1. Introduction
Gastric cancer (GC) is one of the most common types
of malignant tumor, with GC morbidity and mortality worldwide
ranked fourth and second highest, respectively, out of all types of
cancer. It is estimated that ~989,600 individuals were diagnosed
with GC and 738,000 GC patients succumbed to the disease worldwide
in 2008 (1). Thus, GC seriously
threatens human health and is a global cause of mortality. With
improvements in clinical diagnosis and treatment, the five-year
survival rate of patients with early GC has significantly
increased, but the prognosis of patients with advanced GC remains
poor. This disappointing prognosis is mainly due to a lack of
effective therapeutic measures. Although a large number of studies
regarding GC pathogenesis have been conducted, the molecular
mechanism of GC remains poorly understood.
Epigenetic alterations have been shown to exert a
critical role in gastric tumorigenesis. Various types of epigenetic
modifications have been detected, of which DNA methylation was the
first to be elucidated and has been the most widely analyzed. DNA
methylation of CpG islands (CGIs) is a critical mechanism that
results in the ectopic expression of genes, including microRNA
(miRNA) genes (2,3). Increasing evidence has shown that a
number of important cellular functions, such as proliferation,
differentiation and apoptosis, are regulated by miRNAs.
Furthermore, dysregulated miRNAs have been confirmed to be
associated with the development of numerous types of human cancer
(4). In recent years, studies
concerning DNA methylation of miRNAs in GC have been frequently
reported, and these studies deepen the knowledge of how the
epigenetic regulation of miRNAs results in GC pathogenesis,
indicating novel therapeutic strategies for GC. The present review
provides a brief introduction to DNA methylation and miRNAs, and
summarizes the role of promoter-associated methylation of miRNAs in
GC.
2. DNA methylation
DNA methylation and
methyltransferases
DNA methylation, as the best-known epigenetic
modification, occurs when a methyl group (CH3) is added
to the cytosine-C5 position in the CpG dinucleotide (5). In the human genome, the distribution
of CpG dinucleotides (CpGs) is classified into two types: Diffused
distribution and local accumulation. Approximately 80% of CpGs that
usually remain heavily methylated exhibit a diffused distribution
in repetitive DNA sequences, whereas the other CpGs that are always
unmethylated in healthy tissues exhibit local accumulation
(6,7). A ~1 kb genomic region with a CpG
cluster that is usually hypomethylated in healthy cells is known as
a CGI (8). These CpG-rich
sequences, detected in approximately half of human genes, are
predominantly located in the promoter region in which transcription
is initiated, but may also be occasionally identified in the first
exon or in the intronic regions of the gene (9,10).
The methylation of promoter CGIs has been found to exert a critical
role in the regulation of gene expression, genomic imprinting, the
inactivation of the X-chromosome in females and in tumorigenesis
(11).
The addition of methyl groups from
S-adenosylmethionine to C5 is catalyzed by DNA methyltransferases
(DNMTs). The DNMT family includes the following five members:
DNMT1, DNMT2, DNMT3a, DNMT3b and DNMT3L. DNMT1, as the most
abundant DNMT, is involved in maintaining the methylation patterns
by replicating these patterns during the S phase of mitosis
(12), whereas DNMT3a and 3b are
involved in de novo methylation, which is associated with
normal development and disease (13). DNMT1 cooperates with the DNMT3
family to establish and maintain the CGI methylation patterns.
However, DNMT2 exerts limited effects in the methylation of CGIs in
DNA and DNMT3L is deficient in catalytic activity, although the
latter molecule may enhance DNMT3a/3b catalytic activity through
direct binding to the catalytic domains (14). Various DNMT inhibitors have been
employed in attempts to treat a number of human diseases, including
cancer, caused by CGI DNA methylation; 5-aza-2′-deoxycytidine
(5-aza-CdR) may be the most commonly utilized. 5-Aza-CdR is a
cytidine analog, which may be incorporated into DNA nucleotides and
be covalently coupled with DNMTs, resulting in DNMT dysfunction
(15). 5-Aza-CdR has been widely
employed to reactivate tumor-suppressor genes that have been
silenced due to the high expression levels of DNMTs (16).
DNA methylation and GC
Since the first study was published in 1983
(17), the association between DNA
methylation and cancer has been widely investigated. There is
increasing evidence that abnormal DNA methylation is a critical
mechanism in the pathogenesis of cancer. Aberrant methylation
predominantly consists of hypermethylation or hypomethylation. DNA
hypomethylation is primarily global, and usually occurs in
repetitive DNA sequences, such as the Alu and LINE sequences.
However, gene-specific hypomethylation, occurring in certain
distinct regions, particularly promoter-associated CGIs, has also
been observed. Genome-wide hypomethylation may result in
chromosomal instability, reactivation of transposable elements and
loss of imprinting (6,18), and gene-specific hypomethylation is
correlated with the upregulation of oncogenes (19,20).
However, although hypomethylation was first reported earlier than
hypermethylation, the hypermethylation of CGIs in promoter regions
has received more attention in recent decades. Furthermore, the
mechanism of transcription silencing by promoter CGI
hypermethylation is more clearly understood than the carcinogenic
mechanism of DNA hypomethylation. Methylated CGIs promote chromatin
structural stability; the binding of transcription factors to CGIs
is inhibited, which results in the silencing of genes (21). The expression of the majority of
tumor-suppressor and DNA repair genes is regulated by CGI
methylation, and hypermethylation in the promoter region of these
genes may result in the inactivation of genes through transcription
silencing, which contributes to the formation of cancer. In
addition, according to the two-hit hypothesis proposed by Knudson
(22), DNA hypermethylation of
tumor-suppressor genes acts as the second hit following gene
mutation, which is the first. Furthermore, compared with mutations,
unusual methylations in the promoter region are more common and may
be detected more easily. Studies examining numerous types of
cancer, such as gastric and colorectal cancer, have shown that a
change in hypomethylation status does not affect the
hypermethylation of CGIs in the promoter, which suggests no clear
association between genome-wide hypomethylation and regional
hypermethylation (23).
Abnormal methylation in the form of both DNA
hypomethylation and local hypermethylation has been observed in GC
(24,25). In a number of genes, as compared
with genome-wide demethylation, more attention has been focused on
increased methylation in promoter-associated CGIs in GC. Increasing
evidence has indicated that the aberrant DNA methylation of
tumor-suppressor genes is involved in development, progression,
metastasis and invasion of GC (25). At present, numerous protein-coding
tumor suppressor genes have been demonstrated to exhibit abnormal
promoter-associated CGI methylation. These genes are mainly
associated with various cellular processes, including regulation of
the cell cycle, cell differentiation or apoptosis, signal
transduction and DNA repair. In addition, in recent years, the
expression of miRNAs has also been identified to be affected by DNA
methylation, which contributes to tumorigenesis.
3. MicroRNA and cancer
Initially, RNA was identified only as a mediator of
the transition of information from DNA to proteins; however,
increasing evidence has indicated that RNA exerts a key role in
various life processes. miRNA is a type of endogenous,
single-stranded, non-coding small RNA, 18–22 nucleotides (nts) in
length, which is involved in various biological processes and
remains highly conserved during evolution. Since the first report
in 1993 (26) and its true
recognition in the early 2000s, miRNA has been one of the fastest
growing research areas in molecular biology. To date, thousands of
miRNAs have been identified in animals and plants, as well as
viruses. More than 1,000 miRNAs belong to humans, regulating ~30%
of human genes (27).
Subsequent to the downregulation of two miRNAs in
chronic lymphocytic leukemia, miR-15 and miR-16, first being
reported (28), the association
between miRNAs and human cancer has been widely investigated. Due
to the finding that miRNAs commonly require only partial sequence
homology to the 3′-untranslated region (3′-UTR) of target mRNAs, a
single miRNA may have numerous mRNA targets and, conversely, a
single mRNA may also be targeted by numerous miRNAs (29). Therefore, miRNAs may regulate
numerous mRNAs that are closely associated with various types of
cancer. In addition, genome-wide studies have indicated that ~50%
miRNAs are located at cancer-associated genomic regions or fragile
sites of chromosomes (30),
further confirming the closely association between miRNAs and
cancer.
miRNAs may function as tumor suppressor genes
through the regulation of target genes, which subsequently exhibit
lower expression levels. The miR-34 family, itself targeted by p53
via a positive feedback mechanism, is universally inactivated in
various types of cancer. miR-34 molecules act as tumor suppressors
by regulating the expression of the corresponding targets. For
instance, one study found that miR-34a caused the suppression of
silent information regulator 1 (SIRT1), which may function as an
oncogene. Inhibition of SIRT1 may increase p53 activity and in-turn
result in upregulation of miR-34a, to further induce SIRT1
silencing (31).
Conversely, certain miRNAs, such as miR-27a, are
overexpressed in cancer and function as oncogenes. miR-27a has been
found to be overexpressed in ovarian cancer, breast cancer and GC,
and acts as an oncogene through the suppression of targets such as
ZBTB10, Myt-1 and prohibitin (32–34).
Notably, a few miRNAs act as tumor suppressor genes in certain
types of cancer and function as oncogenes in other types of cancer.
For instance, miR-25 has been shown to be downregulated in colon
cancer tissues, as compared with normal mucosal tissues (35), and has demonstrated the ability to
inhibit colon cancer cell growth and migration through
downregulation of a target gene, Smad7, which is involved in the
proliferation and metastasis of colon cancer (35). However, miR-25 has also been
reported to be upregulated in esophageal squamous cell carcinoma
(ESCC) tissues, and miR-25 overexpression was observed to induce
ESCC cell metastasis and invasion via binding to the 3′UTR of
epithelial cadherin (36). These
studies demonstrate that the function of miRNAs may be
tissue-specific.
4. Dysregulation of miRNAs in GC
The dysregulation of miRNAs in GC and the subsequent
effects have been widely analyzed. Abnormal expression of miRNAs
identified in GC has been implicated in the regulation of the cell
cycle, apoptosis, invasion and metastasis. For instance, the
miR-222-221 oncogenic cluster, associated with the cell cycle, has
been reported to be frequently overexpressed in GC (37). miR-222-221 exhibits greater ectopic
expression in GC tissues as compared with normal tissues, and
downregulates the protein levels of p27 and p57 through binding to
target sites; p21, p27 and p57 are all included in the p21 family,
and act as cyclin-dependent kinase (CDK) inhibitors, suppressing
the progression of cell cycle (37). miR-15b, miR-16, miR-181b and miR-34
have the same downstream target, B-cell lymphoma 2 (BCL-2), which
exhibits an antiapoptotic function; overexpression of these miRNAs
inhibits the expression of BCL-2 and induces apoptosis.
Furthermore, through the negative regulation of BCL-2 expression,
miR-15a, miR-16 and miR-181b may contribute to the repression of
multidrug resistance associated with the modulation of apoptosis in
human GC cell lines (38–40). miR-21 negatively regulates
reversion-inducing-cysteine-rich protein with kazal motifs (RECK),
a molecule that represses GC metastasis and angiogenesis, and
miR-21 is also involved in cancer tissue invasion and lymph node
metastasis via reducing the protein levels of phosphatase and
tensin homolog (PTEN), a tumor-suppressor gene (41,42).
Furthermore, overexpression of miR-21 leads to inhibition of
programmed cell death 4 (PDCD4) expression, which results in lymph
node metastasis and venous invasion (43).
The expression features and the functions of miRNAs
have been widely investigated, but the underlying mechanism of
miRNA dysregulation is less well-known. However, aberrant miRNA
expression has been shown to be mediated by a number of mechanisms,
including gene mutation, alteration of the number of DNA copies,
defective transcription and dysregulation of miRNA biogenesis
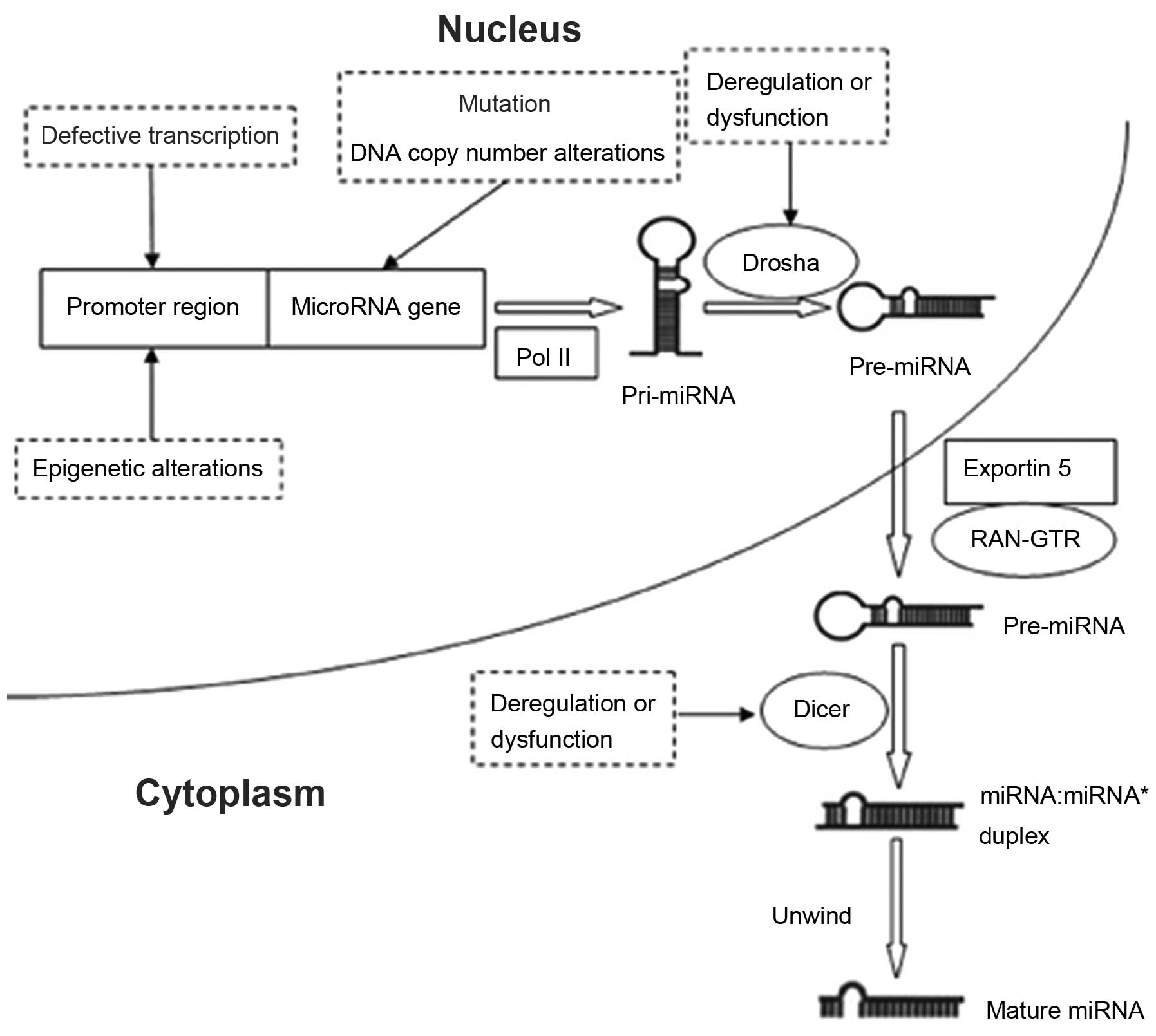
components, as well as epigenetic alteration (44) (Fig.
1). Among these mechanisms, DNA methylation may be pivotal in
the dysregulation of miRNAs, as methylation mainly represses the
expression of tumor-suppressor miRNAs that contain CGIs in promoter
regions. In addition, expression of these miRNAs may be restored
through demethylation, which indicates that demethylation treatment
may serve as a novel therapeutic approach. Thus, DNA methylation of
miRNAs requires further investigation in different types of cancer,
including GC.
5. Aberrant methylation of miRNAs in GC
miR-124a and miR-34b/miR-34c
Recently, aberrant miRNA methylation in GC has
received a great deal of attention. Ando et al (45) confirmed that the promoter regions
of miR-124a-1, miR-124a-2 and miR-124a-3 were methylated in GC cell
lines and samples, which led to the loss of expression of the
miRNAs. However, treatment of the cell lines with the 5-aza-CdR
demethylation drug resulted in restoration of the miRNAs. This
study may have been the first in which DNA methylation was
identified as a mechanism of miRNA silencing in GC. Furthermore,
the authors found that H. pylori infection induced promoter
methylation of the miRNAs and increased the risk of GC. In
addition, in individuals without H. pylori infection, the
methylation levels of miR-124a-1, miR-124a-2 and miR-124a-3 were
significantly higher in the non-cancerous gastric mucosae of
patients with GC than those in the normal mucosae of healthy
individuals, which indicates that miR-124a methylation is involved
in epigenetic field defects. Therefore, the methylation of members
of the miR-124a family may serve a tumor biomarker for the early
diagnosis of GC and treatment of H. pylori infection may
reduce the risk of GC.
In addition to the miR-124a family, miR-34b and
miR-34c have also been observed to be silenced by aberrant
promoter-associated CGI methylation in the majority of GC cell
lines and tissues. Restoration of miR-34b and miR-34c promotes the
repression of cell growth, which demonstrates that miR-34b and
miR-34c function as tumor-suppressor genes (46). The authors also demonstrated that
DNA methylation of miR-34b and miR-34c was associated with H.
pylori infection in normal individuals, and that the
methylation levels of miR-34b and miR-34c in the non-cancerous
gastric mucosae of patients with multiple GC were higher than those
of patients with single GC. Recently, Suzuki et al (47) found that aberrant methylated
miR-34b and miR-34c could be an important predictive biomarker of
metachronous GC risk. Therefore, the aberrant methylation of
miR-34b and miR-34c may be a diagnostic or predictive biomarker,
and the re-expression of miR-34b and miR-34c using demethylation
drugs may be a novel therapeutic strategy for GC or a useful
preventative measure.
miR-181c
DNA methylation as a mechanism of miRNA silencing in
GC has also been identified in miR-181c. Hashimoto et al
(48) observed that miR-181c
expression levels were reduced in 9 of 16 GC samples, as compared
with adjacent non-cancerous mucosae, and that miR-181c was
upregulated following demethylation treatment of GC cells with
5-aza-CdR. In addition, the authors analyzed the methylation status
of miR-181c using bisulfate sequencing and methylation-specific
polymerase chain reaction (MSP) analysis, and found that miR-181c
was silenced following CGI methylation. These results indicate that
reduced miR-181c expression levels are associated with DNA promoter
methylation. As with miR-124a and miR-34b/miR-34c, miR-181c
methylation signals have also been identified in certain
non-cancerous tissues corresponding to GC samples, which implies a
defect in the epigenetic field. In order to investigate the effect
of miR-181c expression, Hashimoto et al (48) transfected two GC cell lines with
pre-miR-181c and observed inhibition of cell growth, indicating
that miR-181c functions as a tumor-suppressor gene. Through further
analysis of the underlying mechanism of action, the authors
observed that miR-181c acts via repression of the NOTCH4 and
v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (KRAS) target
genes. NOTCH4 is involved in cell fate determination and KRAS is
known as a proto-oncogene that belongs to the RAS family. In
conclusion, the study indicated that DNA methylation of miR-181c
may be a biomarker of GC and demethylation with epigenetic drugs
may be a novel therapeutic approach for GC or other types of
cancer. Notably, in this study, miR-181c was only overexpressed in
2 of the 16 GC samples as compared with corresponding non-cancerous
tissues. However, Cui et al (49) reported that increased expression
levels of miR-181c were closely associated with the progression and
prognosis of GC. Thus, further studies are required to clarify the
exact role of miR-181c in the development, progression and
prognosis of GC.
miR-137
Loss of miR-137 expression has been reported in
numerous types of cancer and miR-137 promoter hypermethylation has
been observed as an important determinant of miR-137 downregulation
in colorectal cancer (50).
Therefore, Chen et al (51)
proposed that miR-137 may be downregulated in GC due to increased
methylation of promoter-associated miR-137 CGIs. By use of
quantitative polymerase chain reaction and MSP, the authors found
that the expression levels of miR-137 were frequently lower in GC
tissues than those in the corresponding normal tissues. The authors
further demonstrated that miR-137 was expressed at lower levels in
methylated GC tissues and that demethylation resulted in the
re-expression of miR-137, indicating that miR-137 expression levels
are negatively correlated with promoter methylation. In addition,
the study demonstrated that transfection of AGC and MKN-45 gastric
cancer cell lines with pre-miR-137 inhibited the cell cycle at the
G1-S phase and induced apoptosis, which demonstrates that miR-137
may be involved in GC carcinogenesis. As determined by the results
of target gene prediction and a luciferase reporter assay, the
authors revealed cell division cycle 42 (Cdc42) mRNA to be a direct
target of miR-137. Furthermore, cells transfected with pre-miR-137
exhibited reduced expression levels of Cdc42 and Cyclin D1. The
authors also found that apoptosis was induced in GC cells
transfected with small interfering RNA targeting Cdc42. Therefore,
cell cycle arrest may be caused by the inhibition of Cyclin D1 and
cell apoptosis may result from the inactivation of Cdc42. The study
detected a novel mechanism of GC pathogenesis, which may help in
the identification of a novel potential therapeutic target in
GC.
miR-9 and miR-212
In humans, the miR-9 family has three members,
miR-9-1, miR-9-2 and miR-9-3, which are located on chromosomes 1, 5
and 15, respectively. Epigenetic repression of miR-9 molecules due
to aberrant promoter hypermethylation was first reported in breast
cancer (52). Recently, CGI
hypermethylation-mediated silencing of all three miR-9 family
members was observed in GC; this silencing was reversed in GC cell
lines following treatment with 5-aza-CdR (53). Reactivation of miR-9 family members
promotes tumor suppressor features, including the repression of
cell proliferation and migration. The miR-9 family members may
exert tumor-suppressive roles through the inhibition of target
genes, such as NF-κB1 and RAB34 (54,55).
As with miR-9, the downregulation of miRNA-212 has
been reported to be partly associated with CGI hypermethylation,
which is reversed by 5-aza-CdR treatment (2). In addition, miR-212 may function as a
tumor suppressor through inhibition of the MYC and MECP2 potential
target genes (2). In conclusion,
the demethylation of miR-9 and miR-212 may inhibit the progression
of GC and these molecules may be novel therapeutic targets.
miR-148a
Studies have shown that miR-148a is downregulated
and DNMT1 is overexpressed in gastric cancer cells. However, the
mechanisms underlying the aberrant expression of miR-148a and
DNMT1, and their association in gastric cancer remain unknown
(56,57). The silencing of miR-148a has been
revealed to be associated with promoter hypermethylation in
pancreatic cancer (58).
Therefore, Zhu et al (16)
inferred that miR-148a silencing in GC may also be associated with
aberrant methylation. This study demonstrated that
promoter-associated CGIs were methylated and miR-148a expression
levels reduced in the majority of GC samples. As determined by
TargetScan miRNA target prediction software, miR-148a may directly
target DNMT1, which replicates methylation patterns. Moreover, the
results revealed that overexpressed DNMT1 led to miR-148a
silencing, whereas restoration of miR-148a resulted in the
downregulation of DNMT1. Therefore, there may be a circulating
regulation between miR-148a and DNMT1 expression. Furthermore, the
authors observed that the reactivation of miR-148a repressed cell
growth. In addition, Zheng et al (59) identified that overexpression of
miR-148 suppressed the metastasis and invasion of GC through
repression of a direct target, Rho-associated protein kinase 1
(ROCK1), a potential metastasis promoter. Thus, miR-148a is a
potential biomarker of GC prognosis, and restoration of miR-148a
may exert an important role in inhibiting the development,
metastasis and invasion of GC.
miR-10b
miR-10b exerts an oncogenic role in numerous types
of cancer cell, including breast cancer, malignant glioma,
esophageal cancer and hepatocellular carcinoma (HCC) cells, and
induces metastasis and invasion by regulation of target genes
(60–63). For example, miR-10b is
overexpressed in HCC and inhibits the translation of cell adhesion
molecule 1 by direct targeting, which promotes HCC metastasis and
invasion (60). However, according
to results from methylation array and bisulfate pyrosequencing
analysis, Kim et al (64)
reported that miR-10b was downregulated in GC due to promoter
region hypermethylation. The authors found that treatment of GC
cells with 5-aza-CdR resulted in the re-expression of miR-10b,
which further indicated that miR-10b silencing was closely
correlated with promoter methylation. Microtubule-associated
protein RP/EB family member 1 (MAPRE1), also known as end-binding
protein 1, a molecule mapped to chromosome 20q11.2, is upregulated
in cancer, and induces cell proliferation and represses apoptosis
through the β-catenin/T-cell factor (TCF) signaling pathway, which
indicates that MAPRE1 may function as an oncogene (65). Kim et al (64) identified that miR-10b silencing in
GC cells through methylation led to MAPRE1 overexpression and
induced cell proliferation, whereas restoration of miR-10b resulted
in inhibition of MAPRE1 and reduced the rate of cell growth and
colony formation. In addition, the authors demonstrated using a
luciferase reporter assay that miR-10b binds to the 3′UTR of
MAPRE1. Therefore, MAPRE1 is a direct and functional target gene of
miR-10b, and may repress cell growth through the β-catenin/TCF
signaling pathway in GC. In conclusion, DNA methylation of miR-10b
may act as a biomarker for estimating the risk of GC and the
regulation of miR-10b may have therapeutic potential.
miR-195 and miR-378
Epigenetic inactivation of miR-195 and miR-378 has
recently been reported in GC (66). miRNA silencing in cancer is usually
related to abnormal promoter methylation, and thus Deng et
al (66) hypothesized that the
downregulation of miR-195 and miR-378 is associated with
promoter-associated methylation. The authors observed that the
miR-195 and miR-378 promoters contained CGIs, and that the
demethylation treatment of two GC cell lines with 5-aza-CdR
resulted in the re-expression of these two miRNAs, which confirmed
the hypothesis previously proposed. CDK6 as a direct target of
miR-195 in hepatocellular carcinoma cells has been reported and the
3′UTR of vascular endothelial growth factor (VEGF) has been
demonstrated to contain a potential binding site for miR-378
(67,68). Therefore, Deng et al
(66) inferred that CDK6 may be
the potential target gene of miR-195 in GC and that VEGF may be a
candidate target gene of miR-378. The study results revealed that
the expression levels of CDK6 and VEGF were negatively correlated
with miR-195 and miR-378 expression levels, respectively, with the
latter two molecules inhibiting the expression of the former two.
Furthermore, according to the results of analyses conducted using
two software packages and luciferase reporter assays, the authors
identified CDK6 as a potential direct target of miR-195. CDK6 is an
important regulatory molecule of the G1 cell cycle phase and
downregulation of CDK6 may result in G1-S phase arrest. Aberrant
VEGF expression is associated with angiogenesis, metastasis and
repression of apoptosis. The authors also found that miR-195 and
miR-378 act as tumor-suppressor genes via the inhibition of cell
growth resulting from interruption of the cell cycle. The G0/G1
phase arrest caused by miR-195 may be due to the suppression of
CDK6, but the mechanism of G2/M phase arrest caused by miR-378 is
not clear. In conclusion, the study indicated that
promoter-associated CGI methylation may be a critical factor
resulting in the silencing of miR-195 and miR-378, and that the
restoration of the two miRNAs may have therapeutic potential in
GC.
Other miRNA molecules
Saito et al (69) reported that miR-512-5p became
activated following epigenetic treatment of gastric cancer cells
with 5-aza-CdR and 4-phenylbutyric acid, which indicates that
miR-512-5p may be inactivated by DNA methylation. However, the
authors did not further analyze the miR-512-5p methylation status
in GC. Promoter-associated CGI methylation has been reported to
inhibit the transcriptional activity of miR-196b in GC (20). Unlike the above-described miRNAs,
miR-196b functions as an oncogene and exhibits hypomethylation in
gene promoter regions in primary GC. A recent study revealed that
miR-219-2-3p may act as a tumor suppressor in GC through modulation
of extracellular signal-regulated kinase (ERK) 1/2-associated
signaling pathways (3). In
addition, reduced miR-219-2-3p expression levels may be partly
associated with DNA methylation. The lower expression levels of
miR-219-2-3p are closely associated with tumor staging, and
reactivation may inhibit GC cell proliferation and migration, and
induce apoptosis, indicating the potential for miR-219-2-3p as a
novel therapeutic target and prognostic indicator. Downregulation
of miR-338-3p due to hypermethylation in the promoter region has
also been detected in GC (70).
miR-338-3p exerts antitumor effects through modulation of the
synovial sarcoma X breakpoint protein (SSX2I) oncogene, and the
respective promoter methylation status may be a useful diagnostic
biomarker of GC. The role of DNA methylation of miRNAs in GC
reported in recent years is reviewed in Table I.
 | Table ISummary of methylated miRNAs in
GC. |
Table I
Summary of methylated miRNAs in
GC.
| miRNA | Expression levels
in GC tissues | Target genes | References |
|---|
| miR-124a-1-3 | Downregulated | - | (39) |
| miR-34b/c | Downregulated | CDK4, MET,
CCNE2 | (40,41) |
| miR-181c |
Upregulated/downregulated | NOTCH4, KRAS | (42) |
| miR-137 | Downregulated | Cdc42 | (45) |
| miR-9 | Downregulated | NF-κB1, RAB34 | (47–49) |
| miR-212 | Downregulated | MYC, MeCP2 | (50) |
| miR-148a | Downregulated | DNMT1, ROCK1 | (52,53) |
| miR-10b | Downregulated | MAPRE1 | (58) |
| miR-195 | Downregulated | CDK6 | (60) |
| miR-378 | Downregulated | VEGF | (60) |
| miR-196b | Upregulated | - | (16) |
| miR-512-5p | Downregulated | MCL1 | (63) |
| miR-219-2-3p | Downregulated | - | (64) |
| miR-338-3p | Downregulated | SSX2IP | (65) |
6. Conclusions and future perspectives
At present, the research regarding the aberrant
methylation of miRNAs in GC remains far from complete and studies
have predominantly analyzed tumor-suppressor genes. Therefore, more
studies are required to identify DNA methylation of novel miRNAs in
GC, including oncogenic miRNAs. DNA methylation is a reversible
process and demethylation treatment may have great potential in
cancer treatment. As DNA methylation is mainly catalyzed by members
of the DNMT family, DNMT inhibitors may function as demethylation
drugs and act as anticancer agents. Among these demethylation
inhibitors, 5-aza-CdR is perhaps the most commonly investigated.
However, these demethylation inhibitors not only restore the
expression of tumor-suppressor miRNAs but also increase the
expression levels of particular oncogentic miRNAs, such as
miR-196b. Therefore, as demethylation inhibitors exert a dual
function, further studies are required to improve drug targeting.
For example, current studies have revealed that miRNA mimics, used
in place of miRNAs, effectively alleviated the loss of miRNA
expression and may be a potential therapeutic strategy (71).
In conclusion, the methylation of miRNAs is involved
in GC pathogenesis; therefore, modification of this process is
important in treatment. Thus, more in-depth and extensive studies
concerning the methylation of miRNAs are required. With the
continuous advancement of miRNA methylation research, great
progress in the diagnosis and treatment GC is likely.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu L, Wang F, Xu XF, et al:
Down-regulation of miR-212 expression by DNA hypermethylation in
human gastric cancer cells. Med Oncol. 28(Suppl 1): S189–S196.
2011. View Article : Google Scholar
|
|
3
|
Lei H, Zou D, Li Z, et al:
MicroRNA-219-2-3p functions as a tumor suppressor in gastric cancer
and is regulated by DNA methylation. PLoS One. 8:e603692013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tutar L, Tutar E and Tutar Y: MicroRNAs
and Cancer; an Overview. Curr Pharm Biotechnol. 15:430–437. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Taby R and Issa JP: Cancer Epigenetics. CA
Cancer J Clin. 60:376–392. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dunn BK: Hypomethylation: one side of a
larger picture. Ann NY Acad Sci. 983:28–42. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Frühwald MC and Plass C: Global and
gene-specific methylation patterns in cancer: aspects of tumor
biology and clinical potential. Mol Genet Metab. 75:1–16. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Robertson KD: DNA methylation and human
disease. Nat Rev Genet. 6:597–610. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takai D and Jones PA: Comprehensive
analysis of CpG islands in human chromosomes 21 and 22. Proc Natl
Acad Sci USA. 99:3740–3745. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Herman JG and Baylin SB: Gene silencing in
cancer in association with promoter hypermethylation. N Engl J Med.
349:2042–2054. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bird A: DNA methylation patterns and
epigenetic memory. Genes Dev. 16:6–21. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leonhardt H, Page AW, Weier HU and Bestor
TH: A targeting sequence directs DNA methyltransferase to sites of
DNA replication in mammalian nuclei. Cell. 71:865–873. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Okano M, Bell DW, Haber DA and Li E: DNA
methyltransferases Dnmt3a and Dnmt3b are essential for de novo
methylation and mammalian development. Cell. 99:247–257. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gowher H, Liebert K, Hermann A, Xu G and
Jeltsch A: Mechanism of stimulation of catalytic activity of Dnmt3A
and Dnmt3B DNA-(cytosine-C5)-methyltransferases by Dnmt3L. J Biol
Chem. 280:13341–13348. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Santi DV, Garrett CE and Barr PJ: On the
mechanism of inhibition of DNA-cytosine methyltransferases by
cytosine analogs. Cell. 33:9–10. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu A, Xia J, Zuo J, et al: MicroRNA-148a
is silenced by hypermethylation and interacts with DNA
methyltransferase 1 in gastric cancer. Med Oncol. 29:2701–2709.
2012. View Article : Google Scholar
|
|
17
|
Feinberg AP and Vogelstein B:
Hypomethylation distinguishes genes of some human cancers from
their normal counterparts. Nature. 301:89–92. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eden A, Gaudet F, Waghmare A and Jaenisch
R: Chromosomal instability and tumors promoted by DNA
hypomethylation. Science. 300:4552003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kwon OH, Park JL, Kim M, et al: Aberrant
up-regulation of LAMB3 and LAMC2 by promoter demethylation in
gastric cancer. Biochem Biophys Res Commun. 406:539–545. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsai KW, Hu LY, Wu CW, et al: Epigenetic
regulation of miR-196b expression in gastric cancer. Genes
Chromosomes Cancer. 49:969–980. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Costello JF, Frühwald MC, Smiraglia DJ, et
al: Aberrant CpG-island methylation has non-random and
tumour-type-specific patterns. Nat Genet. 24:132–138. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Knudson AG: Two genetic hits (more or
less) to cancer. Nat Rev Cancer. 1:157–162. 2001. View Article : Google Scholar
|
|
23
|
Ross JP, Rand KN and Molloy PL:
Hypomethylation of repeated DNA sequences in cancer. Epigenomics.
2:245–269. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bae JM, Shin SH, Kwon HJ, et al: ALU and
LINE-1 hypomethylation in multistep gastric carcinogenesis and
their prognostic implications. Int J Cancer. 131:1323–1331. 2012.
View Article : Google Scholar
|
|
25
|
Zhao C and Bu X: Promoter methylation of
tumor-related genes in gastric carcinogenesis. Histol Histopathol.
27:1271–1282. 2012.PubMed/NCBI
|
|
26
|
Lee RC, Feinbaum RL and Ambros V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Calin GA, Dumitru CD, Shimizu M, et al:
Frequent deletions and down-regulation of micro-RNA genes miR15 and
miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci
USA. 99:15524–15529. 2002. View Article : Google Scholar
|
|
29
|
Lewis BP, Shih IH, Jones-Rhoades MW,
Bartel DP and Burge CB: Prediction of mammalian microRNA targets.
Cell. 115:787–798. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Calin GA, Sevignani C, Dumitru CD, et al:
Human microRNA genes are frequently located at fragile sites and
genomic regions involved in cancers. Proc Natl Acad Sci USA.
101:2999–3004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yamakuchi M, Ferlito M and Lowenstein CJ:
miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci
USA. 105:13421–13426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mertens-Talcott SU, Chintharlapalli S, Li
X and Safe S: The oncogenic microRNA-27a targets genes that
regulate specificity protein transcription factors and the G2-M
checkpoint in MDA-MB-231 breast cancer cells. Cancer Res.
67:11001–11011. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu T, Tang H, Lang Y, Liu M and Li X:
MicroRNA-27a functions as an oncogene in gastric adenocarcinoma by
targeting prohibitin. Cancer Lett. 273:233–242. 2009. View Article : Google Scholar
|
|
34
|
Lai Y, Zhang X, Zhang Z, et al: The
microRNA-27a: ZBTB10-specificity protein pathway is involved in
follicle stimulating hormone-induced VEGF, Cox2 and survivin
expression in ovarian epithelial cancer cells. Int J Oncol.
42:776–784. 2013.
|
|
35
|
Li Q, Zou C, Zou C, et al: MicroRNA-25
functions as a potential tumor suppressor in colon cancer by
targeting Smad7. Cancer Let. 335:168–174. 2013. View Article : Google Scholar
|
|
36
|
Xu X, Chen Z, Zhao X, et al: MicroRNA-25
promotes cell migration and invasion in esophageal squamous cell
carcinoma. Biochem Biophys Res Commun. 421:640–645. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim YK, Yu J, Han TS, et al: Functional
links between clustered microRNAs: suppression of cell-cycle
inhibitors by microRNA clusters in gastric cancer. Nucleic Acids
Res. 37:1672–1681. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ji Q, Hao X, Meng Y, et al: Restoration of
tumor suppressor miR-34 inhibits human p53-mutant gastric cancer
tumorspheres. BMC Cancer. 8:2662008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xia L, Zhang D, Du R, et al: miR-15b and
miR-16 modulate multidrug resistance by targeting BCL2 in human
gastric cancer cells. Int J Cancer. 123:372–379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhu W, Shan X, Wang T, Shu Y and Liu P:
miR-181b modulates multidrug resistance by targeting BCL2 in human
cancer cell lines. Int J Cancer. 127:2520–2529. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang Z, Li Z, Gao C, et al: miR-21 plays
a pivotal role in gastric cancer pathogenesis and progression. Lab
Invest. 88:1358–1366. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang BG, Li JF, Yu BQ, Zhu ZG, Liu BY and
Yan M: microRNA-21 promotes tumor proliferation and invasion in
gastric cancer by targeting PTEN. Oncol Rep. 27:1019–1026.
2012.PubMed/NCBI
|
|
43
|
Motoyama K, Inoue H, Mimori K, et al:
Clinicopathological and prognostic significance of PDCD4 and
microRNA-21 in human gastric cancer. Int J Oncol. 36:1089–1095.
2010.PubMed/NCBI
|
|
44
|
Deng S, Calin GA, Croce CM, Coukos G and
Zhang L: Mechanisms of microRNA deregulation in human cancer. Cell
Cycle. 7:2643–2646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ando T, Yoshida T, Enomoto S, et al: DNA
methylation of microRNA genes in gastric mucosae of gastric cancer
patients: its possible involvement in the formation of epigenetic
field defect. Int J Cancer. 124:2367–2374. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Suzuki H, Yamamoto E, Nojima M, et al:
Methylation-associated silencing of microRNA-34b/c in gastric
cancer and its involvement in an epigenetic field defect.
Carcinogenesis. 31:2066–2073. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Suzuki R, Yamamoto E, Nojima M, et al:
Aberrant methylation of microRNA-34b/c is a predictive marker of
metachronous gastric cancer risk. J Gastroentero. 49:1135–1144.
2013. View Article : Google Scholar
|
|
48
|
Hashimoto Y, Akiyama Y, Otsubo T, Shimada
S and Yuasa Y: Involvement of epigenetically silenced microRNA-181c
in gastric carcinogenesis. Carcinogenesis. 31:777–784. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cui M, Yue L, Fu Y, Yu W, Hou X and Zhang
X: Association of microRNA-181c expression with the progression and
prognosis of human gastric carcinoma. Hepatogastroenterology.
60:961–964. 2013.PubMed/NCBI
|
|
50
|
Balaguer F, Link A, Lozano JJ, et al:
Epigenetic silencing of miR-137 is an early event in colorectal
carcinogenesis. Cancer Res. 70:6609–6618. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chen Q, Chen X, Zhang M, Fan Q, Luo S and
Cao X: miR-137 is frequently down-regulated in gastric cancer and
is a negative regulator of Cdc42. Dig Dis Sci. 56:2009–2016. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lehmann U, Hasemeier B, Christgen M, et
al: Epigenetic inactivation of microRNA gene hsa-mir-9-1 in human
breast cancer. J Pathol. 214:17–24. 2008. View Article : Google Scholar
|
|
53
|
Tsai KW, Liao YL, Wu CW, et al: Aberrant
hypermethylation of miR-9 genes in gastric cancer. Epigenetics.
6:1189–1197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wan HY, Guo LM, Liu T, Liu M, Li X and
Tang H: Regulation of the transcription factor NF-kappaB1 by
microRNA-9 in human gastric adenocarcinoma. Mol Cancer. 9:162010.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Luo H, Zhang H, Zhang Z, et al:
Down-regulated miR-9 and miR-433 in human gastric carcinoma. J Exp
Clin Cancer Res. 28:822009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ueda T, Volinia S, Okumura H, et al:
Relation between microRNA expression and progression and prognosis
of gastric cancer: a microRNA expression analysis. Lancet Oncol.
11:136–146. 2010. View Article : Google Scholar
|
|
57
|
Mutze K, Langer R, Schumacher F, et al:
DNA methyltransferase 1 as a predictive biomarker and potential
therapeutic target for chemotherapy in gastric cancer. Eur J
Cancer. 47:1817–1825. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hanoun N, Delpu Y, Suriawinata AA, et al:
The silencing of microRNA 148a production by DNA hypermethylation
is an early event in pancreatic carcinogenesis. Clin Chem.
56:1107–1118. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zheng B, Liang L, Wang C, et al:
MicroRNA-148a suppresses tumor cell invasion and metastasis by
downregulating ROCK1 in gastric cancer. Clin Cancer Res.
17:7574–7583. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Li QJ, Zhou L, Yang F, et al: MicroRNA-10b
promotes migration and invasion through CADM1 in human
hepatocellular carcinoma cells. Tumour Biol. 33:1455–1465. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tian Y, Luo A, Cai Y, et al: MicroRNA-10b
promotes migration and invasion through KLF4 in human esophageal
cancer cell lines. J Biol Chem. 285:7986–7994. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Sasayama T, Nishihara M, Kondoh T, Hosoda
K and Kohmura E: MicroRNA-10b is overexpressed in malignant glioma
and associated with tumor invasive factors, uPAR and RhoC. Int J
Cancer. 125:1407–1413. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ma L, Teruya-Feldstein J and Weinberg RA:
Tumour invasion and metastasis initiated by microRNA-10b in breast
cancer. Nature. 449:682–688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kim K, Lee HC, Park JL, et al: Epigenetic
regulation of microRNA-10b and targeting of oncogenic MAPRE1 in
gastric cancer. Epigenetics. 6:740–751. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Liu M, Yang S, Wang Y, et al: EB1 acts as
an oncogene via activating beta-catenin/TCF pathway to promote
cellular growth and inhibit apoptosis. Mol Carcinog. 48:212–219.
2009. View Article : Google Scholar
|
|
66
|
Deng H, Guo Y, Song H, et al: MicroRNA-195
and microRNA-378 mediate tumor growth suppression by epigenetical
regulation in gastric cancer. Gene. 518:351–359. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Xu T, Zhu Y, Xiong Y, Ge YY, Yun JP and
Zhuang SM: MicroRNA-195 suppresses tumorigenicity and regulates
G1/S transition of human hepatocellular carcinoma cells.
Hepatology. 50:113–121. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Hua Z, Lv Q, Ye W, et al: MiRNA-directed
regulation of VEGF and other angiogenic factors under hypoxia. PLoS
One. 1:e1162006. View Article : Google Scholar
|
|
69
|
Saito Y, Suzuki H, Tsugawa H, et al:
Chromatin remodeling at Alu repeats by epigenetic treatment
activates silenced microRNA-512-5p with downregulation of Mcl-1 in
human gastric cancer cells. Oncogene. 28:2738–2744. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Li P, Chen X, Su L, et al: Epigenetic
silencing of miR-338-3p contributes to tumorigenicity in gastric
cancer by targeting SSX2IP. PLoS One. 8:e667822013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Bader AG, Brown D and Winkler M: The
promise of microRNA replacement therapy. Cancer Res. 70:7027–7030.
2010. View Article : Google Scholar : PubMed/NCBI
|