Introduction
Thalidomide, a derivative of glutamic acid that
exists as an equal mixture of its enantiomers was first introduced
in Europe for the treatment of morning sickness in pregnant
females. Due to its teratogenicity, it was subsequently withdrawn
in the late 1960s (1). However, in
1994, D’Amato et al (2)
suggested that thalidomide inhibited limb development by inhibiting
angiogenesis via the inhibition of basic fibroblast growth factor
(bFGF) and/or vascular endothelial growth factor (VEGF). Following
this study, Kenyon et al (3) demonstrated that thalidomide inhibited
bFGF- and VEGF-induced corneal neovascularization in mice. Bauer
et al (4) revealed that
thalidomide inhibits microvessel formation in a rat aortic ring
angiogenesis assay and slows human aortic endothelial cell
proliferation. Furthermore, thalidomide has been demonstrated to
inhibit tumor necrosis factor-α (TNF-α) synthesis by inducing TNF-α
mRNA degradation, inhibiting the activation of nuclear factor κB
(NF-κB) through a mechanism involving the inhibition of IκB kinase
activity and reducing the level of free radicals that cause
oxidative DNA damage (5). The
anti-angiogenic activity of thalidomide suggested that it may be
effective in the treatment of solid tumors and other
angiogenesis-related diseases, as angiogenesis in solid tumors is
vital for advanced tumor growth and metastasis (6). Currently, thalidomide is one of the
most well known teratogens in medical history and clinically
recognized as an efficient therapeutic agent for different types of
cancer, however, the anti-angiogenic mechanism of thalidomide
remains to be elucidated.
Angiogenesis refers to the formation of new blood
vessels from the endothelium of existing vasculature; this process
is fundamental for tumor growth, progression and metastasis. In
previous years, the inhibition of angiogenesis has been
demonstrated as a promising strategy for the treatment of cancer
and has been successfully transferred from preclinical to clinical
applications (7). In the 1970s,
Folkman hypothesized that targeting the blood supply by inhibiting
blood vessel formation may lead to arrest of tumor growth or even
tumor shrinkage. The physiological basis of this hypothesis is that
tumors cannot exceed 1–2 mm3 in an avascular state.
Following this, intensive and successful studies investigating the
molecular mechanisms of tumor angiogenesis were initiated (8).
The angiogenic switch is considered to be controlled
by a balance between pro- and anti-angiogenic molecules in the
solid tumor microenvironment. The switch occurs as a result of a
net balance of positive and negative regulators. When
pro-angiogenic factors overcome the effect of anti-angiogenic
molecules, the tumor acquires an angiogenic phenotype that leads to
the formation of new blood vessels (9).
Cancer cells can stimulate angiogenesis by producing
several angiogenic factors, including VEGF, angiopoetins, bFGF,
epidermal growth factor, interleukin 8 and transforming growth
factor β (7). The presence of
angiogenic factors alone is not sufficient to initiate new vascular
growth. Pro-angiogenic factors are counterbalanced by a number of
natural anti-angiogenic molecules, including thrombospondin-1,
angiostatin and endostatin (10).
Anti-angiogenic substances can be divided into agents that directly
target endothelial cell recruitment, endothelial cell proliferation
or tube formation, whereas indirect inhibitors target tumor cell
production of pro-angiogenic growth factors or interfere with their
receptors or intracellular signaling pathways (11). Multiple studies have reported that
thalidomide has anti-angiogenic properties, however, the underlying
mechanisms remain to be elucidated (12).
The angiogenesis-related peptide, substance P (SP)
is a member of the tachykinin family, encoded by the
preprotachykinin A (PPT-A) l gene (13). SP is a major neuropeptide involved
in neurogenic inflammation, however, it is also the most
significant neuropeptide in cancer. The PPT-A gene is expressed in
a number of other cell types, including monocytes, human
fibroblasts, keratinocytes, lymphocytes, platelets and tumor cells.
SP also induces angiogenesis and local inflammatory responses,
which may increase cancer progression and metastases (14). SP appears to exert a bidirectional
effect on inflammation, tumor growth and carcinogenesis; these
effects may be due to the counter-balancing effects of SP fragments
and the intact peptide, such that intact peptide is tumorigenic and
induces inflammation, whereas the fragments produced by peptidases
are antitumorigenic and anti-angiogenic (15).
The peptidases, neprilysin (NEP) and A disintegrin
and metalloproteinase (ADAM)10 degrade SP (16,17).
NEP is a membrane-bound, 90–110 kDa, zinc-dependent
metallopeptidase that cleaves amide bonds on the amino side of
hydrophobic amino acids and is normally expressed in a wide range
of tissues and cells. Decreased NEP activity has been linked to
increased growth of certain cancer cells, however, whether its
activity is associated with the growth of cells has yet to be
elucidated (18).
Erin et al (17) revealed that ADAM10 is a
multifunctional, membrane-bound, cell surface glycoprotein, which
has numerous functions in cell growth, differentiation and
motility. The authors demonstrated that ADAM10 hydrolyzes SP;
specifically, ADAM10 produces the same growth-inhibitory products
as SP (i.e. SP 1–7), similarly to NEP.
It is well established that solid tumors frequently
contain a substantial number of cells that are hypoxic. Harrison
et al (19)reported that
the hypoxia exists in tumors. Numerous studies have demonstrated
that hypoxic cells are common in the majority of solid tumors,
including malignant brain tumors, melanomas, soft tissue sarcomas,
prostate cancer, cervical cancer and invasive breast cancer
(20–25). In a clinical setting, particularly
for the treatment of breast cancer, radiotherapy can be
successfully used in combination with other treatments, including
chemotherapy, however, the precise mechanism is not clear (26). For this reason, it is important to
determine the effects of thalidomide plus radiotherapy as a
combined therapy.
Our initial study demonstrated the cytotoxic effects
of thalidomide alone and in combination with radiotherapy in mouse
breast cancer cell lines 4T1 and 4T1 heart metastases
post-capsaicin (4THMpc) using four cytotoxic tests (data
unpublished). Based on these findings, the present study was
designed to investigate the association between SP degrading two
enzymes and treatment with thalidomide alone or thalidomide in
combination with radiotherapy.
Based on the results, it is hypothesized that
thalidomide may activate NEP and ADAM10 enzymatic activity in
vitro and this process may be responsible for its
anti-angiogenic properties.
Materials and methods
Cell culture
The 4T1 breast cancer cell line, which was
originally derived from a breast carcinoma that arose spontaneously
in a Balb-c mouse and the 4THMpc cell line derived from cardiac
metastases of 4T1 cells were used in the present study. The two
cell lines were provided by Dr Nuray Erin at Akdeniz University,
Medicine Faculty (Antalya, Turkey).
The 4T1 and 4THMpc cell lines were grown in
Dulbecco’s modified Eagle’s medium (DMEM)/F12 (Invitrogen Life
Technologies, Carlsbad, CA, USA) supplemented with 5% fetal bovine
serum (FBS), 2 mM L-glutamine, 1 mM sodium pyruvate and 0.02 mM
non-essential amino acids at 37°C with 5% CO2 in a
humidified atmosphere. Confluent cells (90%) were used in all
experiments. All cell lines used in the present study were assessed
and demonstrated to be free of mycoplasma contamination.
Determination of the cytotoxic dose
The cytotoxic effect of thalidomide alone or in
combination with radiotherapy on 4T1 and 4THMpc mouse breast cancer
cell lines was determined via four cytotoxic assessments. The
viability of the control and treated cells was determined using an
MTT colorimetric assay (Promega Corporation, Madison, WI, USA), the
trypan blue dye exclusion method (Sigma, St. Louis, MO, USA) and
the LIVE/DEAD® cell viability assay (Invitrogen Life
Technologies) after 24, 48 and 72 h of incubation. An
EnzCheck® caspase-3 enzyme activity assay (Invitrogen
Life Technologies) was performed to determine whether thalidomide
causes apoptosis.
Determination of ADAM10 enzyme
activity
Confluent cells were trypsinized in a solution of
trypsin-versen and resuspended in DMEM/F12 (Biochrom AR, Berlin,
Germany) medium with 5% FBS (Biochrom). Cells were subsequently
seeded into 100×20 mm petri dishes at a density of 3×105
cell/ml. After 24 h, 40 μg/ml thalidomide was added and after a
further 4 h, radiotherapy labeled petri dishes were irradiated with
45 Gy 60Co. Cells were incubated for an additional 24
h.
Following the incubation period, media was removed
and cells were harvested using 1 ml sterile phosphate-buffered
saline (PBS) and centrifuged at 1,720 × g for 2 min. An assay
buffer (200 μl) containing 0.1% (v/v) Triton-X 100 was added to
each cell pellet, incubated at 4°C for 10 min and subsequently
centrifuged for 10 min at 3,100 × g. The supernatant was collected
and the protein content of samples was determined using the protein
assay kit (Bio-Rad, Hercules, CA, USA) with bovine serum albumin as
standard based on the Bradford method. Samples were stored at −80°C
prior to use.
The ADAM10 enzyme activity was determined with a
fluorimetric assay using a SensoLyte™ 520 TACE (α-Secretase)
Activity Assay kit (Ana Spec Inc., San Jose, CA, USA) according to
the manufacturer’s instructions. A sterile black 96 well plate was
used for measuring the fluorescence intensity. Each sample
contained 100 μg/ml protein and TNF-α converting enzyme substrate
solution to a total volume of 100 μl, which was added to wells
according to test procedure. The fluorescence intensities were
measured at a wavelength of 495/510 nm (excitation/emission) with a
fluorescence/luminescence spectrometer (Model LS55; PerkinElmer
Ltd., Beaconsfield, UK).
Western blot analysis of ADAM10
The alterations in ADAM10 protein expression were
analyzed by standard western blotting techniques. Briefly, 25 μg of
homogenate protein was separated on a 10% polyacrylamide gel by
SDS-PAGE and then transferred onto polyvinylidene difluoride
membranes (Hybond-P, Amersham Pharmacia Biotech, Piscataway, NJ,
USA) with a semi-dry transfer apparatus. The membranes were blocked
with Tris-buffered saline (TBS)-5% milk and incubated at room
temperature with a 1:1,000 dilution of a rabbit polyclonal
anti-ADAM10 antibody (cat no. AB19026; Millipore, Billerica, MA,
USA). The primary antibody was detected with a 1:10,000 dilution of
an anti-mouse peroxidase-conjugated secondary antibody (sc-2005;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and the blots
were visualized using a chemiluminescent substrate (ECL Plus; GE
Healthcare, Amersham, UK) and exposure to film (Sigma Aldrich).
Determination of NEP enzyme activity
NEP enzyme activity was measured using a
fluorometric assay for the generation of free dansyl-D-Ala-Gly
(DAG) from N-dansyl-Ala-Gly-D-nitro-Phe-Gly (DAGNPG), the
substrate for NEP. Confluent cells were trypsinized using
Trypsin-versen and resuspended in DMEM/F12 (Biochrom) medium with
5% FBS (Biochrom). Cells were subsequently seeded into 100×20 mm
petri dishes at a density of 3×105 cell/ml. After 24 h,
40 μg/ml thalidomide was added and following a further 4 h,
radiotherapy-labeled petri dishes were irradiated with 45 Gy
60Cobalt. Cells were incubated for an additional 24 h.
Following the incubation period, media was removed and cells were
mechanically harvested using 500 μl sterile PBS and a cell scraper
(BD Falcon™; BD Biosciences, Franklin Lakes, NJ, USA). Cells were
homogenized in 250 μl of 50 mM Tris-HCl buffer pH 7.4. The
homogenate was centrifuged at 1,000 × g for 10 min at 4°C to remove
crude debris and the supernatant was reserved as a sample for the
assay. Substrate solutions consisting of 1 mM DAGNPG and 10 mM
enalapril in 50 mM Tris-HCl in the absence and presence of 10 mM
phosphoramidon were prepared. The substrate solutions were
preincubated at 37°C for 10 min. The samples (50 μl) were
subsequently incubated at 37°C for 10 min with 100 ml of each
substrate solution. The reaction was stopped by incubating for 10
min at 90°C. The samples were then diluted 1:10 with 50 mM Tris-HCl
and spun for 5 min in a microfuge at 13, 190 × g. The fluorescence
of the supernatant was measured using a LS 55 fluorescence
spectrophotometer (Perkin-Elmer Inc.) at an emission wavelength of
562 nm and an excitation wavelength of 342 nm. Samples and DAG
standards were assayed in triplicate. The protein concentration of
the samples was determined using a Bio-Rad protein assay kit
(Bio-Rad) and measured against bovine serum albumin standards.
Western blot analysis for NEP
In order to elucidate whether changes in NEP
activity in 4T1 and 4THMpc cells were due to changes in NEP protein
content, cell homogenates were analyzed using standard western
blotting techniques (27). The
membranes were subsequently blocked with TBS-5% milk and then
incubated at room temperature with a 1:2,000 dilution of a rabbit
polyclonal antibody known to react with mouse NEP (cat no. AB5458;
Millipore). The primary antibody was detected with 1:20,000
dilution of an anti-mouse peroxidase-conjugated secondary antibody
(sc-2005; Santa Cruz Biotechnology, Inc.) and the blots were
visualized using a chemiluminescent substrate (ECL Plus; GE
Healthcare) and exposure to film (Sigma Aldrich).
Statistical analysis
All data are presented as the mean ± standard error
of the mean. Data analysis was performed using INSTAT version 3.0
software (Graph Pad Software, San Diego, CA, USA). Analysis of
variance (ANOVA) with Dunnett’s multiple comparisons post-hoc test
and Student’s t-test (for comparisons between two groups) were used
for intergroup comparisons. Statistical analyses for the SP
quantity were performed using either ANOVA followed by the
Tukey-Kramer multiple comparison test or Student’s paired t-test on
the percentage of alteration values. The graphs were drawn using
Sigma Plot version 10.0 (SPSS, Inc., Chicago, IL, USA) and
CorelDRAW version X4 (Corel, Co., Minneapolis, MN, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Determining the cytotoxic effects of
thalidomide alone or in combination with radiotherapy
In our previous study, 10 concentrations of
thalidomide and different fractions of irradiation (0,0001–40 μg/ml
thalidomide and 5–45 Gy 60Co) were used to determine the
cytotoxic effects of the drug alone or in combination with
radiotherapy (data not shown). According to four cytotoxicity test
results, 40 μg/ml thalidomide alone or in combination with 45 Gy
60Co irradiation exhibits the most significant cytotoxic
effect on 4T1 and 4THMpc cell lines. The optimum combination for
enzyme activity and SP experiments were selected to be 40 μg/ml
thalidomide and 45 Gy 60Co irradiation.
Determination of ADAM10 enzyme
activity
The effects of 40 μg/ml thalidomide alone or in
combination with 45 Gy 60Co irradiation on ADAM10 enzyme
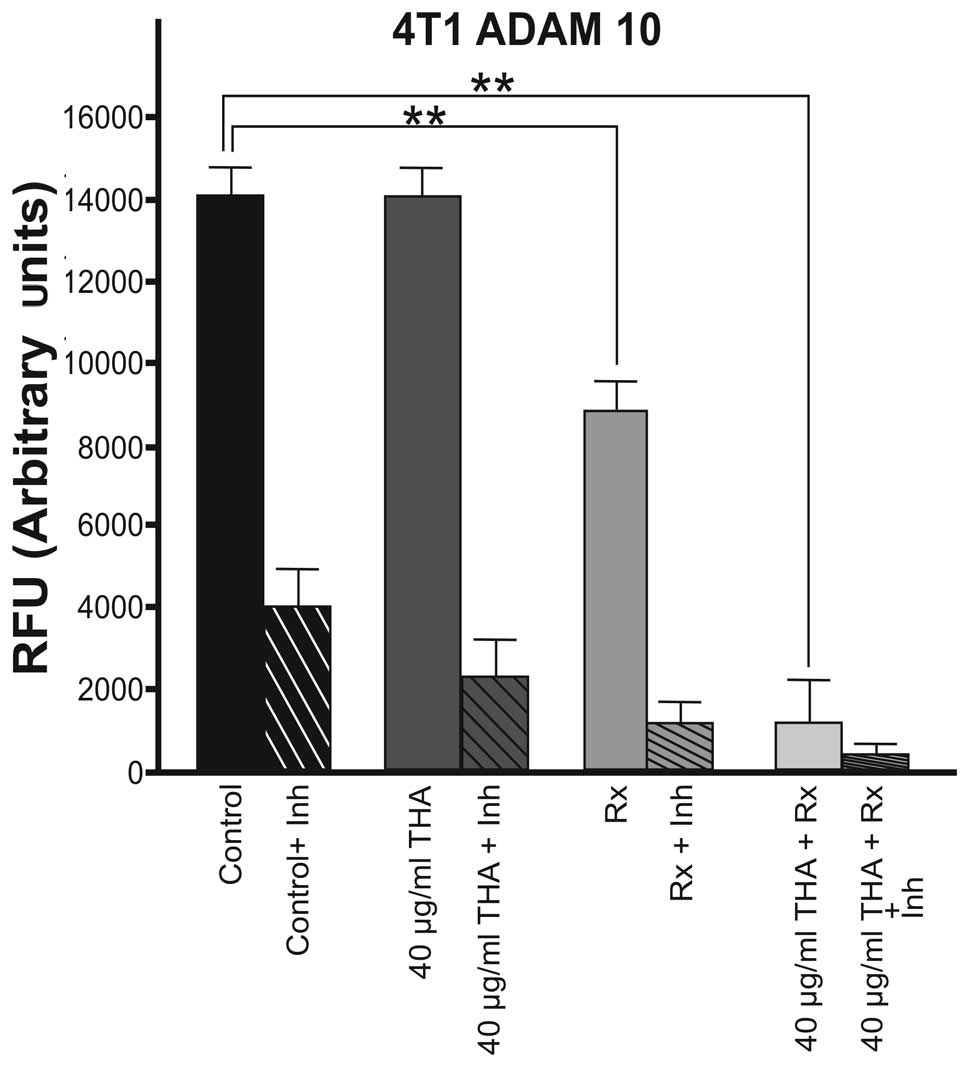
activity in the 4T1 cell line is shown in Fig. 1. Thalidomide (40 μg/ml) alone did
not alter the basal level of ADAM10 activity in 4T1 cells compared
with the control group. By contrast, 60Cobalt
irradiation (45 Gy) alone caused a significant decrease in ADAM10
enzyme activity (P<0.01). Combined therapy also significantly
decreased the ADAM10 enzyme activity in this cell line (P<0.01;
Fig. 1).
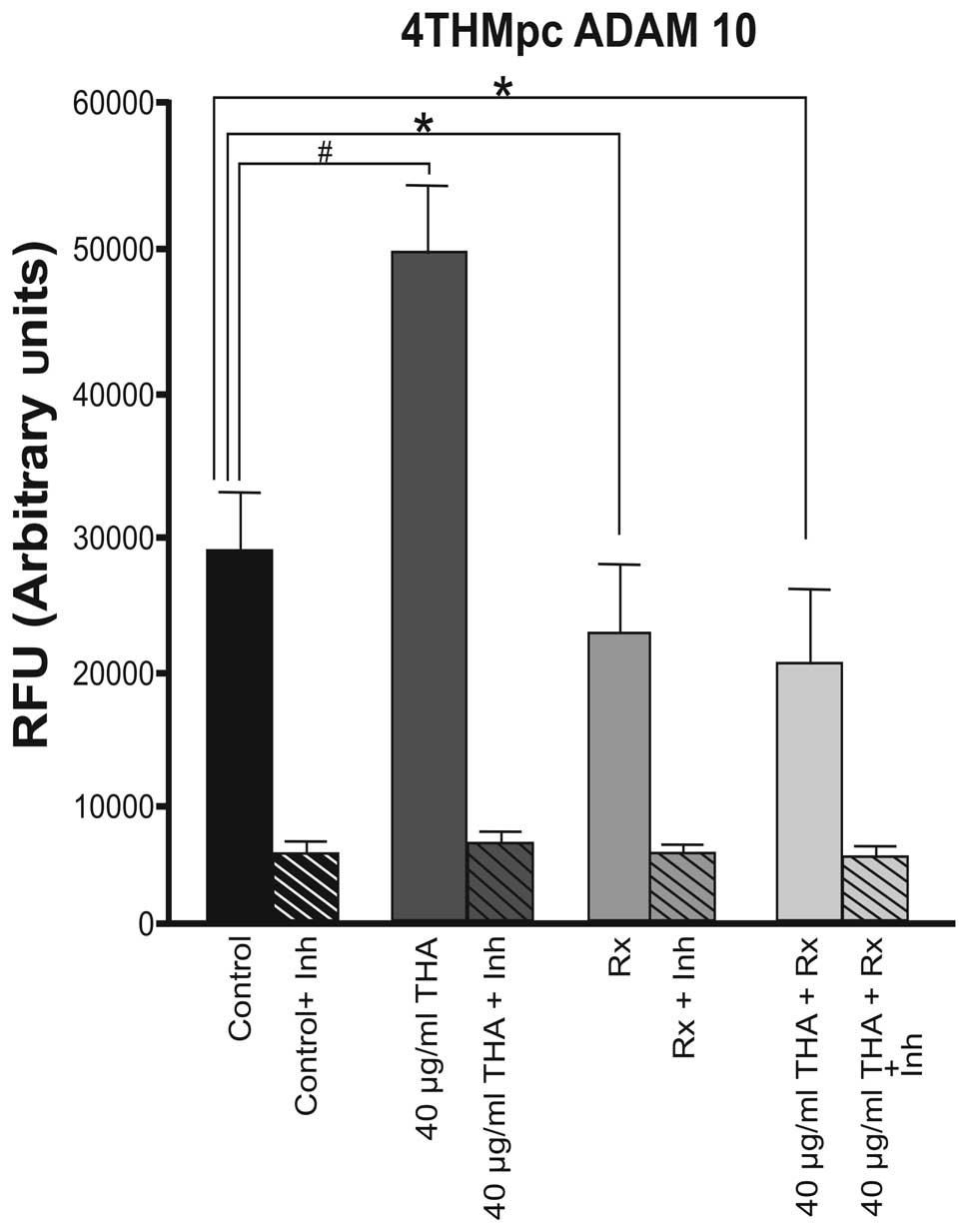
ADAM10 enzyme activity was higher at a basal level
in the 4THMpc cell line compared with 4T1 cells. By contrast to the
4T1 cells, thalidomide increased the activity of the ADAM10 enzyme
in 4THMpc cells. In the 4THMpc cell line, thalidomide increased the
activity of the ADAM10 enzyme in the control group. However,
60Co irradiation (45 Gy) alone or in combination with 40
μg/ml thalidomide decreased the level of ADAM10 enzyme activity to
the basal level (Fig. 2).
Determination of NEP enzyme activity
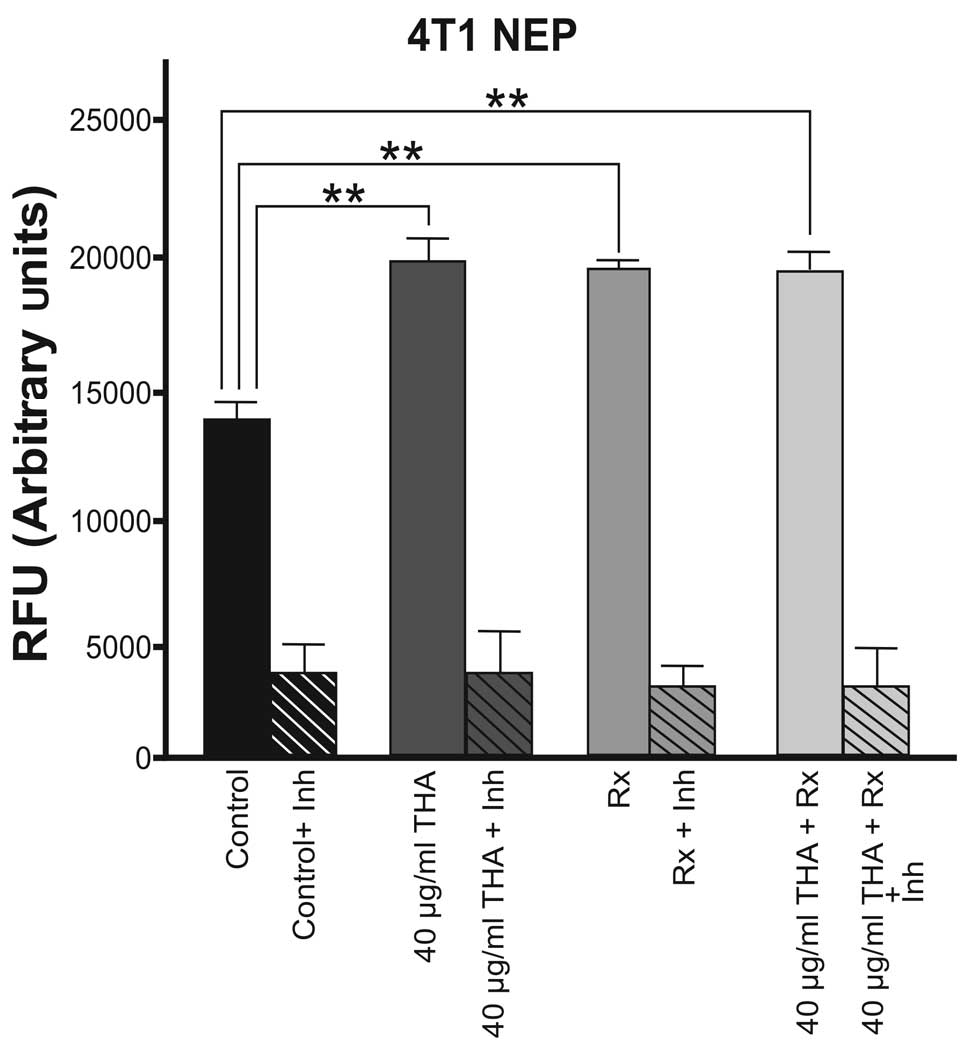
The basal level of NEP enzyme activity was higher in
4T1 cells compared with 4THMpc cells. As shown in Fig. 3, thalidomide and 60Co
irradiation alone and the combined therapy caused an approximately
equal increase in NEP enzyme activity in 4T1 cells.
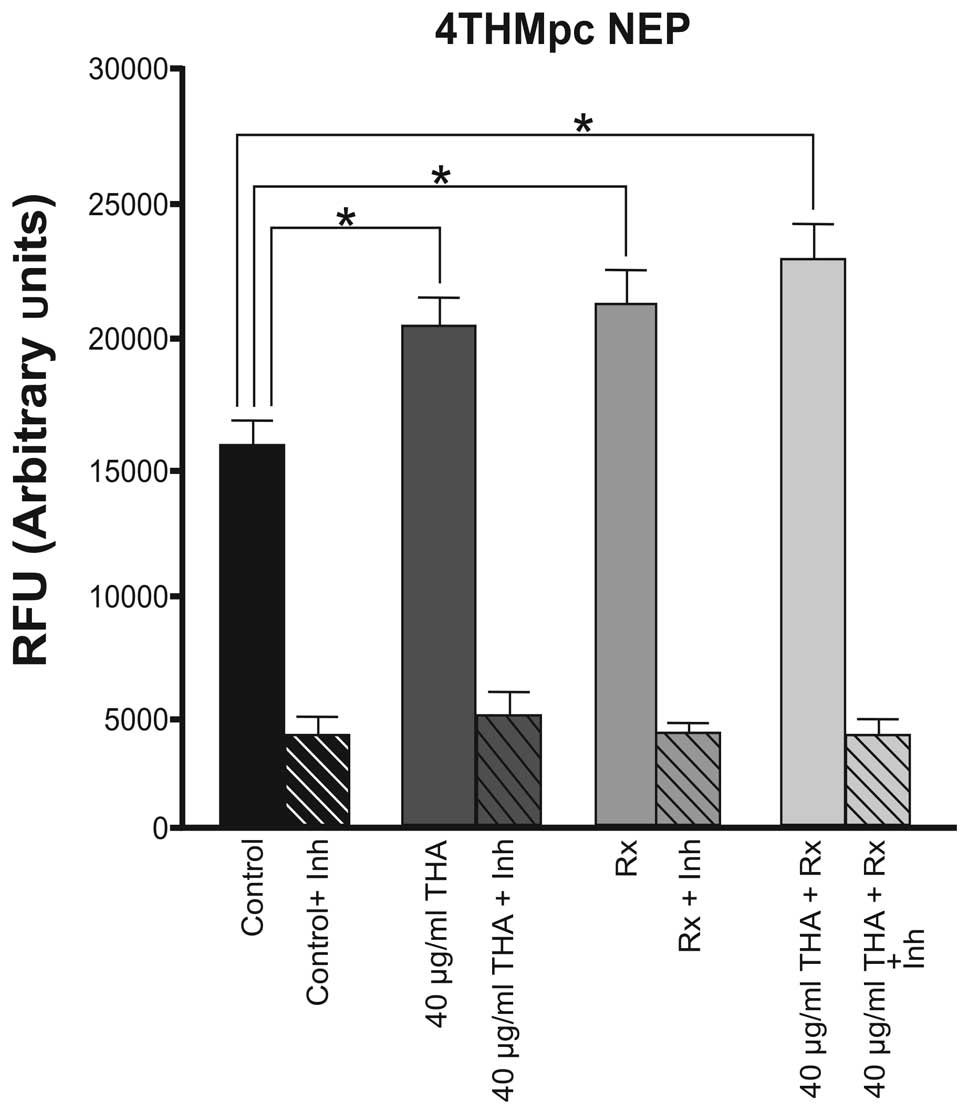
In the 4THMpc cell line, thalidomide and
60Cobalt irradiation alone had a similar effect and
caused an equal increase in the activity of NEP, however, the
enzyme activity was increased further when the combined therapy was
used (Fig. 4).
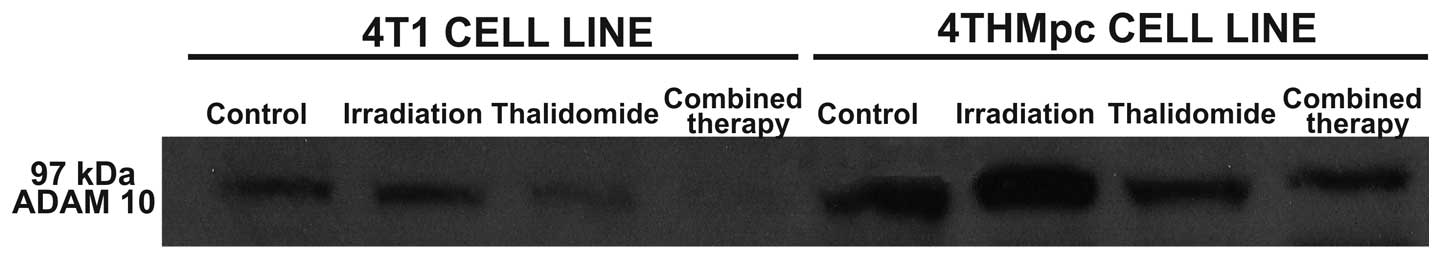
Western blot analysis for ADAM10 and NEP
enzymes
To determine whether the changes in NEP and ADAM10
activities of 4T1 and 4THMpc cells were due to alterations in NEP
and ADAM10 protein concentration, cell homogenates were analyzed by
standard western blotting techniques. As shown in Figs. 5 and 6, determined enzyme activities were
corrected with western blot analysis. The density of the bands
correlated with the activities of the two enzymes.
Discussion
Following the identification of the teratogenic
effects of thalidomide in pregnant females, the drug was
subsequently demonstrated to destroy blood vessels in the fetus
(28). Due to this property,
thalidomide was investigated as an anti-angiogenic drug and its
potential anti-angiogenic and antitumoral properties have been
demonstrated in an animal model (29). Currently, thalidomide is
successfully used for the treatment of multiple myeloma, prostate
cancer and kidney cancer. Research into the potential use of of
thalidomide in other types of cancer continues (30).
Breast cancer is one of the most important global
health concerns, with >1,500,000 new cases and >400,000
mortalities annually, worldwide (27). Thalidomide alone is not effective
in the treatment of metastatic breast cancer, therefore, it must be
combined with another cytotoxic drug or an alternative treatment
(1). For a number of years,
combining a cytotoxic agent with radiotherapy has been of interest
to oncologists. Radiotherapy is the most widely used treatment,
particularly in the treatment of solid tumors. In previous years,
significant results for breast cancer patients were derived from
the use of postoperative systemic therapies and radiotherapy.
Although these two modalities have been extensively used, the exact
mechanisms of their effects in combination remain to be elucidated.
The aim of the present study was to investigate the effect of
thalidomide in combination with radiotherapy on mouse breast cancer
cell lines 4T1 and 4THMpc.
Angiogenesis, the formation of new blood vessels by
the extension or elaboration of pre-existing blood vessels, is
significant in tumor growth, progression and metastasis. The
inhibition of tumor angiogenesis is a promising strategy for the
treatment of cancer, which has been successfully transferred from
preclinical to clinical applications in recent years.
Numerous drugs are utilized clinically as
anti-angiogenic agents and act in different pathways in
angiogenesis. One of the main targets of anti-angiogenic drugs is
to inhibit proteases released from cancer cells. Proteases are a
class of enzymes, which degrade proteins, generating peptides and
amino acids. Cancer cells release proteases and degrade the
extracellular matrix proteins to facilitate migration, metastases
and angiogenesis. These proteases are termed matrix
metalloproteases (MMPs) (31).
ADAMs are a subfamily of MMPs and exist in multiple
organisms from protozoa to humans. ADAMs are critical for multiple
signal transduction pathways and degradative membrane proteins
alter them to their soluble mature forms. One of the most important
ADAMs members is ADAM10 (32). An
increase in ADAM10 protein expression is associated with prostate
and breast cancer, however, the underlying mechanism remains to be
elucidated.
In 4T1 cells, thalidomide (40 μg/ml) alone did not
alter the activity of ADAM10. 60Co irradiation (45 Gy)
alone caused 42% inhibition in ADAM10 activity and the inhibition
increased to 89% when combined therapy was used. By contrast, in
the 4THMpc cell line, thalidomide (40 μg/ml) alone induced a 66.6%
increase in ADAM10 enzyme activity. Radiotherapy alone and
thalidomide with 60Co combined therapy caused a 33.3 and
40% inhibition in ADAM10 activity, respectively. These results were
supported by western blot analysis.
NEP, also known as neutral endopeptidase, is a
metallopeptidase with a zinc-binding motif (33). NEP is an important peptidase in
neuropeptide metabolism. NEP has been demonstrated to activate
bombesin, calsistonine, neurotensin and vasoactive intestinal
peptit and is also important in tumor growth in lung and prostate
cancer (34).
In 4T1 cells, thalidomide alone caused a 40.9%
increase in NEP activity. Radiation therapy alone or in combination
with the drug caused a 40.7% increase in NEP activity. Therefore,
it is suggested that there is no synergistic effect between
radiotherapy and thalidomide.
In the more aggressive 4THMpc cells, thalidomide
alone led to a 26.6% increase in NEP activity. Radiotherapy alone
and combined therapy caused 33.3 and 37% increases in enzyme
activity, respectively. At the same dosage, thalidomide did not
affect the activity of ADAM10, however, NEP enzyme activity was
increased. This result may assist in elucidating the mechanism of
action of the drug. However, in 4THMpc cells, combined therapy did
not alter NEP activity. 4THMpc cells are more aggressive than 4T1
cells and ADAMs are associated with angiogenesis, thus, the
increased activity of ADAM10 in 4THMpc cells compared with 4T1
cells was unexpected.
It is possible that ADAM10 or NEP may degrade SP. SP
and its receptor, neurokinin-1, are associated with mitogenesis,
angiogenesis, migration and metastases. SP was classified as a
neurotransmitter in the 1950s and has an important function in
inflammation and cancer (35,36).
Associations between SP and pancreas, breast and
colon cancer have been reported. SP exists in neuronal cells and
cancer cells (37–39). Based on the hypothesis that all
solid tumors require angiogenesis for nutrition, the new blood
vessel formation triggered by SP and its receptor is the most
important link between angiogenesis and cancer (40). Therefore, eradication of SP or its
receptors is a novel strategy for cancer treatment.
According to the present results, thalidomide at its
toxic dose was determined to exert a significant effect on the
activities of ADAM10 and NEP enzymes in 4T1 and 4THMpc mouse breast
cancer cell lines in vitro.
To the best of our knowledge, the present study is
the first to demonstrate that thalidomide alters the activities of
ADAM10 and NEP enzymes causing significant alterations to the mouse
breast cancer cell lines, 4T1 and 4THMpc. It is hypothesized that
thalidomide may exert its anti-angiogenic property by altering the
activity of the two proteases. However, further studies are
required to elucidate this mechanism.
Acknowledgements
This study was supported by The Scientific and
Technological Research Council of Turkey (grant no. 107T204). The
authors would like to thank Dr Nina Tuncel and all the technicians
in the Radiation Oncology Department for their technical
assistance. The authors would also like to thank all employees of
Akdeniz University Research Unit under the leadership of Professor
Olcay Yeğin for their support during this study. In addition, the
authors are appreciative of Professor Bahriye Uğur Yavuzer for her
useful comments and suggestions on the results of the western blot
analysis and Ms. Duygu Sahintürk Ünal for her excellent technical
assistance.
References
|
1
|
Papaiakovou VE, Bamias A and Dimopoulos
MA: Thalidomide in cancer medicine. Ann Oncol. 15:1151–1160. 2004.
View Article : Google Scholar
|
|
2
|
D’Amato RJ, Loughnan MS, Flynn E, et al:
Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci
USA. 91:4082–4085. 1994. View Article : Google Scholar
|
|
3
|
Kenyon BM, Browne F and D’Amato RJ:
Effects of thalidomide and related metabolites in a mouse corneal
model of neovascularization. Exp Eye Res. 64:971–978. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bauer KS, Dixon SC and Figg WD: Inhibition
of angiogenesis by thalidomide requires metabolic activation, which
is species-dependent. Biochem Pharmacol. 55:1827–1834. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Majumdar S, Lamothe B and Aggarwal BB:
Thalidomide suppresses NF-κB activation induced by TNF and
H2O2, but not that activated by ceramide,
lipopolysaccharides, or phorbol ester. J Immunol. 168:2644–2651.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Macpherson GR, Franks M, Tomoaia-Cotisel
A, et al: Current status of thalidomide and its role in the
treatment of metastatic prostate cancer. Crit Rev Oncol Hematol.
46:49–57. 2003. View Article : Google Scholar
|
|
7
|
Eichhorn ME, Kleespies A, Angele MK, et
al: Angiogenesis in cancer: molecular mechanisms, clinical impact.
Langenbecks Arch Surg. 392:371–379. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Folkman J, Watson K, Ingber D, et al:
Induction of angiogenesis during the transition from hyperplasia to
neoplasia. Nature. 339:58–61. 1989. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jain RK: Normalizing tumor vasculature
with anti-angiogenic therapy: a new paradigm for combination
therapy. Nat Med. 7:987–989. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang Z and Bao SD: Roles of main pro- and
anti-angiogenic factors in tumor angiogenesis. World J
Gastroenterol. 10:463–470. 2004.PubMed/NCBI
|
|
11
|
Eichhorn ME, Strieth S and Dellian M:
Anti-vascular tumor therapy: recent advances, pitfalls and clinical
perspectives. Drug Resist Updat. 7:125–138. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rajkumar SV: Current status of thalidomide
in the treatment of cancer. Oncology (Williston Park). 15:867–874.
2001.
|
|
13
|
Sumner SC, Gallagher KS and Davis DG:
Conformational analysis of the tachykinins in solution: substance P
and physalaemin. J Biomol Struct Dyn. 8:687–707. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Carter MS and Krause JE: Structure,
expression and some regulatory mechanisms of the rat
preprotachykinin gene encoding substance p, neurokinin a,
neuropeptide k, and neuropeptide gamma. J Neurosci. 10:2203–2214.
1990.PubMed/NCBI
|
|
15
|
Erin N and Ulusoy O: Differentiation of
neuronal from non-neuronal Substance P. Regul pept. 152:108–113.
2009. View Article : Google Scholar
|
|
16
|
Harrison S and Geppetti P: Substance p.
Int J Biochem Cell Biol. 33:555–576. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Erin N, Zhao W, Bylander J, et al:
Capsaicin-induced inactivation of sensory neurons promotes a more
aggressive gene expression phenotype in breast cancer cells. Breast
Cancer Res Treat. 99:351–364. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Erin N, Boyer PJ, Bonneau RH, et al:
Capsaicin-mediated denervation of sensory neurons promotes mammary
tumor metastasis to lung and heart. Anticancer Res. 24:1003–1009.
2004.PubMed/NCBI
|
|
19
|
Harrison LB, Chadha M, Hill RJ, et al:
Impact of tumor hypoxia and anemia on radiation therapy outcomes.
Oncologist. 7:492–508. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rampling R, Cruickshank G, Lewis AD, et
al: Direct measurement of pO2 distribution and bioreductive enzymes
in human malignant brain tumors. Int J Radiat Oncol Biol Phy.
29:427–431. 1994. View Article : Google Scholar
|
|
21
|
Lartigau E, Randrianarivelo H, Avril MF,
et al: Intratumoral oxygen tension in metastatic melanoma. Melanoma
Res. 7:400–406. 1997. View Article : Google Scholar
|
|
22
|
Nordsmark M, Hoyer M, Keller J, et al: The
relationship between tumor oxygenation and cell proliferation in
human soft tissue sarcomas. Int J Radiat Oncol Biol Phys.
35:701–708. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Movsas B, Chapman JD, Greenberg RE, et al:
Increasing levels of hypoxia in prostate carcinoma correlate
significantly with increasing clinical stage and patient age: an
Eppendorf pO(2) study. Cancer. 89:2018–2024. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mundt AJ, Connell PP, Campbell T, et al:
Race and clinical outcome in patients with carcinoma of the uterine
cervix treated with radiation therapy. Gynecol Oncol. 71:151–158.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vaupel P, Briest S and Höckel M: Hypoxia
in breast cancer: pathogenesis, characterization and
biological/therapeutic implications. Wein Med Wochenschr.
152:334–342. 2002. View Article : Google Scholar
|
|
26
|
Harrison L and Blackwell K: Hypoxia and
anemia: factors in decreased sensitivity to radiation therapy and
chemotherapy? Oncologist. 9:31–40. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oz ES, Aydemir E, Korcum AF and Fiskin K:
Thalidomide and irradiation combination therapy increases substance
P levels in vitro. Exp Ther Med. 2:529–535. 2011.PubMed/NCBI
|
|
28
|
D’Amato RJ, Loughnan MS, Flynn E, et al:
Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci
USA. 91:4082–4085. 1994. View Article : Google Scholar
|
|
29
|
Bartlett JB, Dredge K and Dalgleish AG:
The evolution of thalidomide and its IMiD derivatives as anticancer
agents. Nat Rev Cancer. 4:314–322. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Downs LS Jr, Rogers LM, Yokoyama Y and
Ramakrishnan S: Thalidomide and angiostatin inhibit tumor growth in
a murine xenograft model of human cervical cancer. Gynecol Oncol.
98:203–210. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vandeputte-Rutten L and Gros P: Novel
proteases: common themes and surprising features. Curr Opin Struc
Biol. 12:704–708. 2002. View Article : Google Scholar
|
|
32
|
Chubinskaya S, Mikhail R, Deutsch A and
Tindal MH: ADAM-10 protein is present in human articular cartilage
primarily in the membrane-bound from and is upregulated in
osteoarthrits and in response to IL-1 alpha in bovine nasal
cartilage. J Histochem Cytochem. 49:1165–1176. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Turner AJ and Hooper NM: The
angiotensin-converting enzyme gene family: genomics and
pharmacology. Trends Pharmacol Sci. 23:177–183. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shipp MA, Richardson NE, Sayre PH, et al:
Molecular cloning of the common acute lymphoblastic leukemia
antigen (CALLA) identifies a type II integral membrane protein.
Proc Natl Acad Sci USA. 85:4819–4823. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Luo W, Sharif TR and Sharif M: Substance
P-induced mitogenesis in human astrocytoma cells correlates with
activation of the mitogenactivated protein kinase signaling
pathway. Cancer Res. 56:4983–4991. 1996.PubMed/NCBI
|
|
36
|
Defea KA, Zalevsky J, Thoma MS, et al:
Beta arrestin-dependent endocytosis of proteinase-activated
receptor 2 is required for intracellular targeting of activated
ERK1/2. J Cell Biol. 148:1267–1281. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bhatia M, Saluja AK, Hofbauer B, Frossard
JL, Lee HS, Castagliuolo I, Wang CC, Gerard N, Pothoulakis C and
Steer ML: Role of substance P and the neurokinin 1 receptor in
acute pancreatitis and pancreatitis-associated lung injury. Proc
Natl Acad Sci USA. 95:4760–4765. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Garcia-Recio S, Fuster G,
Fernandez-Nogueira P, Pastor-Arroyo EM, Park SY, Mayordomo C,
Ametller E, Mancino M, Gonzalez-Farre X, Russnes HG, et al:
Substance P autocrine signaling contributes to persistent HER2
activation that drives malignant progression and drug resistance in
breast cancer. Cancer Res. 73:6424–6434. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Muñoz M and Coveñas R: Neurokinin-1
receptor: a new promising target in the treatment of cancer. Discov
Med. 10:305–313. 2010.PubMed/NCBI
|
|
40
|
Esteban F, Muñoz M, González-Moles MA and
Rosso M: A role for substance P in cancer promotion and
progression: a mechanism to counteract intracellular death signals
following oncogene activation or DNA damage. Cancer Metastasis Rev.
25:137–145. 2006. View Article : Google Scholar : PubMed/NCBI
|