Introduction
Diabetes mellitus is a serious threat to health and
a predominant risk factor in the development of macro- and
microvascular complications. Studies have shown that high glucose
(HG) easily induces endothelial cell (EC) injury and apoptosis,
which in turn results in diabetic vascular complications (1–4). EC
apoptosis has also been closely associated with vascular
disease.
Heat shock protein 27 (HSP27) is important in the
regulation of proliferation and apoptosis in numerous cell types.
Thus, HSP27 may be a potential target for the interventional
treatment of pathological processes associated with cell
proliferation and apoptosis (5,6). The
phosphoinositide 3-kinase (PI3K)-Akt signaling pathway may promote
EC proliferative dysfunction in diabetes. The mitogen-activated
protein kinase (MAPK)/extracellular signal-regulated kinase
(ERK)signaling pathway is involved in proliferation and survival
responses in ECs. Several studies (7–15)
have shown that the MAPK/ERK and PI3K/Akt signaling pathways
regulate the phosphorylation of HSP27. Another previous study has
demonstrated that HSP27 phosphorylation is important in mediating
the migration of vascular smooth muscle cells (VSMCs), and the
MAPK/ERK and PI3K/Akt signaling pathways have also been revealed to
regulate the phosphorylation of HSP27 in VSMCs (16,17).
In the present study, the aim was to obtain information regarding
the signal transduction pathways that regulate HSP27
phosphorylation, proliferation and apoptosis in human umbilical
vein ECs (HUVECs) induced by HG.
Materials and methods
Isolation and culture of human ECs
HUVECs were isolated from the healthy umbilical
veins of healthy female patients following laparotomy at the Fujian
Provincial Hospital (Fuzhou, Fujian, China). The venous lumen was
washed with sterile phosphate-buffered saline (PBS) 2–3 times and
pre-warmed collagenase I (13–15 ml) was injected into the lumen,
which was incubated for 20 min. The lumen was then washed using
30–40 ml sterile PBS. The wash-out was centrifuged at 155 × g for
10 min and the HUVECs were collected subsequent to discarding the
supernatant. The cells were cultured in M199 medium with 20% fetal
bovine serum (FBS). The cultures were maintained at 37°C with a gas
mixture of 5% CO2:95% air. The primary cultures had the
fluid changed 24 h after seeding and were subcultured on reaching
confluence using 0.01% trypsin-EDTA (Sigma-Aldrich, St. Louis, MO,
USA). The ECs were identified by direct immunostaining for factor
VIII. The ECs from the third passages in an actively growing
condition were used for experiments. The present study was
conducted in accordance with the Declaration of Helsinki and with
approval from the Ethics Committee of Fujian Provincial Clinical
College (Fujian Medical University, Fuzhou, China). Written
informed consent was obtained from all patients in this study.
alamarBlue® cell proliferation
assay
HUVECs in a good growth condition were inoculated in
96-well plates with 6,000 cells/well and 150 μl cell culture medium
was added to each well. The day following inoculation, the culture
medium was changed to 60 μl M199 medium with 1% FBS, although the
other components remained unchanged. When the cells had been
cultured in a 37°C incubator for 10–15 h, 90 μl fresh M199 culture
medium with 16% FBS was added. On the following day (within 24 h),
D-glucose was added to the normal culture medium to obtain a final
concentration of 30.5 mmol/l. The medium from the cells cultured
with normal medium was changed every other day. The cells were
incubated in a 37°C and 5% CO2 cell culture incubator.
At 4 h before the end of culture, this medium was removed and 100
μl/well fresh M199 medium with 10% FBS and 10 μl/well
alamarBlue® solution were added. The
alamarBlue® cell proliferation assay was performed
according to the kit manufacturer’s instructions (Merck & Co.,
White House Station, NJ, USA). The cells were incubated for 4 h at
37°C. The 570 and 600 nm absorbance optical density values of each
well were examined with a microplate reader. The
alamarBlue® reduction rates were calculated according to
the manufacturer’s instructions. Five repeated wells were used at
each time point.
Analysis of HUVEC apoptosis
The HUVEC cells were pretreated with quercetin,
LY294002 or U0126 (Sigma-Aldrich) for 45 min before HG was added.
At 48 h after treatment, 1–5×105 cells were collected
using trypsin without EDTA. Following digestion, the cells were
maintained in culture medium with serum to prevent further trypsin
digestion. The cells were then washed twice in PBS, with
centrifugation at 155 × g for 5 min, and resuspended in 500 μl
binding buffer. Subsequently, 5 μl Annexin V-fluorescein
isothiocyanate (FITC; Sigma-Aldrich) and 5 μl propidium iodide (PI;
Biyuntian, Hangzhou, China) were added and the solutions were
mixed. The mixture was incubated at room temperature for 10–15 min
in a cool dark place. Annexin V-FITC binding was analyzed by flow
cytometry (Ex=488 nm; Em=530 nm; Beckman Coulter, Carlsbad, CA,
USA) using a FITC signal detector (usually FL1) and PI staining was
examined using a phycoerythrin emission signal detector (usually
FL2). Experiments were conducted in six-well plates and were
repeated three times.
Western blotting
The HUVEC cells were pretreated with LY294002 (0.1,
1 or 10 μM) or U0126 (0.1, 1 or 10 μM) for 45 min before the
addition of HG. Following treatment, HUVEC cells were washed with
ice-cold PBS and then cellular proteins were obtained. Equal
quantities of protein were then separated by 12% SDS-PAGE and
transferred to polyvinylidene difluoride membranes, which were
incubated at room temperature in Tris-buffered saline and Tween 20
(TBST) containing 5% bovine serum albumin. Subsequent to blocking,
the membranes were incubated with primary rabbit-anti-human
phosphorylated-(p-)HSP27 antibody (1:300; Bioworld Technology,
Inc., Minneapolis, MN, USA) and rat-anti-human total-HSP27 antibody
(1:500; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA)
overnight at 4°C, followed by three washes (10 min each) with TBST.
The membranes were then incubated with secondary antibody (goat
anti-rabbit for p-HSP27 and goat anti-rat for total-HSP27;
Biyuntian) at a dilution of 1:5,000 at room temperature for 70 min,
followed by washing three times (10 min each) with TBST. The
signals were detected using a chemiluminescence kit (Biyuntian) and
quantified by densitometric analysis. Experiments were conducted in
six-well plates and were repeated three times.
Statistical analysis
Statistical analysis was conducted using analysis of
variance with SPSS 11.5 software (SPSS, Inc., Chicago, IL, USA).
Values are expressed as the mean ± standard deviation.
Results
Effect of quercetin on HUVEC
proliferation induced by HG
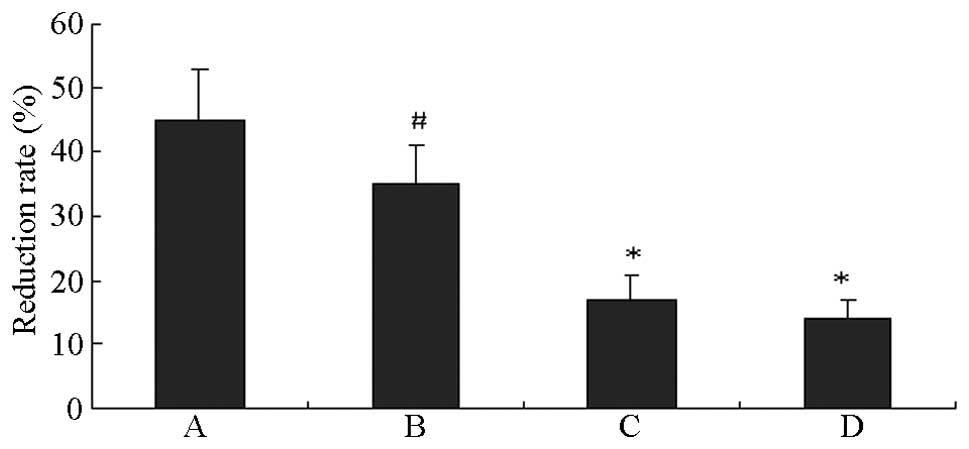
As shown in Fig. 1,
compared with the normal controls, the HUVECs grown in HG exhibited
significantly lower cell proliferation (P<0.01). The
proliferation of HUVECs in HG was reduced by the specific HSP27
inhibitor quercetin in a concentration-dependent manner.
Effect of quercetin on the apoptosis of
HUVECs induced by HG
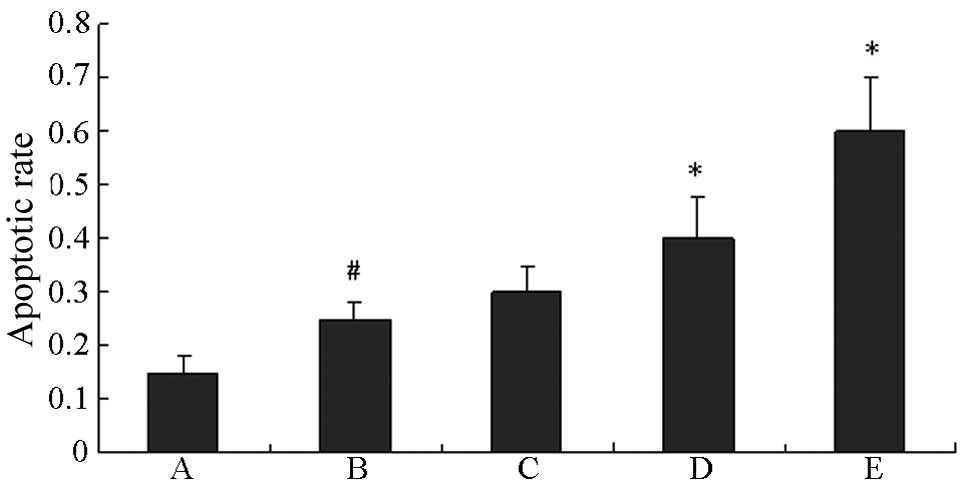
As shown in Fig. 2,
HG significantly promoted the apoptosis of HUVECs compared with the
control (P<0.01). The apoptosis of HUVECs in HG was further
induced by quercetin in a concentration-dependent manner.
Effect of LY294002 and U0126 on HSP27
phosphorylation in HUVECs induced by HG
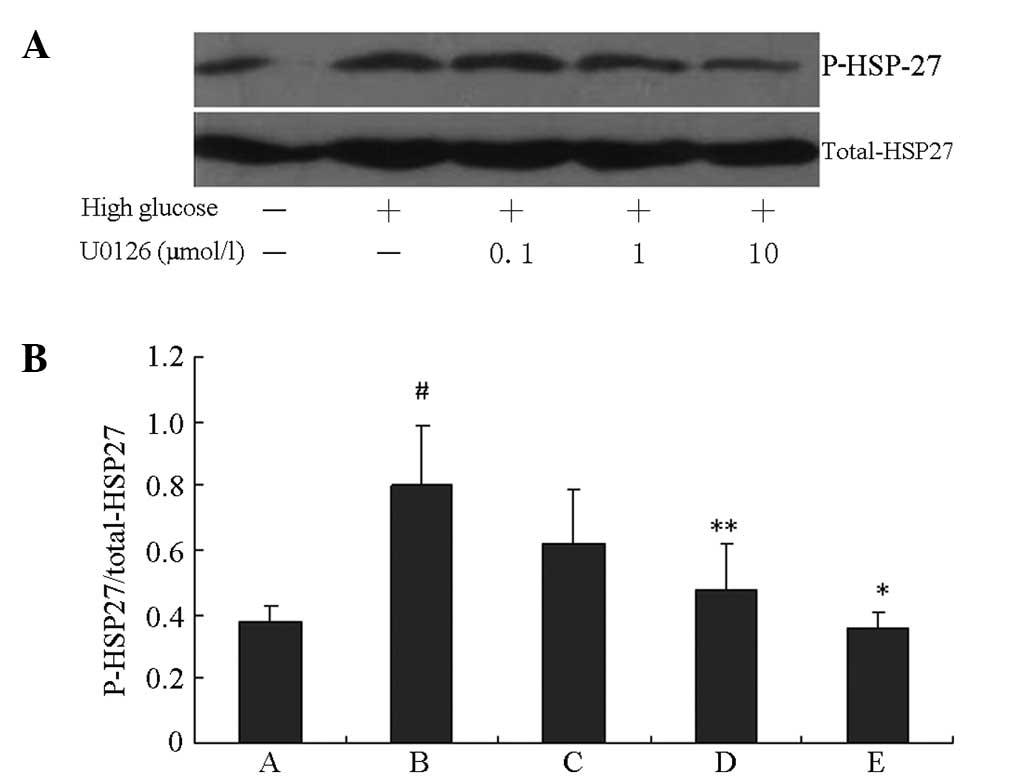
HSP27 phosphorylation induced by HG was blocked by
the specific PI3K inhibitor LY294002 and the specific ERK1/2
inhibitor U0126 in a concentration-dependent manner. As shown in
Figs. 3 and 4, HG induced significant increases in
HSP27 phosphorylation compared with the control group (P<0.01).
The peak inhibition rates of HSP27 phosphorylation induced by HG
were 62.6 and 56.1% following 10 μM LY294002 and U0126 treatments,
respectively (P<0.01 vs. the HG groups).
Effect of LY294002 and U0126 on HUVEC
proliferation induced by HG
HUVECs grown in HG exhibited significantly inhibited
cell proliferation compared with the normal control cells
(P<0.01). The proliferation of HUVECs in HG was further
inhibited by LY294002 and U0126, with peak inhibition rates of 51.4
and 60.0%, respectively (P<0.01 vs. the HG groups; Fig. 5).
Effect of LY294002 and U0126 on HUVEC
apoptosis induced by HG
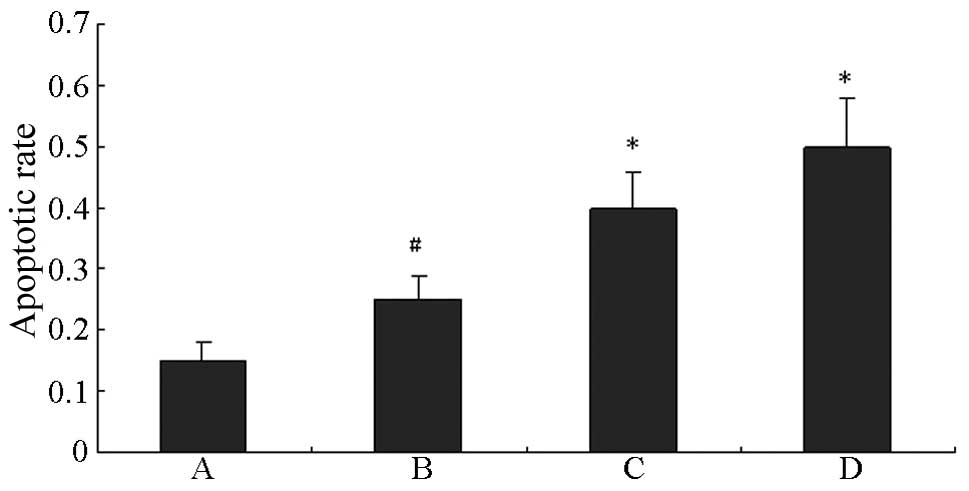
An incubation time period of 48 h was selected as an
intervention time point and flow cytometry was used to detect HUVEC
apoptosis. As shown in Fig. 6,
compared with the normal control group, the HG group exhibited a
significant increase in cell apoptosis (P<0.01). HUVEC apoptosis
following HG was further increased by treatment with either
LY294002 or U0126 (P<0.01 vs. the HG groups).
Discussion
Although HG has been reported to inhibit EC
proliferation and increase apoptosis, the exact mechanism remains
largely unknown. HSP27, with a molecular weight of ~27 kDa, has
been shown to form large aggregates of ≤800 kDa in the cell
cytosol. HSP27 expression is upregulated during the stress response
and correlates with increased survival ability in cells exposed to
cytotoxic stimuli. HSP27 has been shown to prevent the cell death
caused by a variety of toxic agents that promote apoptosis. HSP27
is regulated by means of phosphorylation and dephosphorylation.
Studies have shown that HSP27 is important in the regulation of
proliferation and apoptosis in a number of cell types. HG is known
to activate HSP27; however, since multiple signaling pathways are
activated during HG-induced EC injury, the role of HSP27 activation
in this process requires further clarification. Thus, in the
present study, the effect of quercetin (the specific HSP27
inhibitor) on the proliferation and apoptosis of HUVECs induced by
HG was examined.
A novel method for measuring cell proliferation,
which uses alamarBlue®, has recently become available.
alamarBlue® is a safe, nontoxic aqueous dye that is used
to assess cell viability and cell proliferation. In the present
study, the results of the alamarBlue® assay demonstrated
the apparent toxic effects of HG on HUVECs and suggested that the
specific HSP27 inhibitor quercetin may enhance the inhibition of
HUVEC proliferation by HG.
Currently, apoptosis of ECs is considered to be an
important cause of atherosclerosis during the development of
diabetic chronic vascular diseases. A number of studies have
observed that HG increases the rate of apoptosis in cultured
HUVECs. The results of the present study revealed that
co-administration of quercetin promoted the apoptosis of cultured
HUVECs to a greater extent than HG alone, suggesting that HSP27
phosphorylation protects HUVECs from apoptosis. However, the
underlying mechanism for this remains unclear. Long-term exposure
to HG levels may induce several metabolic changes in ECs; these
include negative feedback mechanisms that regulate upstream
regulatory factors, which counteracts certain effects of glucose
toxicity. This benefits the growth of ECs in HG culture. The
present study demonstrated that the proliferation and apoptosis of
HUVECs induced by HG was affected by quercetin in a
concentration-dependent manner. Therefore, HSP27 phosphorylation
may be part of an important signaling pathway that contributes to
HUVEC proliferation and apoptosis induced by HG. Subsequently, the
signal transduction pathways associated with HSP27 phosphorylation
in HUVECs induced by HG were analyzed.
PI3K and Akt are downstream effectors of insulin
signaling, and are important in ECs, as these signaling pathways
regulate angiogenesis and proliferation (18). In ECs, Akt activation has been
reported to promote cell survival (19,20).
Studies have shown that the PI3K/Akt/nitric oxide signaling pathway
is important in preventing reactive oxygen species-induced EC
injury (21,22). However, whether the PI3K/Akt
signaling pathway regulates the cell apoptosis induced by HG
remains unclear. MAPK/ERK is an important signaling pathway
involved in the regulation of various cellular processes, mainly
proliferation and survival, but also apoptosis under particular
pathological conditions (23). The
MAPK/ERK signaling pathway is mainly involved in mediating growth
factor-stimulated processes, such as division, growth,
differentiation and survival in various cell types, including
HUVECs.
The present study demonstrated that the HSP27
phosphorylation and cell proliferation induced by HG was blocked by
the specific PI3K and ERK1/2 inhibitors LY294002 and U0126, and
that the apoptosis of HUVECs in the LY294002 + HG and U0126 + HG
groups was increased compared with the HG-only group. Therefore,
PI3K/Akt and ERK1/2 may be important signaling pathways that
contribute to HSP27 phosphorylation, and the proliferation and
apoptosis of HUVECs in response to HG. In conclusion, the
inhibition of the PI3K/Akt and ERK1/2 signal transduction pathways
was found to contribute to the suppression of HSP27 phosphorylation
and increased cell apoptosis. As determined by these findings,
these two signaling pathways may be involved in the phosphorylation
of HSP27 and HUVEC apoptosis in response to HG. Therefore,
upregulation of the HSP27 signaling pathway may be considered as a
target in future therapies protecting the diabetic patient from
macrovascular complications. The present study provides further
evidence that HSP27 functions within an anti-apoptotic signaling
pathway and may indicate an alternative therapeutic method in the
treatment of diabetic cardiovascular complications (24,25).
Acknowledgements
This study was supported by the Natural Science
Foundation (grant no. 2013J01272) and the Key Clinical Speciality
Discipline Construction Program of Fujian, China.
References
|
1
|
Chen G, Shen X, Yao J, et al: Ablation of
NF-kappaB expression by small interference RNA prevents the
dysfunction of human umbilical vein endothelial cells induced by
high glucose. Endocrine. 35:63–74. 2009. View Article : Google Scholar
|
|
2
|
Chen G, Chen YF, Chen HF, et al: The
effect of NF-κB pathway on proliferation and apoptosis of human
umbilical vein endothelial cells induced by intermittent high
glucose. Mol Cell Biochem. 347:127–133. 2011. View Article : Google Scholar
|
|
3
|
Schisano B, Harte AL, Lois K, et al: GLP-1
analogue, Liraglutide protects human umbilical vein endothelial
cells against high glucose induced endoplasmic reticulum stress.
Regul Pept. 174:46–52. 2012. View Article : Google Scholar
|
|
4
|
Sun F, Zhou B, Lin X and Duan L: Proteomic
analysis identifies nuclear protein effectors in PKC-delta
signaling under high glucose-induced apoptosis in human umbilical
vein endothelial cells. Mol Med Rep. 4:865–872. 2011.PubMed/NCBI
|
|
5
|
Son TW, Yun SP, Yong MS, et al: Netrin-1
protects hypoxia-induced mitochondrial apoptosis through HSP27
expression via DCC- and integrin α6β4-dependent Akt, GSK-3β, and
HSF-1 in mesenchymal stem cells. Cell Death Dis. 4:e5632013.
View Article : Google Scholar
|
|
6
|
Arany I, Clark JS, Reed DK, Ember I and
Juncos LA: Cisplatin enhances interaction between p66Shc and HSP27:
its role in reorganization of the actin cytoskeleton in renal
proximal tubule cells. Anticancer Res. 32:4759–4763.
2012.PubMed/NCBI
|
|
7
|
Grogan PT, Sleder KD, Samadi AK, et al:
Cytotoxicity of withaferin A in glioblastomas involves induction of
an oxidative stress-mediated heat shock response while altering
Akt/mTOR and MAPK signaling pathways. Invest New Drugs. 31:545–557.
2013. View Article : Google Scholar :
|
|
8
|
McClung HM, Golembieski WA, Schultz CR, et
al: Deletion of the SPARC acidic domain or EGF-like module reduces
SPARC-induced migration and signaling through p38 MAPKHSP27 in
glioma. Carcinogenesis. 33:275–284. 2012. View Article : Google Scholar :
|
|
9
|
Meng G, Sun Y, Fu W, Guo Z and Xu L:
Microcystin-LR induces cytoskeleton system reorganization through
hyperphosphorylation of tau and HSP27 via PP2A inhibition and
subsequent activation of the p38 MAPK signaling pathway in
neuroendocrine (PC12) cells. Toxicology. 290:218–229. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim J, Kang D, Sun BK, Kim JH and Song JJ:
TRAIL/MEKK4/p38/HSP27/Akt survival network is biphasically
modulated by the Src/CIN85/c-Cbl complex. Cell Signal. 125:372–379.
2013. View Article : Google Scholar
|
|
11
|
Ghosh A, Lai C, McDonald S, et al: HSP27
expression in primary colorectal cancers is dependent on mutation
of KRAS and PI3K/AKT activation status and is independent of TP53.
Exp Mol Pathol. 94:103–108. 2013. View Article : Google Scholar
|
|
12
|
Bhargavan B, Fatma N, Chhunchha B, et al:
LEDGF gene silencing impairs the tumorigenicity of prostate cancer
DU145 cells by abating the expression of Hsp27 and activation of
the Akt/ERK signaling pathway. Cell Death Dis. 3:e3162012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qi S, Xin Y, Qi Z, et al: HSP27
phosphorylation modulates TRAIL-induced activation of Src-Akt/ERK
signaling through interaction with β-arrestin2. Cell Signal.
26:594–602. 2014. View Article : Google Scholar
|
|
14
|
Kim J, Kim SY, Kang S, et al: HSP27
modulates survival signaling networks in cells treated with
curcumin and TRAIL. Cell Signal. 24:1444–1452. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen NG, Lu CC, Lin YH, et al: Proteomic
approaches to study epigallocatechin gallate-provoked apoptosis of
TSGH-8301 human urinary bladder carcinoma cells: roles of AKT and
heat shock protein 27-modulated intrinsic apoptotic pathways. Oncol
Rep. 26:939–947. 2011.PubMed/NCBI
|
|
16
|
Chen HF, Xie LD and Xu CS: Role of heat
shock protein 27 phosphorylation in migration of vascular smooth
muscle cells. Mol Cell Biochem. 327:1–6. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen HF, Xie LD and Xu CS: The signal
transduction pathways of heat shock protein 27 phosphorylation in
vascular smooth muscle cells. Mol Cell Biochem. 333:49–56. 2010.
View Article : Google Scholar
|
|
18
|
Ueda K, Nakahara T, Akanuma K, et al:
Differential effects of LY294002 and wortmannin on neurons and
vascular endothelial cells in the rat retina. Pharmacol Rep.
65:854–862. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
You JJ, Yang CH, Yang CM and Chen MS:
Cyr61 induces the expression of monocyte chemoattractant protein-1
via the integrin ανβ3, FAK, PI3K/Akt, and NF-κB pathways in retinal
vascular endothelial cells. Cell Signal. 26:133–140. 2014.
View Article : Google Scholar
|
|
20
|
Chen ML, Lin YH, Yang CM and Hu ML:
Lycopene inhibits angiogenesis both in vitro and in vivo by
inhibiting MMP-2/uPA system through VEGFR2-mediated PI3K-Akt and
ERK/p38 signaling pathways. Mol Nutr Food Res. 56:889–899. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fournier NM, Lee B, Banasr M, Elsayed M
and Duman RS: Vascular endothelial growth factor regulates adult
hippocampal cell proliferation through MEK/ERK- and
PI3K/Akt-dependent signaling. Neuropharmacology. 63:642–652. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oviedo-Boyso J, Cortés-Vieyra R,
Huante-Mendoza A, et al: The phosphoinositide-3-kinase-Akt
signaling pathway is important for Staphylococcus aureus
internalization by endothelial cells. Infect Immun. 79:4569–4577.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shaul YD and Seger R: The MEK/ERK cascade:
from signaling specificity to diverse functions. Biochim Biophys
Acta. 1773:1213–1226. 2007. View Article : Google Scholar
|
|
24
|
Will M, Qin AC, Toy W, et al: Rapid
induction of apoptosis by PI3K inhibitors is dependent upon their
transient inhibition of RAS-ERK signaling. Cancer Discov.
4:334–347. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wakao K, Watanabe T, Takadama T, et al:
Sangivamycin induces apoptosis by suppressing Erk signaling in
primary effusion lymphoma cells. Biochem Biophys Res Commun.
444:135–140. 2014. View Article : Google Scholar : PubMed/NCBI
|