Introduction
Stroke is a leading cause of death in the United
States. Approximately 85% of strokes are ischemic and 15% are
hemorrhagic. Cardioembolic stroke, microvascular disease and
atherothrombosis are the three major etiologies of ischemic stroke
(1). There are known to be two
principal causes of atherosclerotic cerebral infarction (ACI):
Atherosclerosis (AS) and plaque rupture (2). AS is a chronic inflammatory disease
that involves various immune cells, particularly T lymphocytes,
such as CD4+ T-helper cells (3,4). The
increased production of T-helper (Th)17 cells, has previously been
shown to be critical in the pathogenesis of AS and acute coronary
syndrome (5,6). Immune responses occur following acute
ischemic stroke (7). A previous
study recently demonstrated that Th17 cells may be increased in
patients with ACI (8).
Pathological and intervention studies have
implicated microorganisms in the initiation or maintenance of such
inflammation (9,10); however, there is also evidence that
elevated concentrations of the acute phase reactant, C-reactive
protein (CRP), may predict the development of clinical coronary
heart disease over many years (11). These findings suggest that
inflammation may contribute to the earlier stages of ACI.
Furthermore, data from the Physicians’ Health Study suggested that
the beneficial effects of aspirin in reducing cardiovascular risk,
are directly proportional to the degree of elevation of CRP
(12), implicating a
prostanoid-associated mechanism linking inflammation and
atherothrombosis.
The hepatic synthesis of CRP is largely under the
regulation of the proinflammatory cytokine interleukin (IL)-6
(13,14). This cytokine is unusual, in that
its major effects occur at sites distinct from its origin and are
consequent upon its circulating concentrations (15). A previous report from the Rural
Health Study demonstrated that elevated concentrations of IL-6
predict total and cardiovascular mortality over a 5-year follow-up,
with the association being independent of prevalent vascular
disease, smoking and traditional risk factors; and stronger than,
but additive to, that for CRP. A study using animal models strongly
suggested that IL-6 may have a role in neuropathology (16). The present study showed that IL-6
levels were increased in patients with ACI. Furthermore novel
IL-6-expressing γδT cells (γδT6 cells) were identified in patients
with ACI. These γδT6 cells were shown to induce Th17-cell
production in an IL-6 dependent manner. The results of the present
study suggest that the novel γδT6 cells may be a target for
strategic therapies of ACI.
Materials and methods
Patient population
The present study conformed to protocols approved by
the Beijing Institute of Basic Medical Sciences Review Board
(Beijing, China). The study was cross-sectional and blinded. The
patients (50 male and 47 female) were examined at the Beijing
Chaoyang Hospital and 307 Hospital (Beijing, China), where they
were undergoing diagnostic catheterization, between May 2012 and
July 2013. Ethical approval was obtained from the ethics committee
of Beijing Institute of Basic Medical Sciences (Beijing, China) and
informed consent was obtained from the patients, prior to
commencement of the present study The patients were classified into
two groups: Group 1, patients with ACI (22 males and 15 females;
mean age, 56.6±9.9 years); group 2, control subjects (28 males and
32 females; mean age, 54.3±11.1 years), selected on the basis of
recent angiography showing normal carotid arteries. The diagnotic
criteria for ACI was modified from the Trial of Org 10172 in Acute
Stroke Treatment (TOAST) criteria, based on the available clinical,
radiographic and diagnostic information (17). There were no significant
differences between the age ranges of the two groups.
None of the patients included in the present study
were being treated with anti-inflammatory drugs and/or
immunosuppressive agents. Furthermore, none of the patients
suffered from subarachnoid hemorrhage, extradural or subdural
hemorrhage, brain abscess, surgery or trauma, thromboembolism,
disseminated intravascular coagulation, advanced liver disease,
renal failure, malignant disease, other inflammatory disease, or
chronic-immune-mediated disorders.
Blood samples
A total of 10 ml peripheral blood (PB) was collected
from each patient, in a fasting state, on the morning following
admission. The time interval between the onset of symptoms and
blood sampling was <24 h in all cases. All of the samples were
treated with sodium heparin and examined within 4 h. PB mononuclear
cells (PBMCs) were prepared by Ficoll density gradient for flow
cytometric analysis and quantitative polymerase chain reaction
(qPCR). Serum was obtained from the samples, following
centrifugation (900 × g at 4°C for 30 min) and stored at −80°C
until further use.
qPCR analysis
Peripheral blood mononuclear cells qwew extracted
for total RNA with TRIzol solution (Invitrogen Life Technologies).
The final RNA pellets were dissolved in 0.1 mM EDTA (2 μl/mg
original wet weight). Reverse transcription reactions were carried
out on 22 μl of sample using superscript II RNAse H-Reverse
Transcriptase (Invitrogen Life Technologies) in a reaction volume
of 40 μl. All samples were diluted in 160 μl nuclear-free water.
qPCR was employed to quantify human IL-6 gene expression from the
cDNA samples. Human IL-6 was designed using Primer Express version
1.0 software (Applied Biosystems) from the human IL-6 gene
sequences (GenBank/EBML databases; accession no. M54894; http://www.ncbi.nlm.nih.gov/nuccore/M54894). An 81
base-length IL-6 fragment was amplified using the primers: forward
5′-GGTACATCCTCGAC-GGCATCT-3′ and reverse
5′-GTGCCTCTTTGCTGC-TTTCAC-3′. TaqManfluorescent probe, 5′-FAM
(6-carboxyfluorescein)-TGTTACTCTTGTTACATGTCTCCTTTCTCAGGGCT-3′ TAMRA
(6-carboxy-tetramethylrhodamine) (Applied Biosystems) was included
with the primers in each reaction.
Measurement of blood biochemistry
Blood sugar and lipid levels were determined using
an enzymatic method. High sensitive C-reactive protein was measured
using an immunoturbidimetric method. All of the assays were
conducted using an Olympus AU2700 biochemical autoanalyzer (Olympus
Coporation, Tokyo, Japan).
Intracellular cytokine staining and flow
cytometric analysis
The PBMCs (1×106 cells/sample) were
washed with fluorescence-activated cell sorting staining buffer
(phosphate-buffered saline, 2% fetal bovine serum or 1% bovine
serum albumin, 0.1% sodium azide). All of the samples were
incubated for 30 min at 4°C with 5 μg/ml 2.4G2 mouse anti-human Fc
receptor monoclonal antibody (#553142; BD Pharmingen, San Diego,
CA, USA), prior to incubation for 30 min at 4°C with 1:100 diluted
fluorochrome-conjugated mouse anti-human CD3 (#17-0037), CD4
(#11-0048), CD8 (#12-0089), γδTCR (#12-9959), CD11c (#11-0016) and
IL-17 (#12-7178) antibodies (eBioscience, Inc., San Diego, CA,
USA), diluted in fluorescence-activated cell sorting (FACS) buffer
supplemented with 2% anti-Fc receptor. For intracellular cytokine
staining, 50 ng/ml phorbol myristate acetate and 1 mg/ml ionomycin
(Sigma-Aldrich, St Louis, MO, USA) were added and the cells were
incubated for a further 3 h, following which 1 mg/ml brefeldin A
and 2 mM monensin were added. The cells were collected and fixed
for 20 min with 1 ml fixation buffer (Fix and Perm Cell
Permeabilization kit; eBioscience). Following a further wash, the
fixed cells were stained for 30 min at 4°C with 1:100 diluted
fluorescein isothiocyanate-conjugated mouse anti-human interferon
(IFN)γ (#11-7319) and phycoerythrin-conjugated mouse anti-human
IL-17 (#12-7178) monoclonal antibodies (eBioscience, Inc., San
Diego, CA, USA). The cells were incubated for 30 min at 4°C and
were then washed twice and centrifuged (402 × g for 10 min at 4°C).
Data collection and analysis were performed on a FACSCalibur™ flow
cytometer using CellQuest™ software (BD Biosciences, Franklin
Lakes, NJ, USA).
Cytokine analysis by ELISA
The concentration of the cytokine IL-6 was measured
using IL-6 ELISA kits (#DY206-05; R&D Systems, Inc.,
Minneapolis, MN, USA). Briefly, serum was collected by centrifuging
the peripheral blood from healthy individuals or patients (402 × g
for 30 min at room temperature). Subsequently, 100 μl serum (1:10
dilution) was added in triplicate to a 96-well plate for 1 h at
37°C. The plates were then washed and biotin rat anti-human IL-6
monoclonal antibody (5 μg/ml; #840114; R&D Systems, Inc.) was
added to the plates, followed by a further incubation for 1 h at
37°C. The unbound antibodies were removed by washing. The plates
were subsequently incubated with avidin-HRP (1:1,000 dilution) for
1 h at 37°C. All of the antibodies were obtained from eBioscience.
The color was visualized by incubation for 15 min at room
temperature with o-phenylenediamine, and the optical density was
measured at 492 nm, with an ELISA reader (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). Standard curves were established to
quantitate the amount of the respective cytokines.
γδT cell sorting and co-culture with
CD4+T cells
γδT cells were sorted based on CD3 and γδTCR
staining of the samples taken from the controls and the patients
with ACI, by flow cytometry (purity >95). CD4+ T
cells, obtained from the controls, were cultured for 4 days at 37°C
in plates coated with 3 μg/ml mouse anti-human CD3 monoclonal
antibody (#16-0037), and media containing 2 μg/ml mouse anti-human
CD28 monoclonal antibody (#16-0289) (eBioscience, Inc.), in the
presence of γδT cells from controls or patients with ACI
(CD4+ T cells : γδT cells, 4:1). To detect the role of
IL-6 in γδT cells-induced Th17 cell production, neutralizing mouse
anti-human gp130 (IL-6 receptor) monoclonal antibody (50 μg/ml;
#MAB228; R&D Systems, Inc.) was added to the plates and cells
were cultured for 3 days at 37°C.
Statistical analysis
Statistical significance of the differences between
the groups was determined using a t-test. Statistical analyses were
performed using GraphPad Prism version 5.0 (GraphPad Software Inc.,
La Jolla, CA, USA). The values are represented as the means ±
standard deviation. The coefficients of determination
(r2) were calculated in order to evaluate the
correlation between the clinical score with histological
pathological features. A P<0.05 was considered to indicate a
statistically significant difference.
Results
Patients and controls
There were no significant differences in the age,
gender, hypertension, smoking rate, high density
lipoprotein-cholesterol and very low density
lipoprotein-cholesterol concentrations between the two groups. The
fasting blood glucose, total cholesterol and total triglyceride
levels were significantly higher in the patients with ACI, as
compared with the control groups (P<0.05 and P<0.01,
respectively; Table I).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristic | Control (n=60) | ACI (n=37) |
|---|
| Age (years) | 54.3±11.1 | 56.6±9.9 |
| Gender
(male/female) | 28/32 | 22/15 |
| Hypertension, n
(%) | 27 (45) | 18 (48.6) |
| Smoking rate, n
(%) | 36 (60) | 23 (62.2) |
| FBG (mmol/l) | 4.54±0.32 | 4.79±0.58a |
| TC (mmol/l) | 4.17±0.12 | 5.46±0.57b |
| TG (mmol/l) | 1.24±0.21 | 1.52±0.35a |
| HDL-C (mmol/l) | 1.37±0.15 | 1.27±0.17 |
| LDL-C (mmol/l) | 2.62±0.47 | 2.83±0.73 |
| VLDL-C
(mmol/l) | 0.53±0.29 | 0.59±0.34 |
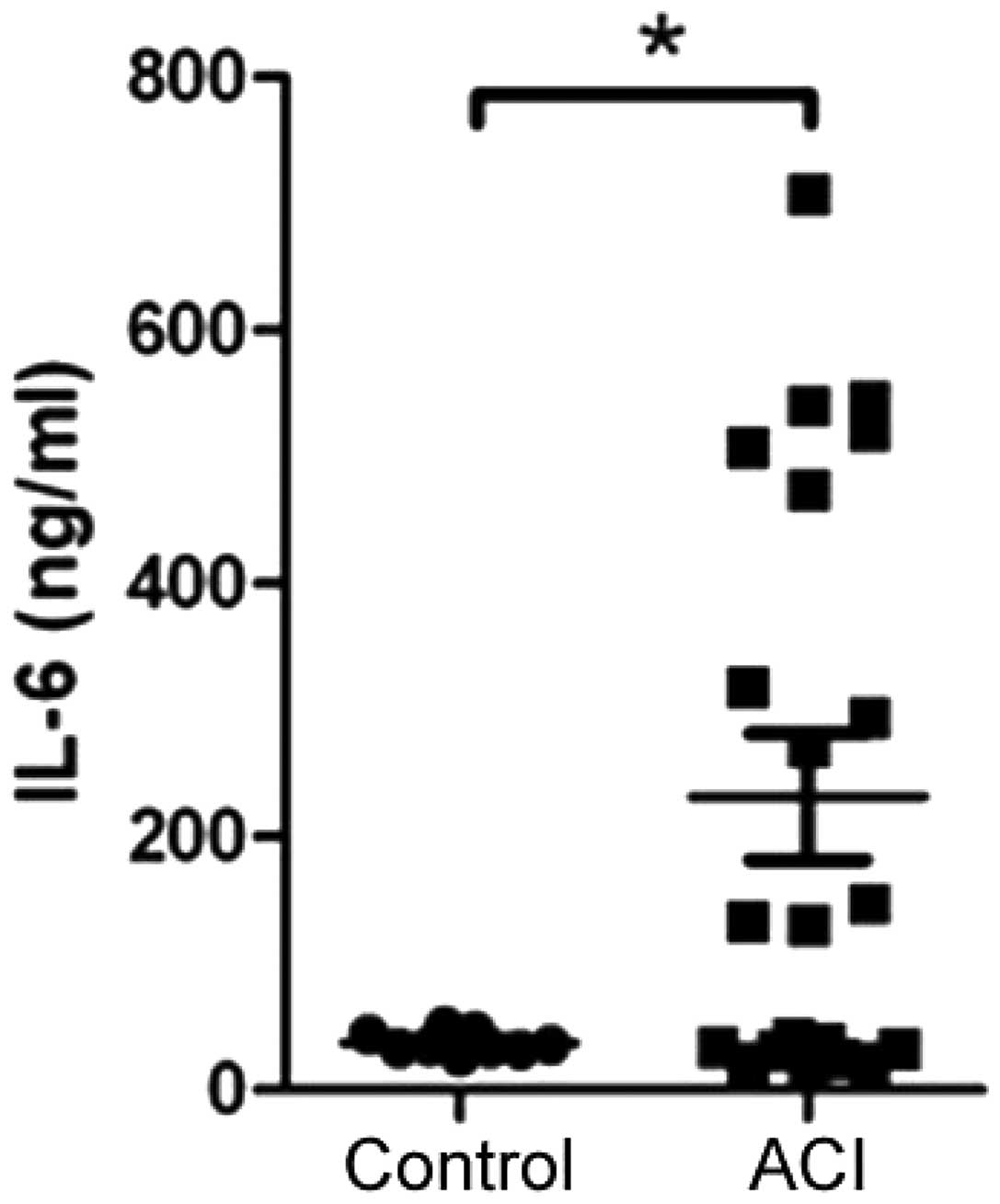
IL-6 levels are increased in patients
with ACI
ACI is thought to be a chronic inflammatory disease,
and IL-6 may have a key role in inducing the inflammatory response
through various mechanisms. Therefore, the levels of IL-6 were
determined in the serum samples taken from the healthy controls and
patients with ACI, by ELISA assay. The patients with ACI had
significantly higher levels of IL-6, as compared with the healthy
controls (Fig. 1).
γδT cells are increased in patients with
ACI
To explore which population of cells induced IL-6 in
the patients with ACI, the immune cells were analyzed by flow
cytometry. PB cells were collected from both the healthy controls
and patients with ACI. The lymphocytes were sorted from the PB
cells using Lymphocyte Separation solution (LTS1077; Tina Jin Hao
Yang Biol Co, Ltd., Tianjing, China). Fluorochrome-conjugated
anti-human CD3, CD4, γδTCR, CD8, CD11c were used to stain the
cells. A FACS analysis showed that the patients with ACI had
slightly reduced percentages of CD3+, CD4+,
CD8+ T cells, as compared with the healthy controls. The
percentage of CD11c+ dendritic cells (DC) was
significantly decreased in the patients with ACI. The percentage of
γδT cells was 0.5% and 2.3% in the PBMCS of the healthy controls
and patients with ACI, respectively (Fig. 2). These results suggest that γδT
cells are significantly increased in patients with ACI.
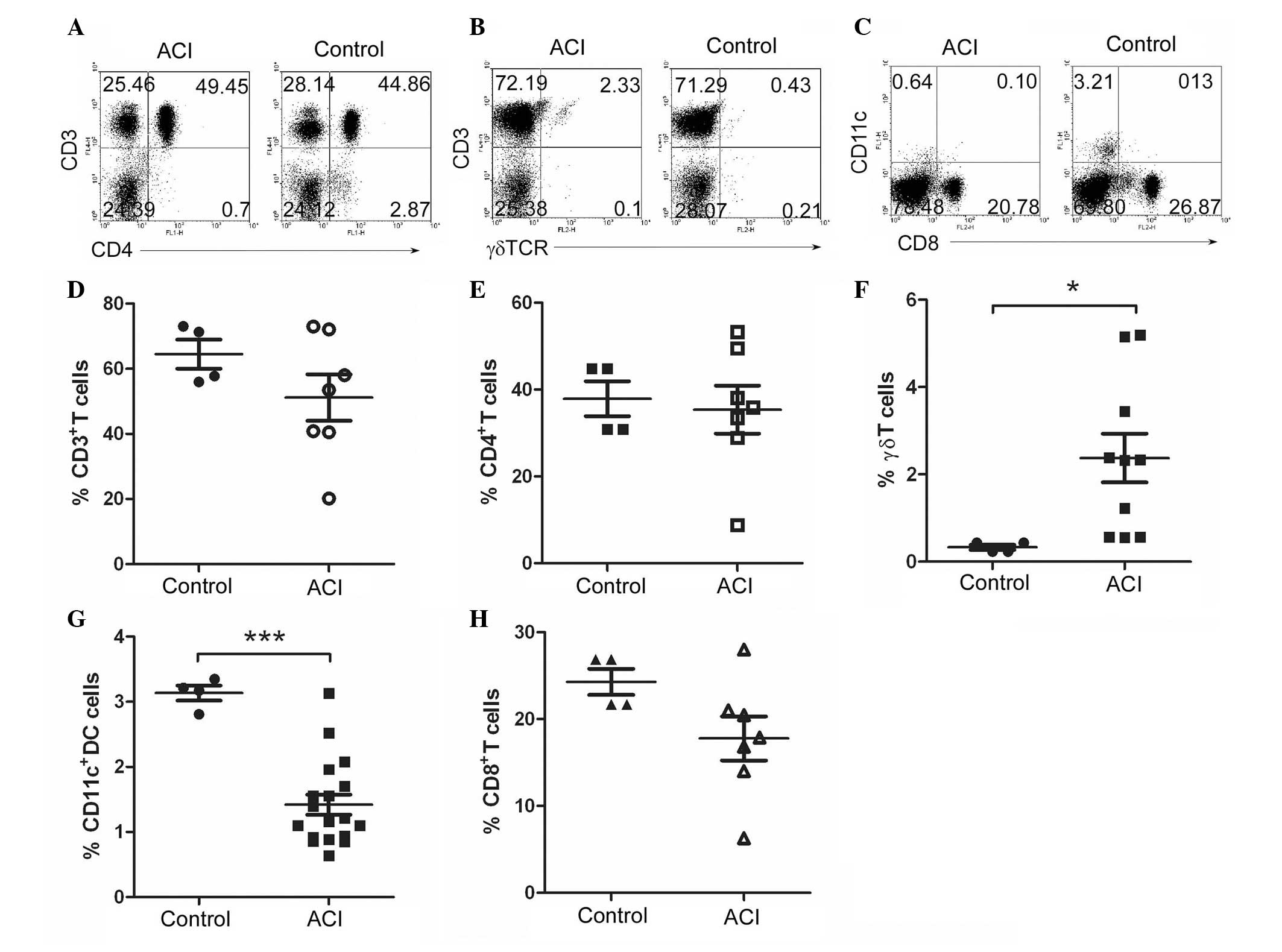 | Figure 2γδT cells increased in patients with
atherosclerotic cerebral infarction (ACI). Peripheral blood
mononuclear cells (PBMCs) were prepared by Ficoll density gradient,
from 60 control and 37 patients with ACI. Fluorochrome-conjugated
anti-human CD3, (A) CD4, (B) γδTCR, CD8, (C) CD11c antibodies were
used to stain the cells. The cells were analyzed by flow cytometry,
the numbers in the quadrants indicate the percentages of (D)
CD3+, (E) CD4+, (F) γδT+, (G)
CD8+-T or (H) CD11c+ dendritic cells.
*P<0.05; ***P<0.001, vs the controls..
The data represents the results of at least four independent
experiments. |
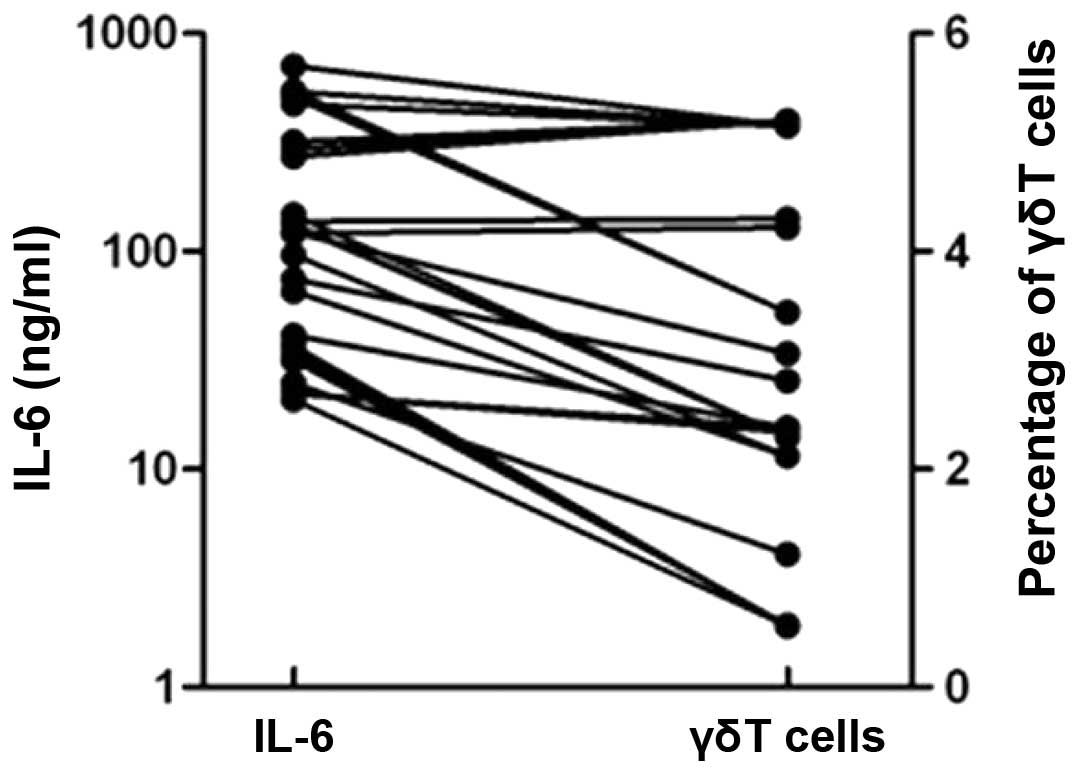
γδT cells secrete high levels of
IL-6
The levels of IL-6 and the percentage of γδT cells
were compared in the patients with ACI. IL-6 was positively
associated with γδT cells in the patients with ACI (Fig. 3).
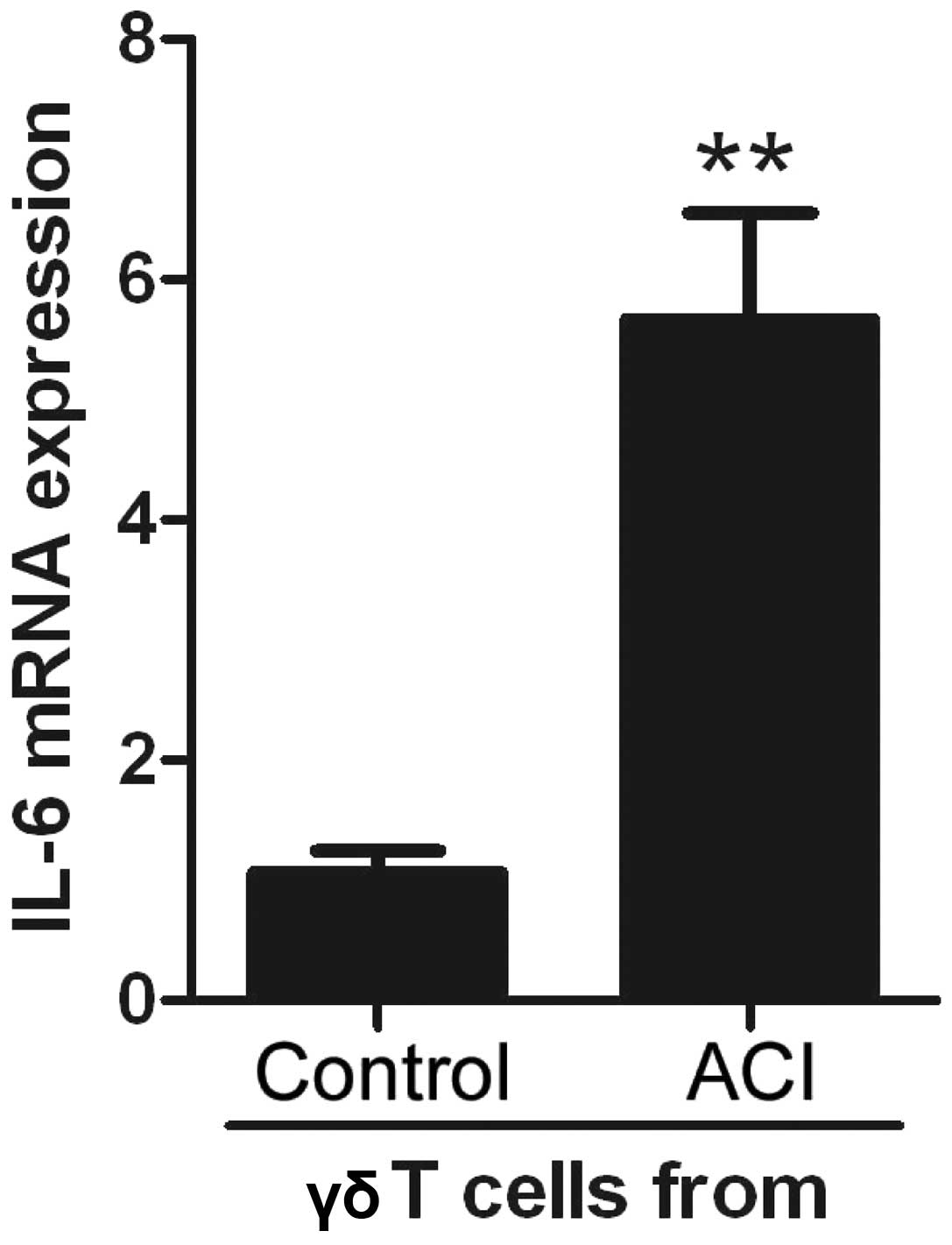
To determine whether γδT cells secreted high levels
of IL-6 in the PBMC from patients with ACI, γδT cells were sorted,
from both groups, by flow cytometry and IL-6 expression levels were
determined by qPCR. The γδT cells from the PBMC of the patients
with ACI significantly increased IL-6 expression levels, as
compared with the controls (Fig.
4). These results suggest that γδT cells are novel
IL-6-expressing cells. Following this finding, these cells were
known as γδT6 cells.
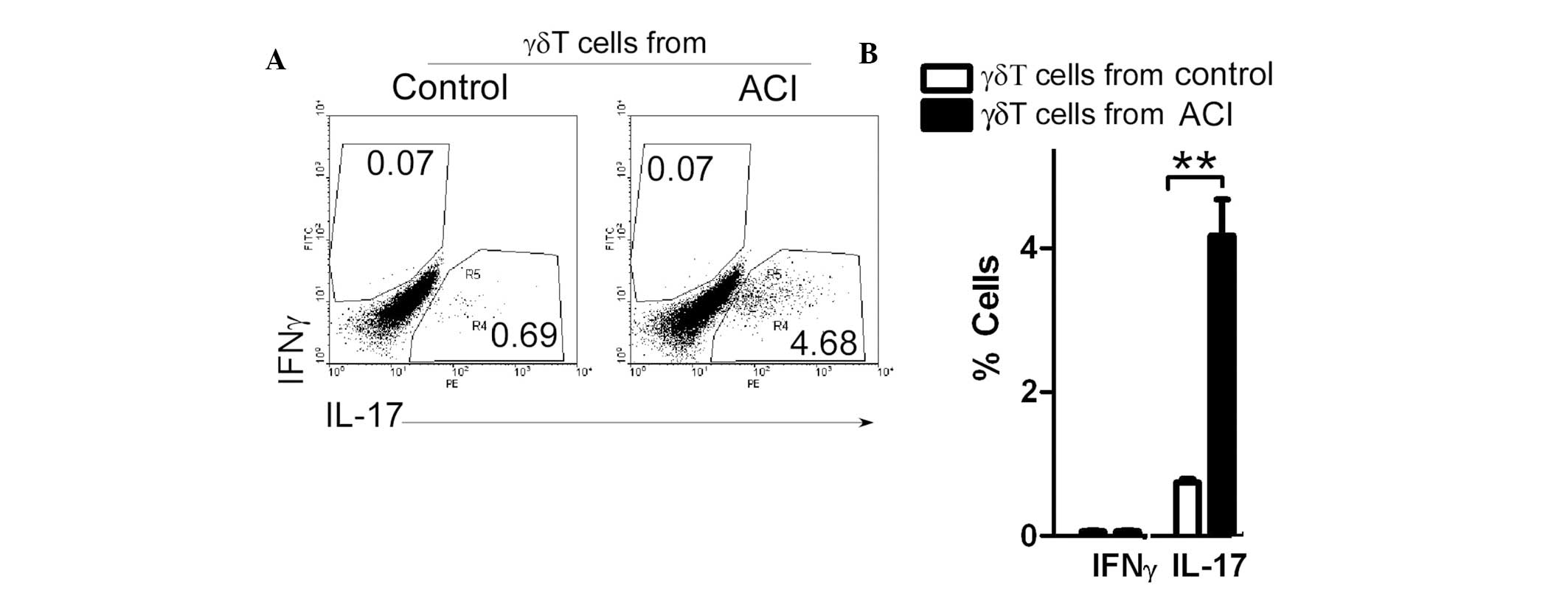
γδT6 cells from patients with ACI induce
Th17-cell production
A recent study demonstrated that Th17 cells are
increased in patients with ACI (8). To determine the role of γδT cells in
Th17-cell production, γδT cells, from both controls and patients
with ACI, were co-cultured with CD4+ T cells obtained
from the controls. Following 4 days of culture, the cells were
collected and stained. The percentage of interferon
(IFN)γ+CD4+Th1 cells was unchanged; however,
co-culture with the γδT cells from the patients with ACI
significantly increased the levels of
IL17+CD4+Th17 cells (Fig. 5). These results suggest that γδT6
cells may be key pro-inflammatory mediators in patients with
ACI.
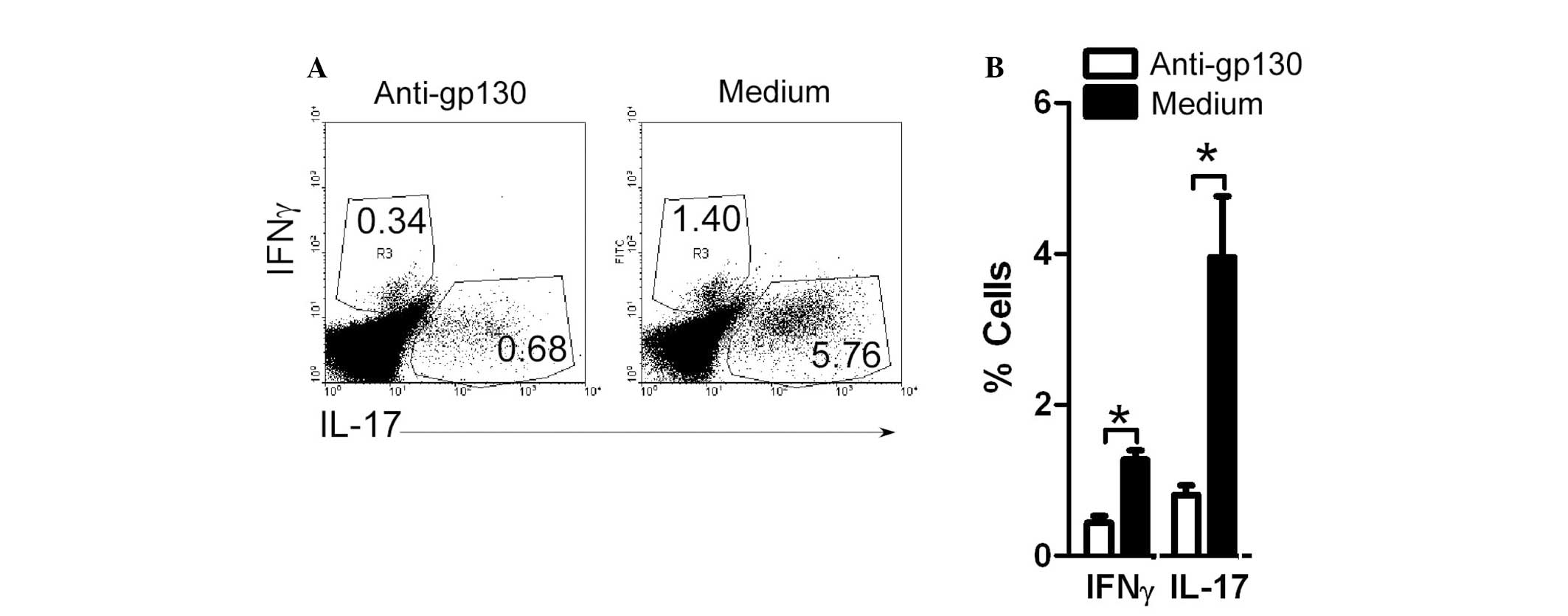
γδT6 cells in ACI patients induce
Th17-cell production in an IL-6 dependent manner
To study the role of IL-6 in γδT6-induced Th17-cell
production, neutralizing anti-gp130 Abs were added to the
co-cultured γδT and CD4+ T cells. Following 4 days of
culture, the cells were collected and stained. The percentage of
IFNγ+CD4+Th1 cells remained unchanged. The
incubation with anti-gp130 significantly reduced the production of
the IL17+CD4+Th17 cells upregulated by γδT6
cells (Fig. 6). These results
suggest that γδT cells in patients with ACI induce Th17-cell
production, in an IL-6 dependent manner.
Discussion
Risk factors of cerebral infarction (CI) are similar
to those associated with atherosclerosis, including diabetes,
tobacco smoking, hypercholesterolemia, hyperlipoproteinemia, high
blood pressure and obesity (1).
There is mounting evidence that inflammation has a role in the
development of CI. Observations have previously been made linking
the presence of infections in the vessel wall with atherosclerosis,
and epidemiological data has also implicated infection in remote
sites, in the aetiology of CI (18). Inflammation leads to localized
recruitment of neutrophils and monocytes, and the presence of
activated macrophages in the cap of atherosclerotic plaques
(19) has led to suggestions that
they may contribute to plaque rupture, through effects on matrix
metalloproteinases (20). The
present study demonstrated that levels of IL-6 were increased in
the serum from patients with ACI. These data suggest that IL-6 may
be an important mechanistic link between various risk factors,
including obesity, occupational stress, and ACI. This may have
clinical implications.
Numerous studies have suggested that IL-6 is derived
from immune cells and is an important determinant of acute phase
activation (15,21). In the present study, in order to
detect which population of cells induced IL-6 secretion, various
immune cells were examined. The γδT cells, but not the other common
immune cells CD3+ T, CD4+ T, CD8+
T, CD11c+ DC, were shown to be increased in the PBMC
from patients with ACI. This finding suggests that γδT cells may
have an important role in the induction of ACI. Previous data
obtained from experimental models of induced autoimmunity, support
the idea that γδT cells, in a niche-restricted manner, accelerate
and enhance the response of tissue antigen-specific T-helper cells.
The function of γδT cells may be particularly relevant at
epithelial surfaces and, perhaps, in neuroectodermal tissue
(22, 23). The functional relevance of γδT
cells in humans has yet to be elucidated. It has previously been
suggested that γδT cells directly shape the inflammatory
infiltrate, for example, by attracting neutrophils (22).
In the present study, the levels of IL-6 were shown
to be positively associated with γδT cells in the patients with
ACI, and the γδT cells from the patients with ACI, significantly
increased the expression levels of IL-6. γδT cells are increasingly
being recognized as having important functional roles in various
disease scenarios, including infection, allergy, autoimmunity and
cancer (22). It has therefore
been hypothesized that γδT cells are not a homogenous population of
cells with a single physiological role. Instead, ever increasing
complexity, in both phenotype and function, is being ascribed to
γδT cells subsets from various tissues and locations, both in mice
and humans (23). Furthermore,
similar to CD4+ T helper cells, subsets of γδT cells can
be defined based on distinct cytokine profiles, with
IFN-γ-producing (γδT1) and IL-17-producing γδT (γδT17) cells having
distinct functional phenotypes. The expression of natural killer
1.1 and CD27, as compared with Scart-2 and C-C chemokine
receptor-6, depends on the commitment of γδT cells to produce IFN-γ
and IL-17, respectively. The present study identified a novel
subset of IL-6-expressing γδT cells: γδT6.
To further explore the function of the novel γδT6
cells, γδT cells from controls and patients with ACI were
co-cultured with CD4+ T cells obtained from the
controls. The γδT6 cells induced the production of the pathogenic
IL-17+Th17 cells. Furthermore, these data suggest that
γδT6 cells from patients with ACI induced Th17-cell production,
dependent on IL-6. In murine models it has previously been
demonstrated that mice with decreased IL-17 levels develop fewer
lesions, and increases in the levels of IL-17 may enhance early
lesion formation. These data suggest a potential role for Th17
cells in the promotion of atherogenesis (24). In human lesions, Th17 may
participate in the inflammatory process of plaque rupture (25). Therefore, Th17 has been
hypothesized to have a role in the development and complications of
atherosclerosis.
Recent findings have demonstrated that a numeral and
functional imbalance of Th17/Treg cells exists in patients with
ACI, suggesting a potential role for the imbalance of these cells
in the onset of ACI (25).
Oxidized-low-density lipoprotein may contribute to plaque
destabilization and rupture by its effects on this balance
(25). The imbalance of Th17/Treg
cells appears to be a novel target for research on the pathogenesis
and treatment of ACI.
In conclusion, the present study identified a novel
IL-6-expressing γδT subset: γδT6, which were increased in patients
with ACI. These γδT6 cells efficiently induced Th17-cell
production. The results of the present study suggest that γδT6
cells may be a target for strategic therapies in ACI patients.
Acknowledgements
The present study was supported by the National
Basic Research Program 973 Grants (no. 2013CB530506), Beijing
Natural Science Foundation (nos. 7132139 and 7132151) and National
Nature and Science Fund (nos. 81272320 and 81172800).
References
|
1
|
Weinberger J: Diagnosis and prevention of
atherosclerotic cerebral infarction. CNS Spectr. 10:553–564.
2005.PubMed/NCBI
|
|
2
|
Parmar JP, Rogers WJ, Mugler JP III, et
al: Magnetic resonance imaging of carotid atherosclerotic plaque in
clinically suspected acute transient ischemic attack and acute
ischemic stroke. Circulation. 122:2031–2038. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Binder CJ, Chang MK, Shaw PX, et al:
Innate and acquired immunity in atherogenesis. Nat Med.
8:1218–1226. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hansson GK and Libby P: The immune
response in atherosclerosis: a double-edged sword. Nat Rev Immunol.
6:508–519. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Q, Wang Y, Chen K, et al: The role of
oxidized low-density lipoprotein in breaking peripheral Th17/Treg
balance in patients with acute coronary syndrome. Biochem Biophys
Res Commun. 394:836–842. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xie JJ, Wang J, Tang TT, et al: The
Th17/Treg functional imbalance during atherogenesis in ApoE(−/−)
mice. Cytokine. 49:185–193. 2010. View Article : Google Scholar
|
|
7
|
Haeusler KG, Schmidt WU, Foehring F, et
al: Immune responses after acute ischemic stroke or myocardial
infarction. Int J Cardiol. 155:372–377. 2012. View Article : Google Scholar
|
|
8
|
Li Q, Wang Y, Yu F, et al: Peripheral
Th17/Treg imbalance in patients with atherosclerotic cerebral
infarction. Int J Clin Exp Pathol. 6:1015–1027. 2013.PubMed/NCBI
|
|
9
|
Patel P, Mendall MA, Carrington D, et al:
Association of Helicobacter pylori and Chlamydia pneumoniae
infections with coronary heart disease and cardiovascular risk
factors. BMJ. 311:711–714. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kuo CC, Gown AM, Benditt EP and Grayston
JT: Detection of Chlamydia pneumoniae in aortic lesions of
atherosclerosis by immunocytochemical stain. Arterioscler Thromb.
13:1501–1504. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kuller LH, Tracy RP, Shaten J and Meilahn
EN: Relation of C-reactive protein and coronary heart disease in
the MRFIT nested case-control study. Multiple Risk Factor
Intervention Trial. Am J Epidemiol. 144:537–547. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ridker PM, Cushman M, Stampfer MJ, Tracy
RP and Hennekens CH: Inflammation, aspirin, and the risk of
cardiovascular disease in apparently healthy men. New Engl J Med.
336:973–979. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bataille R and Klein B: C-reactive protein
levels as a direct indicator of interleukin-6 levels in humans in
vivo. Arthritis Rheum. 35:982–984. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Heinrich PC, Castell JV and Andus T:
Interleukin-6 and the acute phase response. Biochem J. 265:621–636.
1990.PubMed/NCBI
|
|
15
|
Harris TB, Ferrucci L, Tracy RP, et al:
Associations of elevated interleukin-6 and C-reactive protein
levels with mortality in the elderly. Am J Med. 106:506–512. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Erta M, Quintana A and Hidalgo J:
Interleukin-6, a major cytokine in the central nervous system. Int
J Biol Sci. 8:1254–1266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fure B, Wyller TB and Thommessen B: TOAST
criteria applied in acute ischemic stroke. Acta Neurol Scand.
112:254–258. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yudkin JS, Kumari M, Humphries SE and
Mohamed-Ali V: Inflammation, obesity, stress and coronary heart
disease: is interleukin-6 the link? Atherosclerosis. 148:209–214.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alexander RW: Inflammation and coronary
artery disease. N Engl J Med. 331:468–469. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Galis ZS, Sukhova GK, Lark MW and Libby P:
Increased expression of matrix metalloproteinases and matrix
degrading activity in vulnerable regions of human atherosclerotic
plaques. J Clin Invest. 94:2493–2503. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tappia PS, Troughton KL, Langley-Evans SC
and Grimble RF: Cigarette smoking influences cytokine production
and antioxidant defences. Clin Sci (Lond). 88:485–489. 1995.
|
|
22
|
Korn T and Petermann F: Development and
function of interleukin 17-producing γδT cells. Ann NY Acad Sci.
1247:34–45. 2012. View Article : Google Scholar
|
|
23
|
Pang DJ, Neves JF, Sumaria N and
Pennington DJ: Understanding the complexity of γδ T-cell subsets in
mouse and human. Immunology. 136:283–290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song L and Schindler C: IL-6 and the acute
phase response in murine atherosclerosis. Atherosclerosis.
177:43–51. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hashmi S and Zeng QT: Role of
interleukin-17 and interleukin-17-induced cytokines interleukin-6
and interleukin-8 in unstable coronary artery disease. Coron Artery
Dis. 17:699–706. 2006. View Article : Google Scholar : PubMed/NCBI
|