Introduction
The mammalian testis serves two main functions: The
synthesis of steroids and the production of spermatozoa. This
second role is achieved through a process of transcriptional,
translational and posttranslational regulation (1). A number of spermatogenesis-related
genes, which are involved in specific phases of germ cell
development, have been identified during the past few years, and
certain genes have been shown to be important in spermatogenesis
(2–3). However, spermatogenesis-derived
factors involved in this process and the underlying molecular
mechanisms remain largely obscure.
Cell division and proliferation, which are the most
basic features of living organisms, are crucial in cell survival.
Apoptosis, or programmed cell death, is the process by which excess
or dysfunctional cells are eliminated, and is critical to maintain
tissue homeostasis (4). Preserving
the balance between cell proliferation and death is essential in
sustaining differentiated tissue homeostasis and also forms a
defense mechanism against pathogens (5). Nuclear factor (NF)-κB is a
transcription factor that is important in regulating inflammation,
immunity, cell proliferation and apoptosis (6–9).
Homo sapiens G-patch domain containing 2 (GPATC2;
GenBank accession no. BC042193.1) also termed CT110, PPP1R30 and
RP11-361K17.1, was first cloned by The National Institutes of
Health Mammalian Gene Collection program in October, 2002, which is
a multi-institutional effort to identify and sequence a cDNA clone
containing a complete open reading frame for each human and mouse
gene (10). It has been shown
using northern blot analysis that the GPATC2 gene is highly
expressed in the testes (11). The
functions and mechanisms of action of the GPATC2 gene have not yet
been elucidated. As no suitable male germ cell lines are currently
available, a somatic cell line was used to investigate the function
of GPATC2 in apoptosis, cell proliferation and the cell cycle.
The aim of the present study was to investigate the
expression of GPATC2 in different human cell lines and rat tissues,
and to determine the sub-cellular localization of the GPATC2
protein in 293T cells. In addition, the function of GPATC2 in 293T
cell cycle progression was studied in order to provide novel
insight into the breadth of expression of GPATC2 and its role in
cell proliferation.
Materials and methods
Plasmid construction
The generation of vector pCMV-SPORT6-GPATC2 has been
cloned by The Chinese National Human Genome Research Center (CHGB;
Beijing, China), full-length GPATC2 the coding sequence was
obtained by double enzyme digestion from pCMV-SPORT6-GPATC2 and
cloned into the pcDNA3.1/myc-His (12) B vectors (pcDB; Invitrogen,
Carlsbad, CA, USA), enhanced green fluorescent protein plasmid-N1
(pEGFP-N1) and pEGFP-C3, using the same enzyme restriction sites
for EcoRI and BamHI.
Preparation of small interfering RNAs
(siRNA)
The target sequence of the siRNA was
5′-GGAGCTGGTTCATGACCTT-3′ for human GPATC2. Sense and antisense
oligonucleotides with the internal loop were synthesized by Takara
Bio Inc., (Tokyo, Japan). These were annealed and ligated into the
BamHI and HindIII sites of pSilencer 4.1-CMVneo
(Ambion, Carlsbad, CA, USA) to construct the GPATC2-specific siRNA
expression plasmid, according to the manufacturer’s instructions.
pSilencer 4.1-CMVneo expressing scrambled siRNAs (Ambion) was used
as a control.
Immunofluorescence microscopy
293T cells were transfected with si-GPATC2 or
si-control and were grown on glass chamber slides. The cells were
fixed using 4% paraformaldehyde in phosphate-buffered saline (PBS)
for 30 min, permeabilized in 0.1% Triton X-100 for 30 min and then
blocked with 0.5% bovine serum albumin in PBS for 30 min at room
temperature (RT). After washing with PBS, the cells were incubated
with polyclonal mouse anti-human GPATC2 antibody (H00055105-B01;
Abnova, Walnut, CA, USA) for 2 h at 37°C. After washing with PBS
and Tween-20, the cells were incubated with rabbit anti-mouse
fluorescein isothiocyanate-conjugated secondary antibody (F-4143;
Sigma, St. Louis, MO, USA) and stained with
4′,6-diamidino-2-phenylindole (DAPI; Roche, Basel, Switzerland).
Images were visualized and captured using an Olympus microscope
(Olympus, Tokyo, Japan).
Cell culture and transient
transfection
H520, Hela, Hepg2, H1299, THP-1, Jurkas, U937,
786-O, HL60, A498, Caki, G401, MCF-7, T47d and 293T cells (American
Type Culture Collection, Manassas, VA, USA) were maintained in
Dulbecco’s modified Eagle’s medium or RPMI-1640 (HyClone, Logan,
UT, USA) supplemented with 10% fetal bovine serum (Gibco-BRL, Grand
Island, NY, USA) under 5% CO2 at 37°C. 293T cells seeded
at a density of 1×104 cells/well in 96-well plates or
3×105 cells/well in 6-well plates and were transfected
with empty vector pcDB or pcDB-GPATC2 using Vigorous transfection
reagent (Vigorous Instruments Co., Ltd., Beijing, China), according
to the manufacturer’s instructions.
RNA isolation and reverse
transcription-polymerase chain reaction (RT-PCR)
Male Sprague-Dawley rats were purchased from Peking
University Laboratory Animal Center (Beijing, China). The study was
approved by the Ethics Committee of Hebei United University
(Tangshan, China). Rats were sacrificed by cervical dislocation.
Appendix, liver, heart muscle, lung, small intestine, skeletal
muscle, pancreas, brain, kidney, stomach and testis tissues were
obtained from three rats of the same age. Total mRNA was extracted
from all tissues and cell lines using the TRIzol RNA isolation
protocol (Gibco-BRL). Samples (0.25 μg) were used for cDNA
synthesis using the PrimeScript RT Master mix (Perfect Real Time)
(Takara Bio Inc.). GPATC2 and GAPDH internal control transcripts
were amplified by RT-PCR using the following primers: Forward:
5′-GCGCAGGCCGTCATCAAC-3′ and reverse: 5′-TCCCACCAGGGCACAACTC-3′ for
human GPATC2; forward: 5′-GCCAGTATCCCGAGTTTAT-3′ and reverse:
5′-GCCCGACTCCTTCTCATA-3′ for rat GPATC2; and forward:
5′-AAGAAGGTGGTGAAGCAGGC-3′ and reverse: 5′-TCCACCACCCTGTTGCTGTA-3′
for GAPDH.
Subcellular localization
293T cells seeded at a density of 1×104
cells/well in 96-well plates were transfected with pEGFP-N1-GPATC2
or pEGFP-C3-GPATC2. After 24 h, cells were washed with PBS, fixed
with 4% paraformaldehyde for 10 min at RT, washed again with PBS
and stained with DAPI (5 μg/ml in PBS) at RT for 5 min. Cells were
visualized using an inverted fluorescence microscope (Nikon TE
2000-U, Nikon, Tokyo, Japan).
Dual-luciferase reporter assay
293T cells were seeded into 96-well plates at
1×104 cells/well. After 24 h, cells were co-transfected
with 80 ng/well pcDB-GPATC2 or pcDB, 40 ng pNF-κB-Luc plasmid
containing the firefly luciferase reporter gene (PathDetect,
Stratagene, La Jolla, CA, USA) and 4 ng internal control pRL-TK
plasmid containing the renilla luciferase gene (Promega
Corporation, Madison, WI, USA). Each transfection was conducted in
triplicate wells. After 24 h, cells were lysed in standard lysis
buffer (Promega Corporation) and cell lysates were assayed for
firefly and renilla luciferase activities using the dual-luciferase
reporter (DLR) Assay system (Promega Corporation), according to the
manufacturer’s instructions. Light emission was measured using a
GENios Pro reader (Tecan, Männedorf, Switzerland). Luciferase
activity was normalized to renilla luciferase activity. Duplicate
experiments were performed.
Cell counting kit-8 (CCK-8) assay
293T cells transfected with pcDB or pcDB-GPATC2 were
seeded into 96-well plates at a density of 2×103
cells/well. At the indicated time point, the cell counting kit
proliferation assay (Dojindo, Tokyo, Japan) was performed by adding
10 μl CCK-8 solution to each well and incubating at 37°C for 2 h.
Absorbance at 450 nm was then measured using a microplate plate
reader (Synergy 2 Multi-Mode Microplate Reader, BioTek, Winooski,
VT, USA).
Flow cytometry
293T cells were synchronized by serum starvation for
12 h and then transfected with 2.5 μg pcDB or pcDB-GPATC2. Cells
were trypsinized 48 h after GPATC2 transfection, washed twice with
PBS and fixed with ice-cold 70% ethanol. Fixed cells were pelleted,
washed and resuspended in PBS. Samples were then treated with 1 μl
of 10 mg/ml DNase free RNase A (Promega Corporation) incubated at
37°C for 30 min and then 50 μl of 300 mg/ml propidium iodide
(Boehringer, Mannheim, Germany) containing Triton X-100 was added
and cells were incubated in the dark for 30 min. Cell cycle data
were obtained by flow cytometry (FACSCalibur, BD Biosciences,
Franklin Lakes, NJ, USA).
Results
Characterization and expression profile
of the GPATC2 gene
The putative GPATC2 protein is comprised of 376
amino acids with a deduced molecular mass of ~43 kDa and a
theoretical isoelectric point of 7.65. The putative GPATC2 protein
has no transmembrane helices as predicted by the program TMHMM
Server v.2.0 (http://www.cbs.dtu.dk/services/TMHMM/), and no other
domains, including signal peptides, were found by the InterProScan
program (http://www.ebi.ac.uk/Tools/InterProScan/). Human
GPATC2 is located on chromosome 1q41. As shown in Fig. 1A, GPATC2 mRNA was widely expressed
in the 15 human cell lines examined, which represented different
lineages, as measured by RT-PCR. This technique was also performed
to analyze GPATC2 mRNA expression in 11 different rat tissues,
including appendix, liver, heart muscle, lung, small intestine,
skeletal muscle, pancreas, brain, kidney, stomach and testis
tissues. As shown in Fig. 1B,
GPATC2 mRNA was expressed in a number of these tissues, although
the GPATC2 mRNA relative expression level was significantly higher
in the testis than it was in other tissues.
 | Figure 1Expression profile of human GPATC2.
(A) RT-PCR analysis of GPATC2 expression in cell lines. GAPDH was
used as a control. Lane 1, H520 cells; lane 2, Hela cells; lane 3,
293T cells; lane 4, Hepg2 cells; lane 5, H1299 cells; lane 6, THP-1
cells; lane 7, Jurkas cells; lane 8, U937cells; lane 9, 786-O
cells; lane 10, HL60 cells; lane 11, A498 cells; lane 12, Caki
cells; lane 13, G401cells; lane 14, MCF-7 cells; and lane 15, T47d
cells. (B) RT-PCR analysis of GPATC2 expression in normal rat
tissues. GAPDH was used as a control. Lane 1, rat testis; lane 2,
rat appendix; lane 3, rat liver; lane 4, rat heart muscle; lane 5,
rat lung; lane 6, rat small intestine; lane 7, rat skeletal muscle;
lane 8, rat pancreas; lane 9, rat brain; lane 10, rat kidney; and
lane 11, rat stomach. GPATC2, G-patch domain containing 2; GAPDH,
glyceraldehyde 3-phsophate dehydrogenase; RT-PCR, reverse
transcription polymerase chain reaction. |
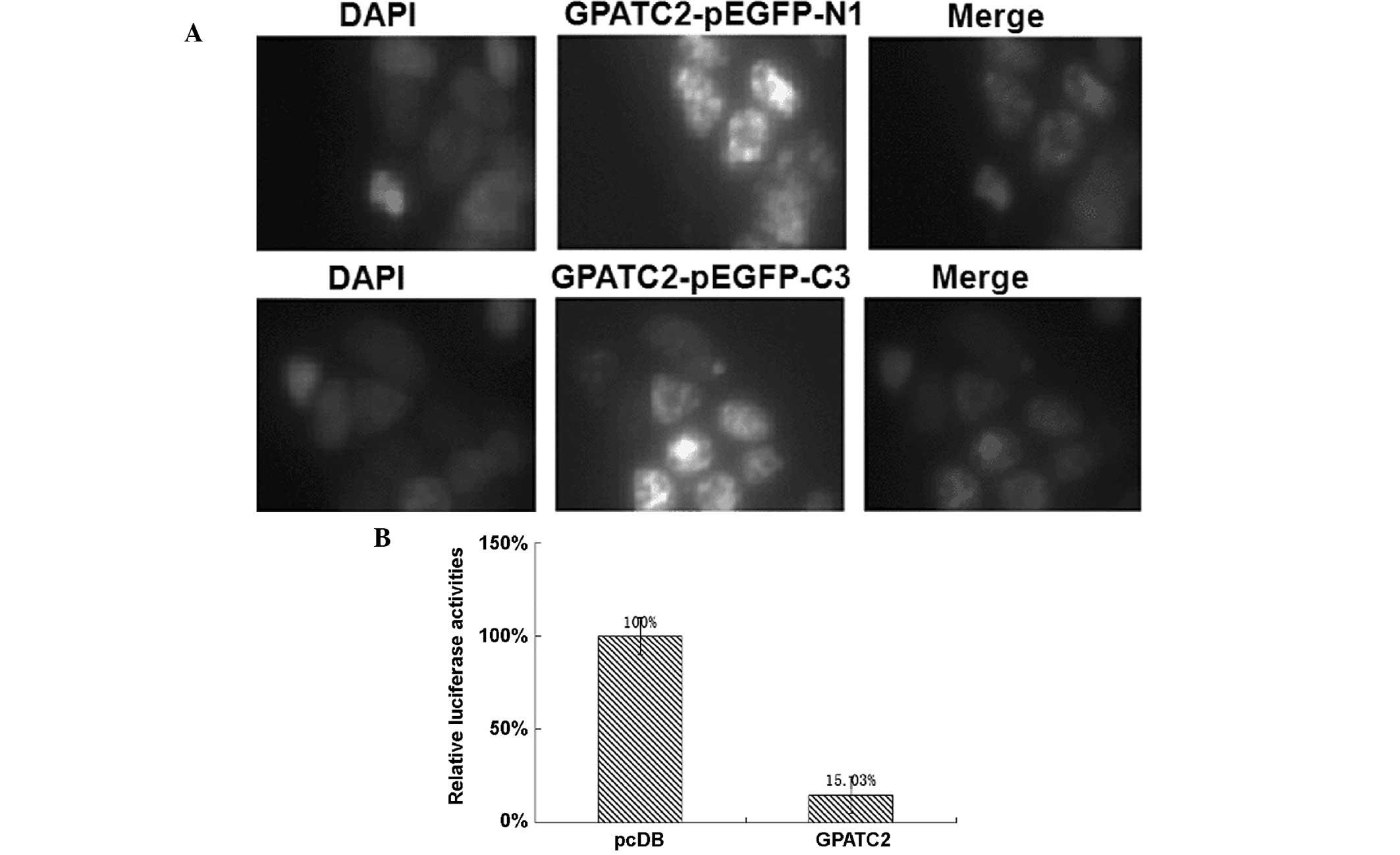
Over-expressed GPATC2 localizes to the
nucleus and inhibits NF-κB
To determine the sub-cellular localization of
GPATC2, GPATC2 cDNA was fused to the 3′ end of the green
fluorescent protein (GFP) cDNA in the pEGFP-C3 vector and to the 5′
end of the GFP cDNA in the pEGFP-N1 vector. As shown in Fig. 2A, GPATC2-EGFP was predominantly
expressed in the nucleus of 293T cells. The DLR assay system showed
that GPATC2 over-expression in 293T cells inhibited the NF-κB
reporter gene compared with the control group (Fig. 2B).
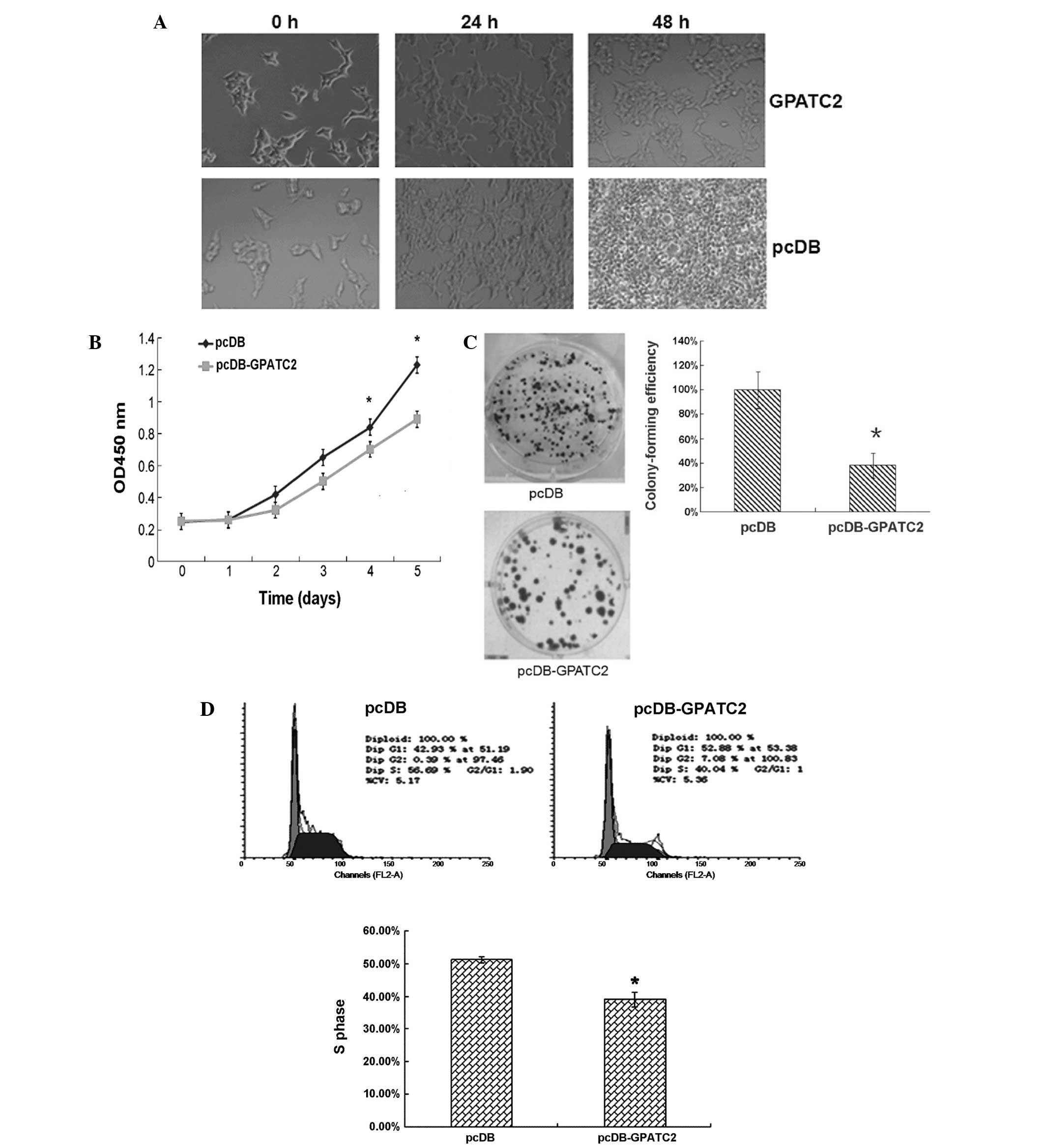
GPATC2 over-expression inhibits cell
proliferation
As shown in Fig. 3A and
B, cell numbers decreased significantly 24 h after GPATC2
over-expression was established compared with empty vector
controls. As shown in Fig. 3C,
colony-forming efficiency was 3.0-fold higher in controls cells
than in cells over-expressing GPATC2 (Fig. 3C). This indicates a
growth-inhibiting effect of GPATC2 on 293T cells. Furthermore, flow
cytometry showed that a reduced proportion of 293T cells were in S
phase 48 h after GPATC2 transfection, with 39.2% of cells
over-expressing GPATC2 compared with 51.3% of controls in S phase
(Fig. 3D). In addition, GPATC2
over-expression increased the proportion of cells in the
G0/G1phase. These data suggest that GPATC2
over-expression significantly inhibits cell cycle G1-S
phase progression in 293T cells.
GPATC2 knockdown by RNA interference
promotes cell proliferation
Based on the above results, it was hypothesized that
knockdown of GPATC2 using RNA interference results in an increased
number of cells in S phase. In order to test this hypothesis, an
siRNA expression plasmid targeting GPATC2 or a scrambled control
siRNA plasmid was transiently transfected into 293T cells. The
reduction in GPATC2 expression was demonstrated by the use of
immunofluorescence (Fig. 4A). As
shown in Fig. 4B, in 293T cells
depleted of GPATC2, cell numbers increased significantly after two
days, compared with the empty vector-transfected cells. These data
suggest that GPATC2 knockdown significantly promotes cell
proliferation in 293T cells.
Discussion
GPATC2 exhibits high RNA expression in rat testis,
an important organ involved in the synthesis of steroids and
production of spermatozoa. Spermatogenesis is a complex biological
process involving the mitotic proliferation of spermatogonia and
the meiotic division of spermatocytes, followed by the morphogenic
differentiation of spermatids to mature spermatozoa. This intricate
process is reflected in the complex gene expression in the testis
(13–14). This study shows that GPATC2 is a
nuclear factor, which suggests that it may be important in
spermatogenesis.
NF-κB is a generic term for a dimeric transcription
factor formed by the heterodimerization or homodimerization of
members of the Rel/NF-κB family (7). NF-κB-regulated genes are important
for cell differentiation, embryonic development, the immune
response and inflammation (15–17),
as well as the development, progression and drug resistance of
cancer cells (18,19). The present study demonstrated that
GPATC2 inhibits the NF-κB pathway, suggesting that it may be an
important regulator of a variety of cell functions. Furthermore,
the results showed that GPATC2 inhibits 293T cell cycle progression
by decreasing the number of cells in S phase, indicating that
GPATC2 may be an important regulator of cell cycle progression. Lin
et al (11) demonstrated
that the interaction of GPATC2 protein with hPrp43, an
RNA-dependent ATPase, significantly enhanced the ATPase activity of
hPrp43 and induced a growth-promoting effect on mammalian cells.
However, the mechanism through which GPATC2 inhibits 293T cell
proliferation requires further elucidation.
In conclusion, GPATC2, which is highly expressed in
the testis and is largely localized to the nucleus of 293T cells,
is capable of inhibiting NF-κB and 293T cell cycle progression
in vitro. However, the mechanism of GPATC2 function may be
far more complex in vivo and further investigations are
required to elucidate the role and molecular mechanisms of GPATC2
in the process of spermatogenesis.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81170616, 81072093,
30671092 and 81302323); and the Natural Science Foundation of Hebei
Province (grant nos. C2009001260, C2012401039, H2013209194,
C2013209024 and C2014209140).
References
|
1
|
Bettegowda A and Wilkinson MF:
Transcription and post-transcriptional regulation of
spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 365:1637–1651.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu ML, Cheng YM and Jia MC: LM23 is
essential for spermatogenesis in Rattus norvegicus. Front Biosci
(Elite Ed). 2:187–194. 2010. View
Article : Google Scholar
|
|
3
|
De Gendt K, Swinnen JV, Saunders PT, et
al: A Sertoli cell-selective knockout of the androgen receptor
causes spermatogenic arrest in meiosis. Proc Natl Acad Sci USA.
101:1327–1332. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hale AJ, Smith CA, Sutherland LC, et al:
Apoptosis: molecular regulation of cell death. Eur J Biochem.
237:8841996.PubMed/NCBI
|
|
5
|
Kim JH, Yang YI, Lee KT, et al:
Costunolide induces apoptosis in human endometriotic cells through
inhibition of the prosurvival Akt and nuclear factor kappa B
signaling pathway. Biol Pharm Bull. 34:580–585. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gebel HM, Braun DP, Tambur A, et al:
Spontaneous apoptosis of endometrial tissue is impaired in women
with endometriosis. Fertil Steril. 69:1042–1047. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karin M and Ben-Neriah Y: Phosphorylation
meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev
Immunol. 18:621–663. 2000. View Article : Google Scholar
|
|
8
|
Huxford T, Malek S and Ghosh G: Structure
and mechanism in NF-kappa B/I kappa B signaling. Cold Spring Harb
Symp Quant Biol. 64:533–540. 1999. View Article : Google Scholar
|
|
9
|
Baldwin AS Jr: The NF-kappa B and I kappa
B proteins: new discoveries and insights. Annu Rev Immunol.
14:649–683. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Strausberg RL, Feingold EA, Grouse LH, et
al: Mammalian Gene Collection Program Team: Generation and initial
analysis of more than 15,000 full-length human and mouse cDNA
sequences. Proc Natl Acad Sci USA. 99:16899–16903. 2002. View Article : Google Scholar
|
|
11
|
Lin ML, Fukukawa C, Park JH, et al:
Involvement of G-patch domain containing 2 overexpression in breast
carcinogenesis. Cancer Sci. 100:1443–1450. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sekido R, Murai K, Funahashi J, et al: The
delta-crystallin enhancer-binding protein delta EF1 is a repressor
of E2-box-mediated gene activation. Mol Cell Biol. 14:5692–5700.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dufau ML, Tsai-Morris C, Tang P and Khanum
A: Regulation of steroidogenic enzymes and a novel testicular RNA
helicase. J Steroid Biochem Mol Biol. 76:187–197. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Walker WH: Molecular mechanisms of
testosterone action in spermatogenesis. Steroids. 74:602–607. 2009.
View Article : Google Scholar
|
|
15
|
Karin M: Nuclear factor-kappaB in cancer
development and progression. Nature. 441:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luo JL, Maeda S, Hsu LC, et al: Inhibition
of NF-kappaB in cancer cells converts inflammation-induced tumor
growth mediated by TNFalpha to TRAIL-mediated tumor regression.
Cancer Cell. 6:297–305. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Luo JL, Tan W, Ricono JM, et al: Nuclear
cytokine-activated IKKalpha controls prostate cancer metastasis by
repressing Maspin. Nature. 446:690–694. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ikezoe T, Yang Y, Bandobashi K, et al:
Oridonin, a diterpenoid purified from Rabdosia rubescens, inhibits
the proliferation of cells from lymphoid malignancies in
association with blockade of the NF-kappa B signal pathways. Mol
Cancer Ther. 4:578–586. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Karin M, Cao Y, Greten FR and Li ZW:
NF-kappaB in cancer: from innocent bystander to major culprit. Nat
Rev Cancer. 2:301–310. 2002. View
Article : Google Scholar : PubMed/NCBI
|