Introduction
Systemic inflammatory response syndrome (SIRS) is
the clinical expression and action of numerous complex intrinsic
mediators of the acute phase reaction; SIRS is triggered by events,
including infection, trauma, pancreatitis and surgery (1) and may result in multiple organ
dysfunction syndrome. Current studies suggest that SIRS is caused
by an imbalance of the pro-inflammatory and anti-inflammatory
homeostasis mechanisms within the body. The release of high levels
of pro-inflammatory cytokines into the blood, including
interleukin-1, interferon-γ and phospholipase A2, promotes the
production of oxygen free radicals, lipid metabolites and lysosomal
enzymes, resulting in a waterfall or cascade effect. While it
appears that immune disorders and inflammatory responses cannot be
controlled (2,3), regulating the body’s inflammatory
responses and blocking these pathways in a timely and effective
manner is the key to the successful treatment of patients (4,5).
As the NALP3 inflammasome is an important signal
receptor and potential therapeutic target in a number of diseases,
studies on NALP3 are becoming increasingly important. The NALP3
inflammasome is a macromolecular protein complex, which consists of
NALP3, ASC, caspase-1 and Cardinal-8 (6–9).
NALP3 is expressed primarily in the cells with a phagocytic
function, for example monocyte-macrophage cell and granulocytes. In
addition, certain immune cells express NALP3, including T and B
cells. Within the body, NALP3 is primarily distributed in the
epithelium of skin, joints, ears, eyes, bladder and ureter
(10). When cells are stimulated
by external factors, including microbial toxin, peptidoglycan and
cathepsin B, intracellular NALP3, ASC, caspase-1 and CARD-8 come
together to form a complex protein, the NALP3 inflammasome.
Caspase-1 is activated, splicing and activating the
pro-inflammatory cytokine interleukin (pro-IL)-1β and pro-IL-18
(11). The NALP3 inflammasome is
an important signal receptor that is activated by a number of types
of pathogens and danger signals within the body, resulting in an
immune response. NALP3 is involved in the development of numerous
inflammatory diseases, including inflammatory bowel disease
(12), hypersplenism (13), acute pancreatitis associated lung
injury (14) and oral inflammatory
diseases (15). In addition,
certain non-infectious inflammatory diseases have close
associations with the NALP3 inflammasome, including the rare
autoimmune disease and gout (16).
Based on the above mentioned existing literature,
the current study selected the representative P388D1 mouse
macrophage cell line as the research focus. Adenosine triphosphate
(ATP), an activator of the P2X7 receptor, was used to treat the
cells. To elucidate the association between the P2X7 receptor and
the NALP3 gene in murine P388D1 macrophage-like cells at the
molecular level, gene knockout technology was used to manipulate
the NALP3 gene and P2X7 receptor. In this study, the role and
significance of the NALP3 gene in inflammatory diseases was
investigated by observing the activation pathways of NALP3 and its
specific expression changes in inflammatory cells.
Materials and methods
Main cell solution and reagents
The murine macrophage-like lymphoma cell line P388D1
was purchased from the Institute of Biochemistry and Cell Biology
of the Chinese Academy of Sciences (Shanghai, China). RPMI-1640 dry
medium was obtained from Gibco-BRL (Grand Island, NY, USA). Fetal
calf serum (FCS) was purchased from Sijiqing Biological Engineering
Materials Co. Ltd (Hangzhou, China). P2X7-small interfering RNA
(siRNA) and NALP3-siRNA were purchased from Shanghai GenePharrma
Co. Ltd (Shanghai, China). Polyclonal rabbit antibody against rat
NALP-3 was provided by Abcam Ltd (Hong Kong, China), and
horseradish peroxidase (HRP) conjugated goat anti-rabbit antibody
was obtained from ZhongShan Goldenbridge Biotechnology (Beijing,
China). TRIzol® was purchased from Takara Co., Ltd.
(Dalian, China).
Cell culture and transfection
P388D1 cells were cultured in RPMI-1640 medium,
supplemented with 10% (v/v) FCS, 100 U/ml penicillin (Beyotime
Institute of Biotechnology, Shanghai, China), and 100 mg/ml
streptomycin (Beyotime Institute of Biotechnology). All cells were
cultivated at 37°C in a humidified atmosphere containing 5%
CO2. The cells were placed into Petri dishes containing
six coverslips, onto which cells were plated at a density of
~4×105 per coverslip and cultured for 24 h prior to
transfection. The medium was refreshed prior to transfection with
4.0 μg isolated P2X7-siRNA and/or NALP3-siRNA. Cells were
transfected according to the manufacturer’s instructions. Control
cells were transfected with an empty vector. Three groups of P388D1
cells were obtained, cells only transfected with NALP3-siRNA, cells
that were transfected with P2X7-siRNA and NALP3-siRNA and cells
transfected with an empty vector (control).
ATP-induced expression of NALP-3
Control, cells transfected with NALP3-siRNA or cells
transfected with P2X7-siRNA and NALP3-siRNA were collected from the
flask separately. Following counting with a cell counting kit
(Beyotime Institute of Biotechnology), ~6×106 cells were
cultured with added ATP (1 mmol/l; Takara Co., Ltd.) which is the
natural agonist of the P2X7 receptor. Meanwhile, a separate
parallel control experiment of cells with added adenosine
diphosphate (ADP) was performed.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was harvested from the P388D1 cells using
TRIzol®. The cDNA sequence was produced via reverse
transcription under the following conditions: 30°C for 10 min, 42°C
for 30 min, 99°C for 5 min and 5°C for 5 min. The resulting cDNA
(10 μl) was used to amplify the coding sequence of NALP-3. The
sequences of the primers used in the qPCR reactions were as
follows: Forward, 5′-CTGTGTGTGGGACTGAAGCAC-3′ and reverse,
5′-GCAGCCCTGCTGTTTCAGCAC-3′ for rat NALP-3; forward,
5′-ATCTGGCACCAAACACCTTCTACAATGAGCTGCG-3′ and reverse,
5′-CGTCATACTCCTGCTTGCTGATCCACATCTGC-3′ for β-actin. PCR was
performed in 35 cycles of: 95°C for 1 min, annealing at 60°C for 1
min and extension at 72°C for 1 min, terminating at 4°C at the end
of the reaction. The transcripts were successfully cloned and
sequencing confirmed the target amplification. The PCR products
were separated on a 1% agarose gel which contained 0.5 μl/ml
ethidium bromide and visualized under ultraviolet light.
Western blotting
Control, cells transfected with NALP3-siRNA or cells
transfected with P2X7-siRNA and NALP3-siRNA were centrifuged at
1000 × g for 5 min, and the supernatant was pooled. The cells were
resuspended in phosphate-buffered saline, counted, and
~6×106 cells were aliquoted. These cells were added to
100 μl radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology) prior to centrifugation at 12,000 × g at 4°C for 10
min. Following removal of the supernatants, the total protein was
mixed with loading buffer (1:1; Takara Co., Ltd.) and heated to
100°C for 5 min prior to loading. Tris-glycine SDS-PAGE gels
(concentration, 10%) were used for separation. The total protein
was transferred onto a polyvinylidene fluoride (PVDF; OK-kingding
Membrane Structure Technology Co., Ltd., Shenzhen, China) membrane
with transfer buffer. The PVDF membrane was blocked at 37°C with 5%
dry milk in tris-buffered saline for 2 h, and incubated with rabbit
anti-rat polyclonal antibody (1:200) at 4°C overnight. Following
washing with Tris buffered saline in Tween 20, the membrane was
incubated with HRP-conjugated goat anti-rabbit immunoglobulin G
(IgG) secondary antibody (1:1,000) at 37°C for 2 h.
Statistical analysis
All data are expressed as the mean (χ̄) ± standard
deviation (SD). P<0.05 was considered to indicate a
statistically significant difference. All the experiments and
statistical analyses were performed four times.
Results
Identifying the expression of NALP3 in
P388D1 murine macrophage-like cells
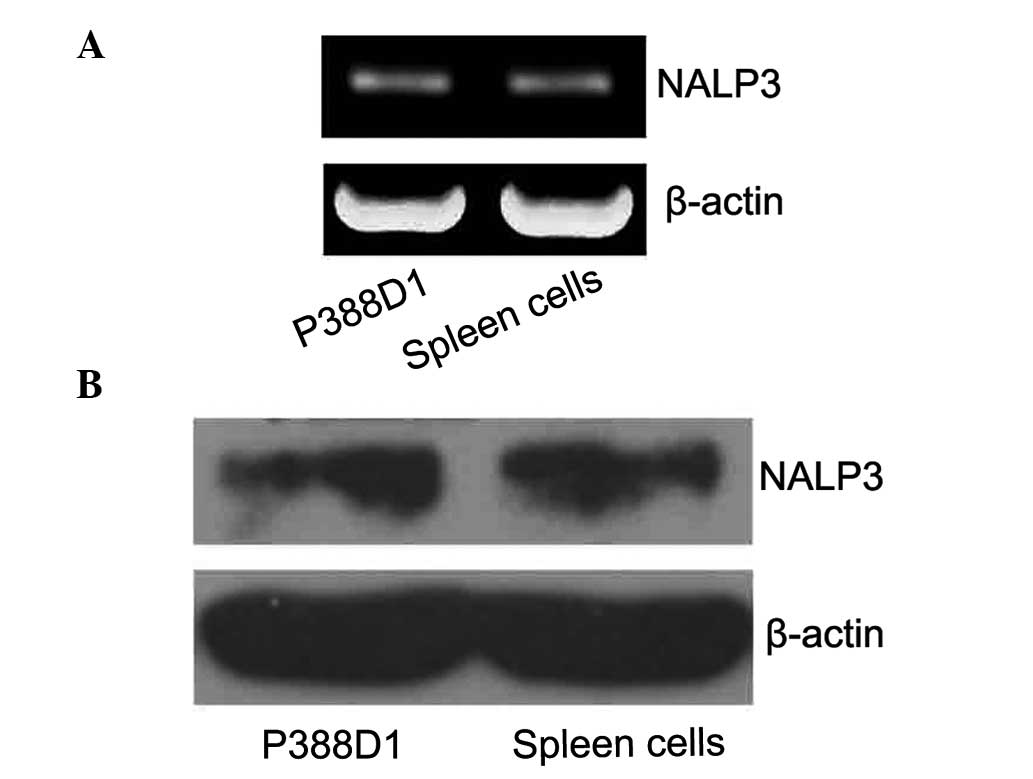
To confirm the expression of NALP3 in P388D1 cells
without activating the P2X7 receptor, the expression levels of the
NALP3 mRNA were detected by RT-qPCR. The related literature reports
the band for NALP3 is at ~252 bp, specifically for the RT-qPCR
amplification products of NALP3. β-actin was used as the internal
control with a fragment size of ~838 bp. The P388D1 cells were
observed to have NALP3 gene expression (Fig. 1A). As the expression of NALP3 mRNA
was detected, western blotting was used to detect the expression of
the NALP3 protein. The target band was observed on film at 94 KDa.
These results demonstrated that the murine P388D1 macrophage-like
cells express NALP3 protein (Fig.
1B).
Identifying the expression of NALP3 in
P388D1 murine macrophage-like cells following treatment with
ATP
We know that ATP is the P2X7 receptor agonist
(17). To determine whether or not
the P2X7 receptor mediates the upregulation of NALP3 expression,
the expression levels of NALP3 were investigated in P388D1 cells in
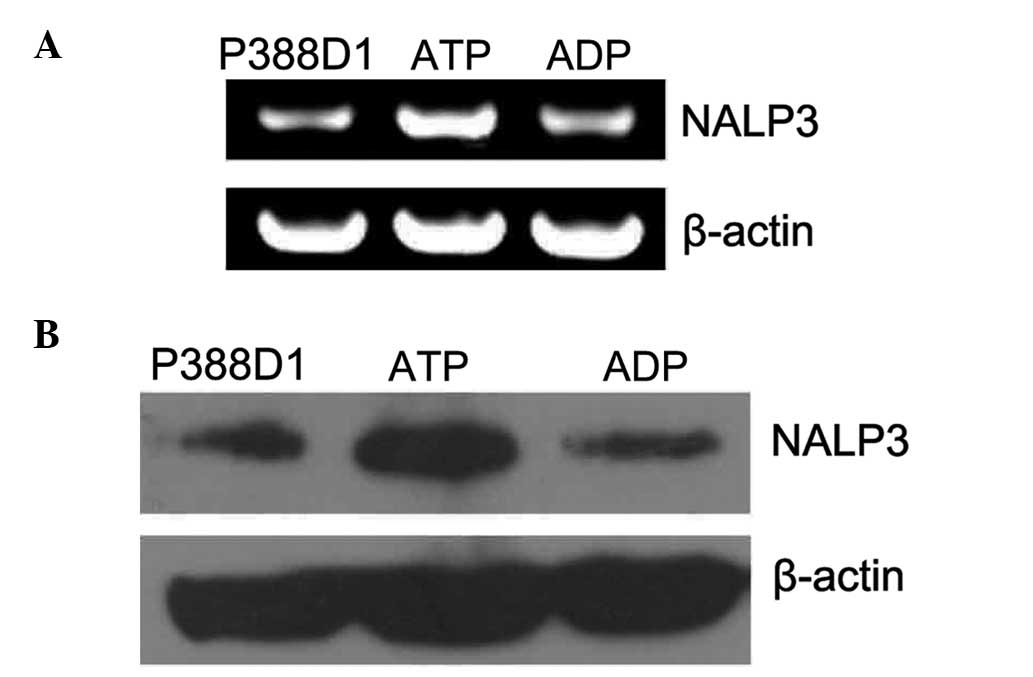
which ATP had activated the P2X7 receptor. Firstly, the expression
of NALP3 mRNA in P388D1 cells treated with ATP for 24 h was
examined using RT-qPCR, the products of which were separated by 1%
agarose gel electrophoresis. This revealed that the expression
levels of NALP3 RT-qPCR amplification products (~252 bp) were
markedly increased compared with the levels in the normal untreated
P388Dl cells (Fig. 2A). The
control group was treated with ADP, and the expression level of
NALP3 mRNA showed no significant change compared with that of the
untreated P388D1 cells (P<0.05, χ̄ ± SD, n=4) (Fig. 3A). Secondly, the expression levels
of NALP3 protein in P388D1 treated with ATP for 24 h were
investigated using western blotting (Fig. 2B). The target band was located at
93 KDa on the film and the NALP3 protein expression levels were
found to be increased compared with those of the untreated normal
P388Dl cells. The expression level of the NALP3 protein showed no
significant change following treatment of cells with ADP compared
with those of the normal untreated P388Dl cells (P>0.05, χ̄ ±
SD, n=4) (Fig. 3B). Therefore, the
results show that the expression levels of NALP3 increased at the
gene and protein levels following the activation of the P2X7
receptor by ATP.
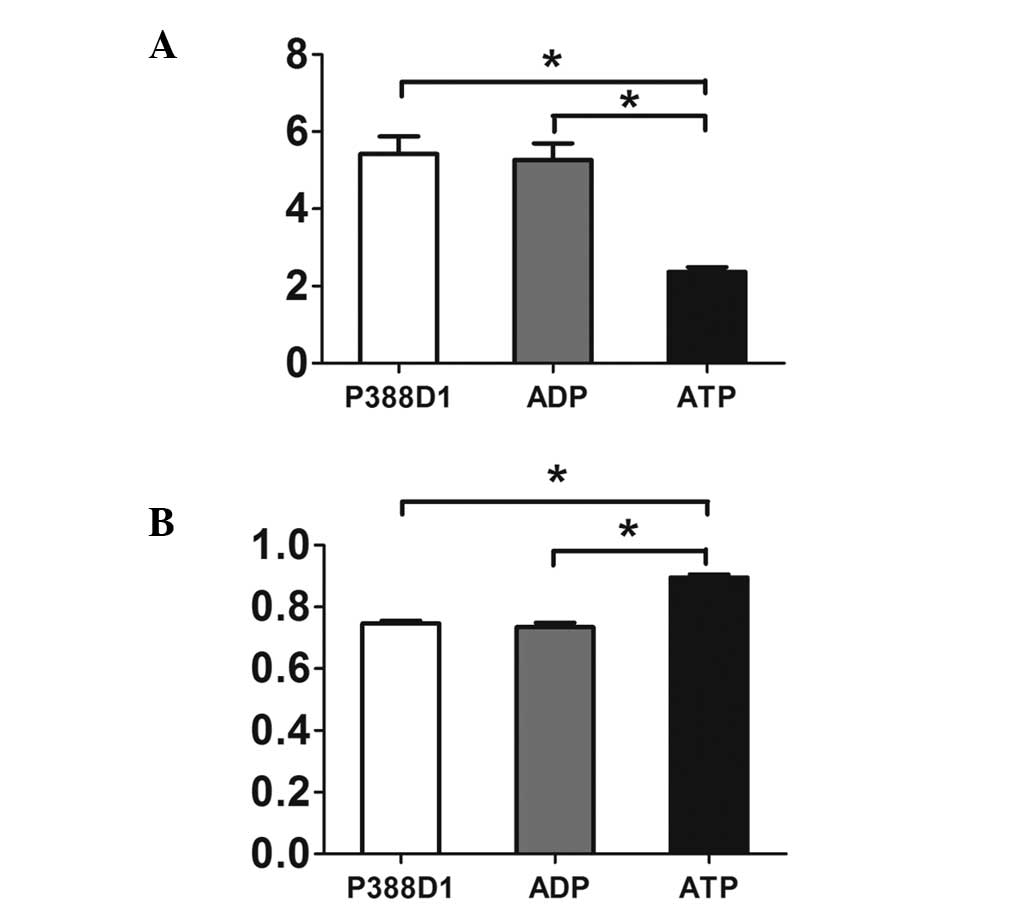 | Figure 3The expression of NALP3 was increased
at the gene and protein levels during the activation of the P2X7 by
ATP. (A) Semi-quantitative analysis showed that compared with
normal untreated P388Dl cells and the control group added with ADP,
the expression of NALP3 in the group added with ATP increased
markedly at the gene level (P<0.05, mean ± SD, n=4). (B) A
similar phenomenon appeared when the three groups were compared at
the protein level. Therefore, the expression of NALP3 was increased
at the protein level during the activation of ATP (P<0.05, mean
± SD, n=4). Due to the different gray values in reverse
transcription polymerase chain reaction and western blotting, a
high relative value in the histogram above reflects (A) a low level
of RNA, while (B) a high level of protein. SD, standard deviation;
ADP, adenosine diphosphate; ATP, adenosine triphosphate. |
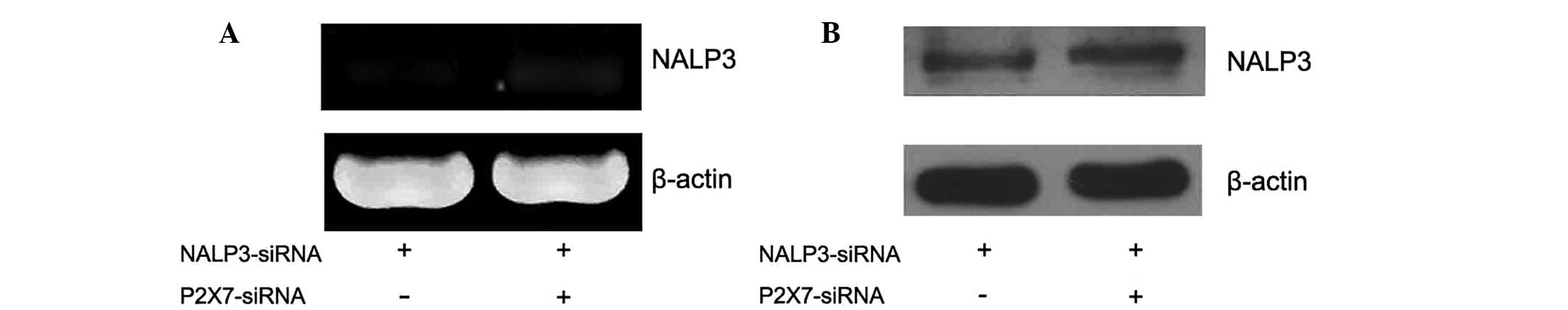
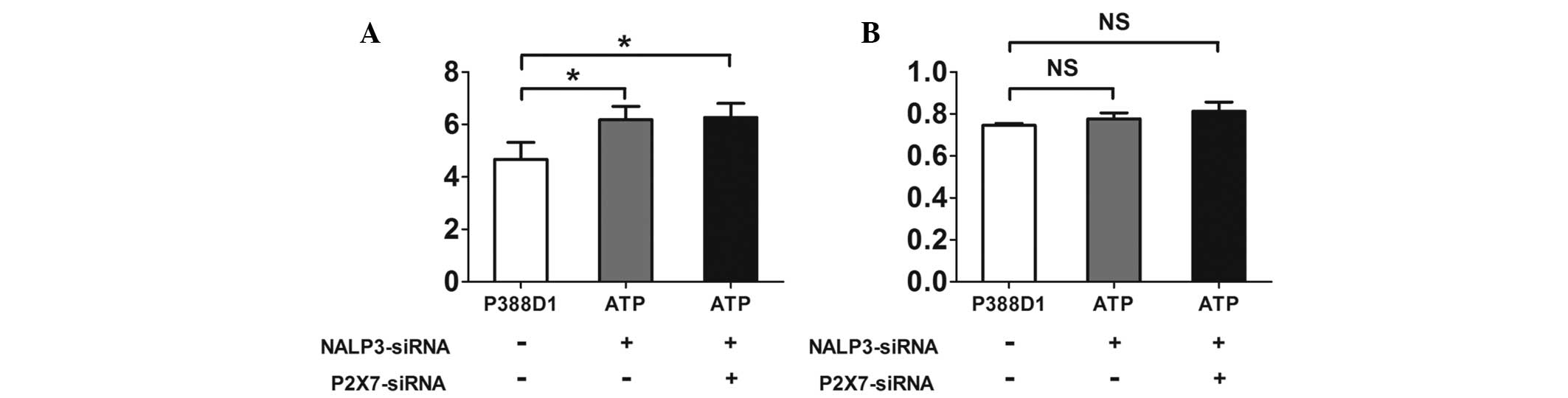
In P388D1 cells cotransfected with the
NALP3-siRNA and P2X7-siRNA plasmids the expression levels of the
NALP3 mRNA decreased, while the expression of NALP3 protein
remained the same
Transfection technology was used to elucidate the
association between the P2X7 receptor and NALP3 expression levels.
Two groups of P388D1 cells were simultaneously transfected with
NALP3-siRNA and P2X7-siRNA prior to the addition of ATP. The
expression levels of NALP3 mRNA were observed using RT-qPCR
(Fig. 4A), but the results showed
the expression levels of NALP3 mRNA were reduced in the two groups
compared with those of the untreated normal P388Dl cells
(P<0.05, χ̄ ± SD, n=4) (Fig.
5A). The expression levels of NALP3 protein were detected by
western blotting. P388D1 cells were transfected with the
NALP3-siRNA plasmid prior to ATP treatment (Fig. 4B). There was no significant
difference between the expression levels of the NALP3 protein in
the transfected cells compared with those of the untreated normal
P388Dl cells. In the cells simultaneously transfected with the
P2X7-siRNA and NALP3-siRNA prior to ATP treatment, the expression
levels of the NALP3 protein showed no significant difference
compared with those of the normal P388Dl cells (P>0.05, χ̄±SD,
n=4) (Fig. 5B).
Discussion
The results of this study demonstrated that when the
P2X7 receptor exists on the cell surface, its activation by ATP
induces the upregulation of NALP3 expression levels. In other
words, the P2X7 receptor and NALP3 gene may have a conduction
effect in the inflammatory response. The results of these
experiments are consistent with the expected results.
There have been a number of studies undertaken in
this area. A previous study determined that ATP activates the P2X7
receptors via pannexin-1, a hemichannel protein in the cell
membrane (18). It has also been
determined that when ligands recognized by NALP3 enter the cell
through the pannexin-1 channel, they activate caspase-1 causing
IL-18 and IL-1β to mature and be secreted (19). The aim of the current study was to
systematically analyze the regulation of NALP3 expression by the
P2X7 receptor in murine P388D1 macrophage-like cells.
To explore the role of the P2X7 receptor in the
regulation of NALP3 expression in murine P388D1 macrophage-like
cells, the expression levels of NALP3 mRNA and protein were
analyzed by RT-qPCR and western blotting. The results of the two
techniques demonstrated that the P388D1 cells expressed NALP3 mRNA
and protein, without the activation of the P2X7 receptor.
Notably, a previous study indicated that ATP has an
important role in the activation of the P2X7 receptor (20). In order to study the association
between the P2X7 receptor and NALP3, an ATP concentration of 1 mM
was used as the native agonist of the P2X7 receptor. P388D1 cells
were treated with ATP for 24 h, followed by detection of the
expression of NALP3 by RT-PCR and western blotting. The expression
levels of NALP3 mRNA and protein increased compared with those of
the normal P388D1 cells. This result shows that activating the P2X7
receptor induces the upregulation of NALP3 expression at the gene
and protein levels. In contrast, when cells were treated with ADP
instead of ATP as a control experiment, the detected expression
levels of NALP3 showed no significant change in gene and protein
expression levels compared with those of the normal untreated P388D
cells. The results of a previous study have shown that ATP
activates the NALP3 inflammasome by stimulating the P2X7 receptor,
which leads to a sudden drop in the levels of K+
(21). ATP activates the P2X7
receptor and rapidly reduces the opening of K+ channels.
The activated P2X7 receptor recruits pannexin-1 and mediates a
gradual increase in the permeability of the transition pore to open
gradually (22), which is finally
recognized in the plasma by NALP3.
The current study aimed to determine the association
between the P2X7 receptor and NALP3 at the molecular level. It has
previously been determined that there are a diverse range of
signaling molecules that induce the activation of the NALP3
inflammation complex, hence, there are a variety of ways to
activate NALP3 (23,24). The P2X7 receptor, activated by ATP,
induces the expression of NALP3. Additionally, the formation of
pannexin-1, lysosomal damage and the induction of ROS are all able
to induce the expression of NALP3.
In the current study, it was revealed that the NALP3
protein has a certain level of expression in normal P388D1 cells.
NALP3 expression may be induced by other means, but at this level
it did not activate the P2X7 receptor. Gene silencing technology
was used to knockout the NALP3 gene, and ATP was added to the
cells. The expression levels of NALP3 were reduced in the knockout
cells compared with those of the untreated normal P388Dl cells,
demonstrating that the transfection had been effective. Secondly,
the NALP3 gene and P2X7 receptor were knocked out simultaneously
and ATP was added. The expression levels of NALP3 in this group
were detected using RT-qPCR and western blotting, and then compared
with the expression levels in the normal P388D1 cells. The results
showed that the expression level of the knockout cells was reduced
at the gene level, but was not significantly different at the
protein level compared with the normal untreated P388D1 cells.
Hence, while other paths can induce NALP3 expression, the
expression of NALP3 protein showed no increase following the
removal of the P2X7 receptor. Upon activation of the P2X7 receptor
by ATP the expression levels of NALP3 protein increased compared
with those of the normal group.
It should be noted that this study detected the
expression of NALP3 only, it did not detect the levels of the NALP3
inflammasome. However, NALP3 is a family member of the NALP3
inflammasome, and it has a similar structure and function to the
other NALP3 inflammasome family members (6,8,9,25).
As the levels of NALP3 were detected in this study, these can be
used to infer that the P2X7 receptor may be involved in the
activation of the NALP3 inflammasome. However, further studies are
required to confirm this hypothesis.
In conclusion, the results of this study
demonstrated that the activity of the P2X7 receptor is associated
with the expression of NALP3. All the results indicate that the
activated P2X7 receptor in murine P388D1 macrophage-like cells
mediates the upregulation of NALP3 expression of NALP3, which is
closely associated with the activation of the NALP3 inflammasome.
The results of this study indicate that in the inflammatory
reaction, macrophages that participate in the activation of the
P2X7 receptor may have an important role in activating the NALP3
inflammasome.
Acknowledgements
This study was supported by grants from the NSFC
(no. 81372669), the State Key Development Program of Basic Research
of China (no. 2012CB822103) and the Department of Science and
Technology for Liaoning (no. 2012225020).
References
|
1
|
Bone RC, Balk RA, Cerra FB, et al:
Definitions for sepsis and organ failure and guidelines for the use
of innovative therapies in sepsis. The ACCP/SCCM Consensus
Conference Committee American College of Chest Physicians/Society
of Critical Care Medicine. Chest. 101:1644–1655. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nyström PO: The systemic inflammatory
response syndrome: definitions and aetiology. J Antimicrob
Chemother. 41(Suppl A): 1–7. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang XP and Tian H: Pathogenesis of
pancreatic encephalopathy in severe acute pancreatitis.
Hepatobiliary Pancreat Dis Int. 6:134–140. 2007.PubMed/NCBI
|
|
4
|
Ono S, Ichikura T and Mochizuki H: The
pathogenesis of the systemic inflammatory response syndrome and
compensatory antiinflammatory response syndrome following surgical
stress. Nihon Geka Gakkai Zasshi. 104:499–505. 2003.(in Japanese).
PubMed/NCBI
|
|
5
|
Levels JH, Lemaire LC, van den Ende AE, et
al: Lipid composition and lipopolysaccharide binding capacity of
lipoproteins in plasma and lymph of patients with systemic
inflammatory response syndrome and multiple organ failure. Crit
Care Med. 31:1647–1653. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kihlmark M, Rustum C, Eriksson C, et al:
Correlation between nucleocytoplasmic transport and
caspase-3-dependent dismantling of nuclear pores during apoptosis.
Exp cell Res. 293:346–356. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martinon F, Pétrilli V, Mayor A, et al:
Gout-associated uric acid crystals activate the NALP3 inflammasome.
Nature. 440:237–241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Antonopoulos C, Cumberbatch M, Dearman RJ,
et al: Functional caspase-1 is required for Langerhans cell
migration and optimal contact sensitization in mice. J Immunol.
166:3672–3677. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aravind L, Dixit VM and Koonin EV: The
domains of death: evolution of the apoptosis machinery. Trends
Biochem Sci. 24:47–53. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kummer JA, Broekhuizen R, Everett H, et
al: Inflammasome components NALP 1 and 3 show distinct but separate
expression profiles in human tissues suggesting a site-specific
role in the inflammatory response. J Histochem Cytochem.
55:443–452. 2007. View Article : Google Scholar
|
|
11
|
Kanneganti TD, Ozören N, Body-Malapel M,
et al: Bacterial RNA and small antiviral compounds activate
caspase-1 through cryopyrin/Nalp3. Nature. 440:233–236. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Villani AC, Lemire M, Fortin G, et al:
Common variants in the NLRP3 region contribute to Crohn’s disease
susceptibility. Nature Genet. 41:71–76. 2008. View Article : Google Scholar
|
|
13
|
Xia Z, Wang G, Wan C, et al: Expression of
NALP3 in the spleen of mice with portal hypertension. J Huazhong
Univ Sci Technolog Med Sci. 30:170–172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hartwig W, Werner J, Jimenez RE, et al:
Trypsin and activation of circulating trypsinogen contribute to
pancreatitis-associated lung injury. Am J Physiol. 277:G1008–G1016.
1999.PubMed/NCBI
|
|
15
|
Bostanci N, Emingil G, Saygan B, et al:
Expression and regulation of the NALP3 inflammasome complex in
periodontal diseases. Clin Exp Immunol. 157:415–22. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ting JP, Kastner DL and Hoffman HM:
Caterpillers, pyrin and hereditary immunological disorders. Nature
Rev Immunol. 6:183–195. 2006. View
Article : Google Scholar
|
|
17
|
Codocedo JF, Godoy JA, Poblete MI, et al:
ATP induces NO production in hippocampal neurons by P2X(7) receptor
activation independent of glutamate signaling. PloS One.
8:e576262013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kanneganti TD, Lamkanfi M, Kim YG, et al:
Pannexin-1-mediated recognition of bacterial molecules activates
the cryopyrin inflammasome independent of Toll-like receptor
signaling. Immunity. 26:433–443. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Willingham SB, Allen IC, Bergstralh DT, et
al: NLRP3 (NALP3, Cryopyrin) facilitates in vivo caspase-1
activation, necrosis, and HMGB1 release via inflammasome-dependent
and-independent pathways. J Immunol. 183:2008–2015. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arandjelovic S, McKenney KR, Leming SS, et
al: ATP induces protein arginine deiminase 2-dependent
citrullination in mast cells through the P2X7 purinergic receptor.
J Immunol. 189:4112–4122. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Surprenant A, Rassendren F, Kawashima E,
et al: The cytolytic P2Z receptor for extracellular ATP identified
as a P2X receptor (P2X7). Science. 272:735–738. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pelegrin P and Surprenant A: Pannexin-1
mediates large pore formation and interleukin-1beta release by the
ATP-gated P2X7 receptor. EMBO J. 25:5071–5082. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Duncan JA, Gao X, Huang MT, et al:
Neisseria gonorrhoeae activates the proteinase cathepsin B to
mediate the signaling activities of the NLRP3 and ASC-containing
inflammasome. J Immunol. 182:6460–6469. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qu Y, Ramachandra L, Mohr S, et al: P2X7
receptor-stimulated secretion of MHC class II-containing exosomes
requires the ASC/NLRP3 inflammasome but is independent of
caspase-1. J Immunol. 182:5052–5062. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cerwenka A, Baron JL and Lanier LL:
Ectopic expression of retinoic acid early inducible-1 gene (RAE-1)
permits natural killer cell-mediated rejection of a MHC class
I-bearing tumor in vivo. Proc Natl Acad Sci USA. 98:11521–11526.
2001. View Article : Google Scholar : PubMed/NCBI
|