suppresses
lipopolysaccharide-induced matrix metalloproteinase-2 activity in
rat aortic endothelial cells via Ras-MEK1/2 signaling. Int J
Cardiol. 158:54–58. 2012. View Article : Google Scholar
|
|
27
|
Asano T, Wakisaka M, Yoshinari M, et al:
Peroxisome proliferator-activated receptor gamma1 (PPARgamma1)
expresses in rat mesangial cells and PPARgamma agonists modulate
its differentiation. Biochim Biophys Acta. 1497:148–154. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rangan G, Wang Y and Harris D: NF-kappaB
signalling in chronic kidney disease. Front Biosci (Landmark Ed).
14:3496–3522. 2009. View
Article : Google Scholar
|
|
29
|
Wang Y, Zhang MX, Meng X, et al:
Atorvastatin suppresses LPS-induced rapid upregulation of Toll-like
receptor 4 and its signaling pathway in endothelial cells. Am J
Physiol Heart Circ Physiol. 300:H1743–H1752. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zahner G, Schaper M, Panzer U, et al:
Prostaglandin EP2 and EP4 receptors modulate expression of the
chemokine CCL2 (MCP-1) in response to LPS-induced renal glomerular
inflammation. Biochem J. 422:563–570. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang L, Pang S, Deng B, et al: High
glucose induces renal mesangial cell proliferation and fibronectin
expression through JNK/NF-κB/NADPH oxidase/ROS pathway, which is
inhibited by resveratrol. Int J Biochem Cell Biol. 44:629–638.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jiang F, Zhang Y and Dusting GJ: NADPH
oxidase-mediated redox signaling: roles in cellular stress
response, stress tolerance, and tissue repair. Pharmacol Rev.
63:218–242. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oudit GY, Liu GC, Zhong J, et al: Human
recombinant ACE2 reduces the progression of diabetic nephropathy.
Diabetes. 59:529–538. 2010. View Article : Google Scholar :
|
|
34
|
Korchak HM and Kilpatrick LE: Roles for
beta II-protein kinase C and RACK1 in positive and negative
signaling for superoxide anion generation in differentiated HL60
cells. J Biol Chem. 276:8910–8917. 2001. View Article : Google Scholar
|
|
35
|
Wei XF, Zhou QG, Hou FF, Liu BY and Liang
M: Advanced oxidation protein products induce mesangial cell
perturbation through PKC-dependent activation of NADPH oxidase. Am
J Physiol Renal Physiol. 296:F427–F437. 2009. View Article : Google Scholar
|