Introduction
Prostate cancer (CaP) ranks first among the top ten
most common types of cancer in males, and second highest as a cause
of cancer-related mortalities. It was estimated that in the United
States in 2014 there would be ~233,000 newly diagnosed patients
with CaP and ~29,480 CaP-related mortalities (1). Although the majority of patients with
localized low-risk CaP survive for a long time without any
intervention, the remainder experience advanced disease with a high
incidence of deterioration, recurrence and metastasis, all of which
are associated with a poor prognosis and a higher risk of mortality
(2). Currently, conventional
imaging technologies are not sufficiently sensitive to detect small
lesions or metastases in the early stage of the disease, providing
little information for the identification of aggressive and
indolent disease (3). Therefore,
it is of great importance to develop cancer-specific molecular
imaging techniques to improve CaP management.
Near-infrared fluorescence (NIRF) imaging is an
attractive novel modality for cancer detection and the acquisition
of real-time pathophysiological information. This imaging technique
requires NIRF probes with an emission wavelength in the
near-infrared region, similar to the contrast agents applied prior
to positron emission tomography. There are a number of important
requisites for these probes, including excellent optical
characteristics, suitable biocompatibility and a cancer targeting
ability (4). Two organic
polymethine cyanine dyes have previously been described as ideal
NIRF probes, IR-783 and its derivative MHI-148. These dyes
accumulate selectively at tumor sites but not in normal tissues,
possibly due to the differential expression of organic anion
transporting peptides (OATPs) in cancer cells (5). OATPs are 12-transmembrane
glycoproteins originating from the SLCO gene superfamily (6) that are expressed in several
epithelial tissues throughout the body. In addition, OATP
overexpression affects cancer development, including OATP1B3 in
prostate cancer (7,8).
IR-783 and MHI-148 have great potential in the
detection of malignancies without the requirement for additional
conjugation with cancer-specific moieties, a method that has been
widely used in the application of other NIRF probes (9–11).
To further expand their clinical value in prostate cancer
detection, the underlying mechanism and the feasibility of NIRF
dye-mediated imaging was explored in different experimental
settings, including both in vivo and in vitro
studies. The aim of the present study was to investigate the
feasibility of NIRF dye-mediated prostate cancer imaging, using
IR-783 cyanine dyes. The dye uptake and subcellular co-localization
in human prostate cancer cells PC-3, DU-145 and LNCaP and normal
prostate epithelial cells RWPE-1 was tested.
Materials and methods
Chemical reagents
IR-783 cyanine dye was purchased from Sigma-Aldrich
(St. Louis, MO, USA). MHI-148 was synthesized and purified as
previously described (12). All
dyes were prepared as stock solutions (1 mM) in dimethyl sulfoxide
(DMSO; Sigma-Aldrich) and stored at 4°C in the dark. The dyes were
diluted in serum free medium to an appropriate working solution and
filtered through 0.2 μm filters prior to use.
Cell lines and cell culture
PC-3, DU-145 and LNCaP human prostate cancer and
RWPE-1 normal prostate epithelial cell lines were purchased from
the American Type Culture Collection (ATCC, Manassas, VA, USA) and
grown according to ATCC recommendations. Each of the recommended
media (RPMI-1640 for LNCaP, F-12 Ham’s Kaighn’s modification medium
for PC-3 and minimal essential medium for DU-145; Invitrogen Life
Technologies, Carlsbad, CA, USA) contained 10% fetal bovine serum
(Gibco-BRL, Carlsbad, CA, USA) and penicillin (100
IU/ml)/streptomycin (100 μg/ml) and the cells were cultured in a
humidified atmosphere with 5% CO2 at 37°C.
In vitro study of dye uptake in cultured
cells
The cell staining procedures were undertaken as
described previously (12). In
brief, suspensions of PC-3, DU-145, LNCaP, and RWPE-1
(1×104/well) cells were placed into four-chamber slides
(Nalgen Nunc International, Penfield, New York, USA) and cultured
for 24 h. Following the removal of the culture medium, working
solutions of IR-783 or MHI-148 dyes (20 μM) were added. The slides
were incubated at 37°C for 30 min and then washed twice with
phosphate-buffered saline (PBS). The cells were counterstained
using DAPI at 37°C for 10 min, followed by a two PBS washes and a
10-min fixation with 4% paraformaldehyde (Sigma-Aldrich). The
slides were covered with glass coverslips using aqueous mounting
medium (Sigma-Aldrich) and observed under a confocal laser
microscope (OLYMPUS FV1000; Olympus, Tokyo, Japan) with an
excitation wavelength of 633 nm and an emission wavelength of
670–810 nm (5).
Subcellular localization of the dyes in the prostate
cancer cells was detected according to a previously established
protocol (12). Briefly,
commercially available probes, Mito Tracker Orange CMTMRos and Lyso
Tracker Green DND-26 (Molecular Probes, Camarillo, CA, USA), were
used to track cytoplasmic mitochondria and lysosomes. Following
DAPI staining, the slides were placed in 500 nM CMTMRos for 30 min
at 37°C followed by repeated rinsing. Subsequently, 200 nM DND-26
was added for 60 min at 37°C. Following repeated washes and
mounting, the slides were observed under a confocal microscope
(OLYMPUS FV1000; Olympus).. The emission/excitation wavelengths for
CMTMRos were 504 nm/511 nm and for DND-26 were 554 nm/576 nm.
Images captured in the same visual field in varying light
conditions were merged for co-localization analysis of the NIRF
cyanine dyes.
Prostate cancer cells were pre-incubated with
different OATP inhibitors to determine the underlying mechanisms
attributed to their specific uptake and accumulation of cyanine
dyes. Nonspecific OATP inhibitor bromosulfophthalein (BSP, 250
μmol/l), OATP1 inhibitor rifampicin (20 μmol/l), selective OATP1B1
inhibitor 17β-estradiol (EST, 20 μmol/l), and selective OATP1B3
inhibitor cholecystokinin octapeptide (CCK-8, 20 μmol/l) were added
to the prostate cancer cells for 5 min, which was followed by the
previously mentioned staining procedures (13–15).
The uptake and accumulation of the dyes with or without inhibitors
was observed under a confocal microscope (OLYMPUS FV1000; Olympus).
For comparative studies, flow cytometry was applied to determine
the fluorescence intensity of each group. The prostate cancer cells
(1×104) were cultured in 6-well plates for 24 h,
followed by staining with the NIRF dyes as previously described.
Following a final PBS wash, the fluorescence of each tube was
measured using a flow cytometer (FACS Aria; BD Biosciences), with
excitation/emission wavelengths of 633 nm/780 nm for NIRF dye
detection. The relative fluorescence intensity in each group was
calculated as a percentage of the fluorescence intensity of the
group without inhibitor application.
Detection of prostate cancer cells in
human blood samples
An experimental model was established to evaluate
the feasibility of NIRF dye-mediated imaging of prostate cancer
cells in human blood. Heparinized human blood samples were obtained
from healthy volunteers following the approval of the Institutional
Ethics Committee. Informed consent was obtained from all
individuals. The single-cell suspension of prostate cancer cells
was pre-labeled with DAPI, mixed into human blood
(10–104/ml) and NIRF dye (20 μmol/l) was added. The
mixture was gently vortexed several times and the mononuclear and
cancer cells were isolated via the gradient centrifugation method
using Ficoll-Paque™ solution (GE Healthcare, Little Chalfont,
United Kingdom) as previously described (16). The cells were then labeled with
NIRF dyes as previously discussed (12). Subsequently, the mixture of normal
and cancer cells was fixed and observed under a confocal microscope
to identify the prostate cancer cells via the colored staining. The
total number of prostate cancer cells in the blood was counted by
flow cytometry.
NIRF imaging of human prostate cancer
tissues
Samples of human prostate cancer tissue and the
adjacent noncancerous tissues were obtained with informed consent
from five patients at Xijing Hospital to test the possibility of
direct and selective NIRF dye staining in ex vivo settings.
Retrieved tissues were stained with cyanine dyes for NIRF imaging
using the IVIS Lumina II imaging station (Caliper Life Sciences,
Hopkinton, MA, USA). The nonspecific IR-800 dye (Sigma) was used as
a control. For confocal microscopy, the tissues were embedded in
optimum cutting temperature medium (Sakura Finetek, Torrance, CA,
USA). Frozen 10 μm sections were cut and stained with DAPI and the
mounted sections were analyzed with a confocal microscope to
observe the NIRF signals. For hematoxylin and eosin (H&E)
staining, tissues were fixed in 4% paraformaldehyde and embedded in
paraffin. Following H&E staining, the tissue sections (5 μm)
cut from the paraffin blocks were examined and imaged by an expert
pathologist (17) using an Olympus
DP-72 digital camera (Olympus). Furthermore, following anesthesia
with 2% isoflurane in 100% oxygen at a delivery rate of 1.5 l/min
(18), slices of freshly harvested
tumor specimens (1 mm × 2 mm × 2 mm) were implanted subcutaneously
to the dorsolateral part of 4–6 weeks old athymic nude mice (n=3),
which were obtained from the experimental animal center of the
Fourth Military Medical University (Shaanxi, China) and had free
access to food and water under a normal light/dark cycle. All
animal experiments were conducted in accordance with the Animal
Care and Use Committee Guidelines of the Fourth Military Medical
University. The implanted mice were left for 24 h and then NIRF
dyes were injected intraperitoneally at a dose of 0.375 mg/kg.
During the following five days, mice bearing prostate cancer
implants underwent NIRF imaging using the IVIS Lumina II imaging
station.
Evaluating NIRF imaging in mice models of
prostate cancer
Human prostate cancer cells (1×106) were
implanted either subcutaneously, intraosseously or orthotopically
into athymic nude mice (n=3, respectively) following the procedures
previously reported (19). Once
the tumors reached ~5–10 mm in diameter as assessed by macroscopic
observation, palpation or X-ray, cyanine dyes were injected
intraperitoneally at a dose of 0.375 mg/kg. Tumor-loaded mice were
anesthetized 24 h post-NIRF dye injection. The whole body NIRF
imaging of the mice was undertaken using an IVIS Lumina II imaging
station. Following the imaging, mice underwent painless euthanasia
by isoflurane overdose. The tissue distribution of the dyes within
the mice was assessed. Frozen tissue sections together with
paraffin-embedded tissue sections were obtained for immediate
confocal imaging and H&E staining as described above.
Data processing and statistics
Numerical data are expressed as the means ± the
standard error of the mean (SEM). The statistical significance of
data was determined by Student’s t test. SPSS 16.0 software (SPSS
Inc., Chicago, IL, USA) was used for statistical analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Cultured prostate cancer cells
selectively uptake and accumulate NIRF dyes
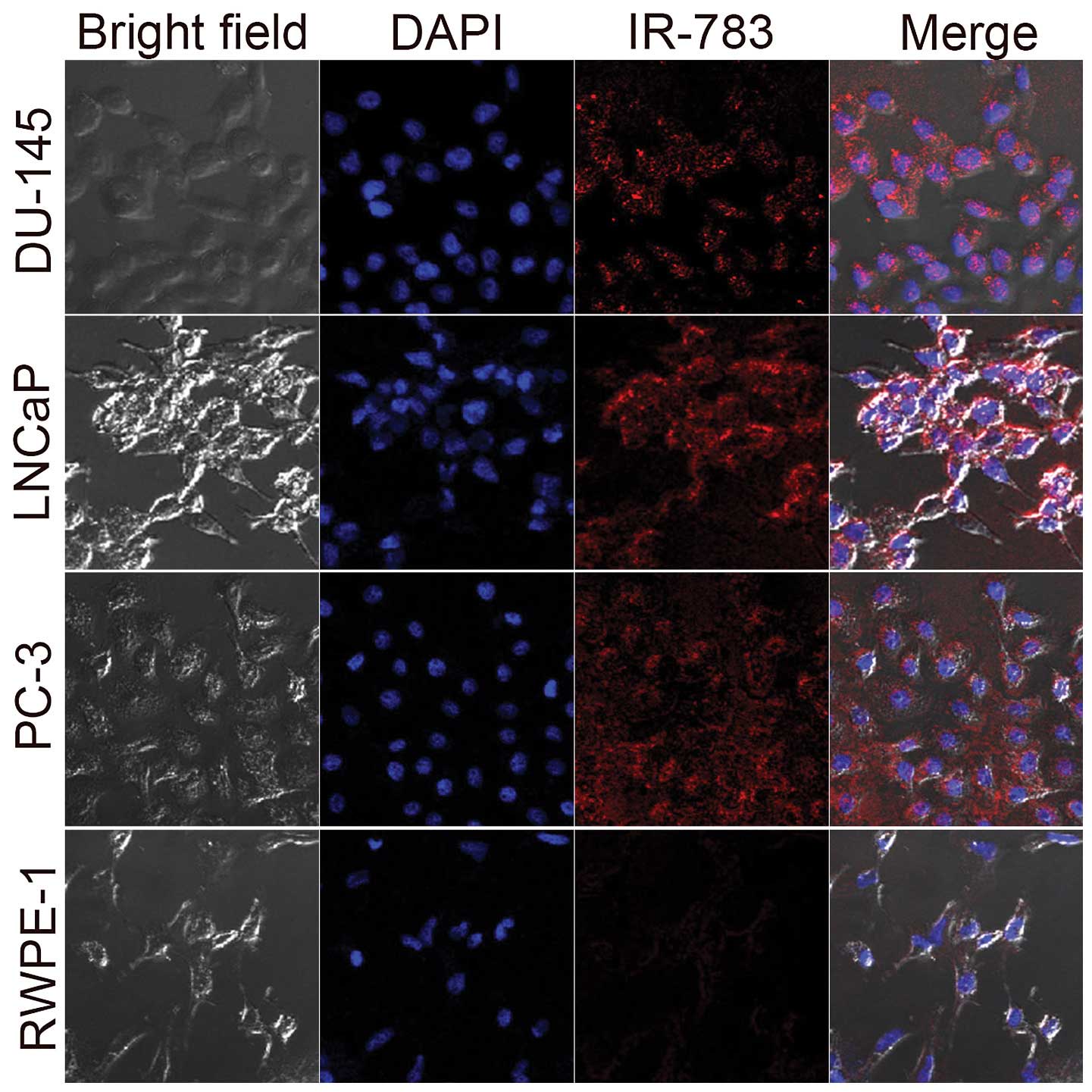
PC-3, DU-145, LNCaP human prostate cancer cells and
RWPE-1 normal human prostate epithelial cells were selected for
in vitro studies to evaluate the NIRF dye uptake and
retention by prostate cancer cells. Three images were captured of
the same visual field of the prepared slides using confocal
microscopy, including transparent, DAPI and NIRF imaging patterns
(Fig. 1). Selective uptake and
accumulation of NIRF dyes was observed in all prostate cancer
cells. However, there was only a weak NIRF signal detected in
RWPE-1 cells. These results confirm the findings of a previous
study that these dyes have a unique cancer targeting ability
(5).
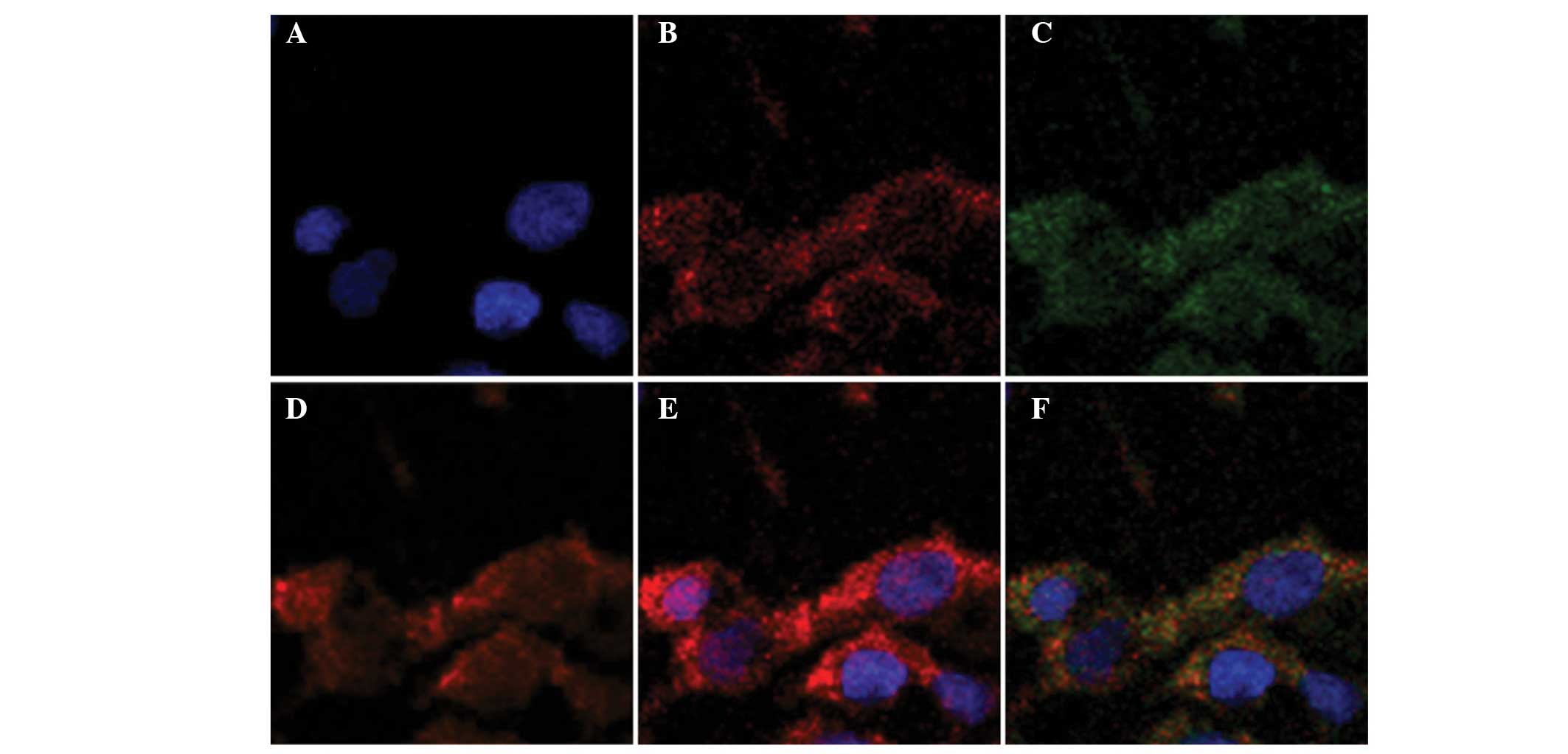
Previous studies have demonstrated the preferential
retention of NIRF dyes in the lysosomes and mitochondria of the
ARCaPM cell line, a highly metastatic subclone of ARCaP cell line
derived from the ascitic fluid of a patient with prostate cancer
(12). The present study
investigated subcellular co-localization of NIRF dyes in three
commonly used prostate cancer cell lines, PC-3, DU-145 and LNCaP.
Merged images revealed a large overlap of cytoplasmic staining
among the NIRF dyes in the lysosomes and mitochondria, indicating
that a substantial portion of these dyes accumulate in the
lysosomes and mitochondria of a number of prostate cancer cells
(Fig. 2).
Previous studies have demonstrated that the
inhibition of OATPs greatly affects the uptake of NIRF dyes,
however, the explicit mechanism remains elusive (11,20).
Elevated expression levels of OATP1B3 have been observed in
prostate cancer, therefore, the current study employed four types
of OATP inhibitor to test whether this subfamily of OATPs was
involved in NIRF uptake in prostate cancer cells (6,21,22).
There were remarkable disparities in the uptake and accumulation of
these dyes when different inhibitors were applied (Fig. 3). Nonspecific OATP inhibitor BSP
induced the greatest impact on the NIRF signal, as revealed by the
reduction in fluorescence intensity (26.4%±5.7%), compared with
that of the control. The OATP1 inhibitor rifampicin (RIF) strongly
diminished the fluorescence intensity (36.5±6.4%) compared with
that in the control. Similarly, the selective OATP1B3 inhibitor
CCK-8 significantly reduced the NIRF signal (39.2%±4.8%) compared
with that of the control, although to a lesser extent than BSP or
RIF. However, the selective OATP1B1 inhibitor EST had a minimal
effect on the intensity of the fluorescence signal compared with
that of the control group (Fig.
3). These results indicate that the selective uptake of NIRF
dyes relies primarily on the transporting functions of OATP1B3.
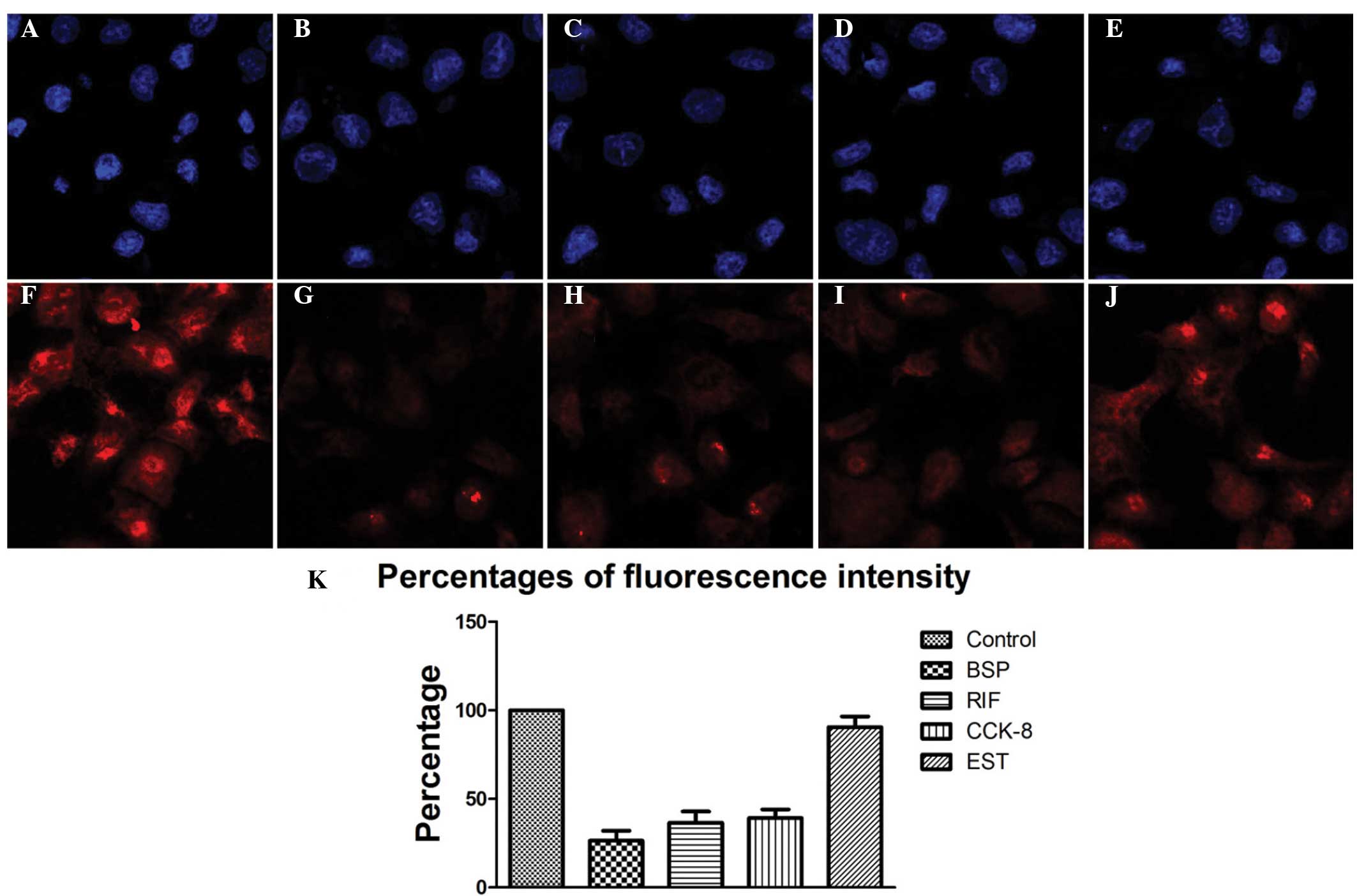 | Figure 3PC-3 prostate cancer cells were
treated with different organic anion transporting peptide (OATP)
inhibitors followed by staining with DAPI (blue) or IR-783 (red).
(A, F) Control group with no treatment, (B, G) nonspecific OATP
inhibitor bromosulfophthalein (BSP) group, (C, H) OATP1 inhibitor
rifampicin (RIF) group, (D, I) selective OATP1B3 inhibitor
cholecystokinin octapeptide (CCK-8) group, (E, J) selective OATP1B1
inhibitor 17β-estradiol (EST) group (magnification, ×200). (K)
Fluorescence intensity in each group evaluated by flow
cytometry. |
NIRF dye mediates the detection of
circulating tumor cells in human blood samples
Prostate cancer cells have the potential to migrate
to distant organs via the circulatory system (23–25).
Fluorescent probes with excitation/emission wavelengths in the
visible region have been tested for the feasibility and reliability
of detecting circulating tumor cells (CTC) in preclinical studies
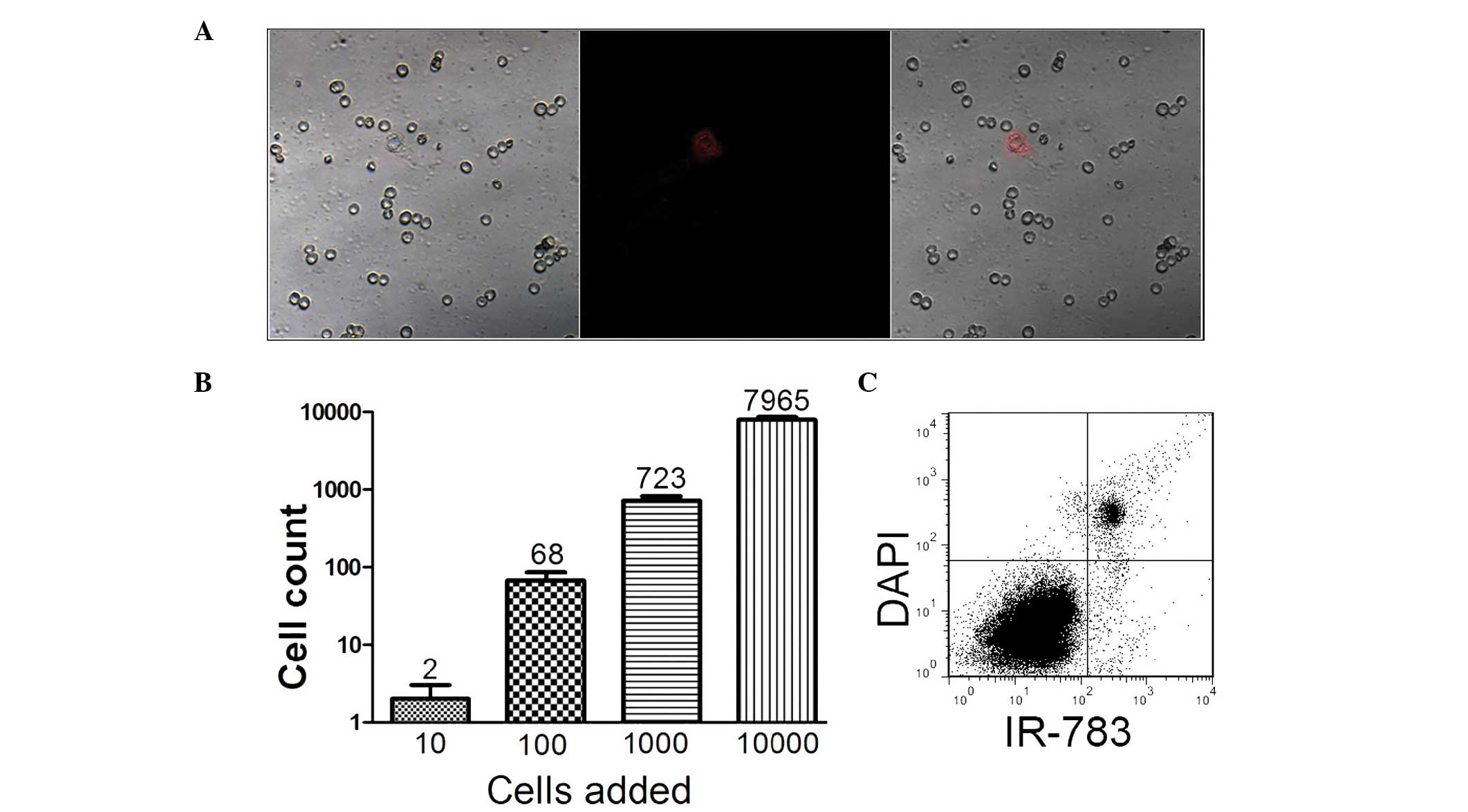
(16). An experimental model
mimicking the detection procedure has previously been established
to verify whether these NIRF dyes may be exploited for CTC
detection (5). In the current
study, blood samples were spiked with different numbers of prostate
cancer cells (10–104/ml). It was revealed that prostate
cancer cells could be recognized with particular NIR fluorescence
in isolated mononuclear cell mixtures, even at concentrations as
low as 10 cells/ml in the blood (Fig.
4). Additionally, the results of the flow cytometric analysis
support the viability of NIRF dye application in the detection of
CTC in prostate cancer.
NIRF imaging of human prostate cancer
tissues using cyanine dyes
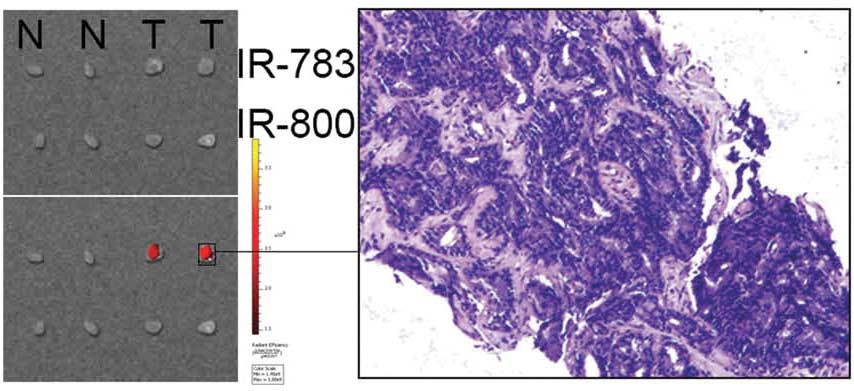
NIRF imaging was performed on human prostate cancer
tissues, with the aim of determining if IR-783-mediated NIRF
imaging remains effective at detecting prostate cancer cells in
surgical samples. Samples of human prostate cancer tissues and the
adjacent normal tissues were stained using IR-783 or IR-800. A
strong NIRF signal was detected in the prostate cancer tissues but
not in the normal tissues of the IR-783 group (Fig. 5). This result was confirmed by
confocal microscopy and H&E staining. However, no difference
was found in the IR-800 group, possibly owing to its lower
stability and binding specificity to cancer cells. In addition,
slices (1 mm × 2mm × 2mm) of prostate cancer tissue that were
implanted subcutaneously into mice were detected by IR783,
demonstrating the potential of IR-783-mediated NIRF imaging for
clinical application.
NIRF imaging of prostate cancer
xenografts in mice models using cyanine dyes
Subcutaneous, intraosseous and orthotopical models
of prostate cancer using athymic nude mice were established to
validate the possible in vivo applications of NIRF imaging
for the detection and observation of prostate cancer cells. NIRF
dyes were administered and, 24 h later, high signal to background
ratios were observed between the xenografts and the mouse models
(Fig. 6). Bio-distribution
analysis indicated that the metabolism of the NIRF dye was
primarily through the excretions of bile, urine and feces.
Furthermore, the NIRF signal from the prostate cancer cells
remained detectable for >1 week. In sections of the xenografts
retrieved from the sacrificed mice, the presence of prostate cancer
cells and cancer specific uptake of NIRF dyes was confirmed.
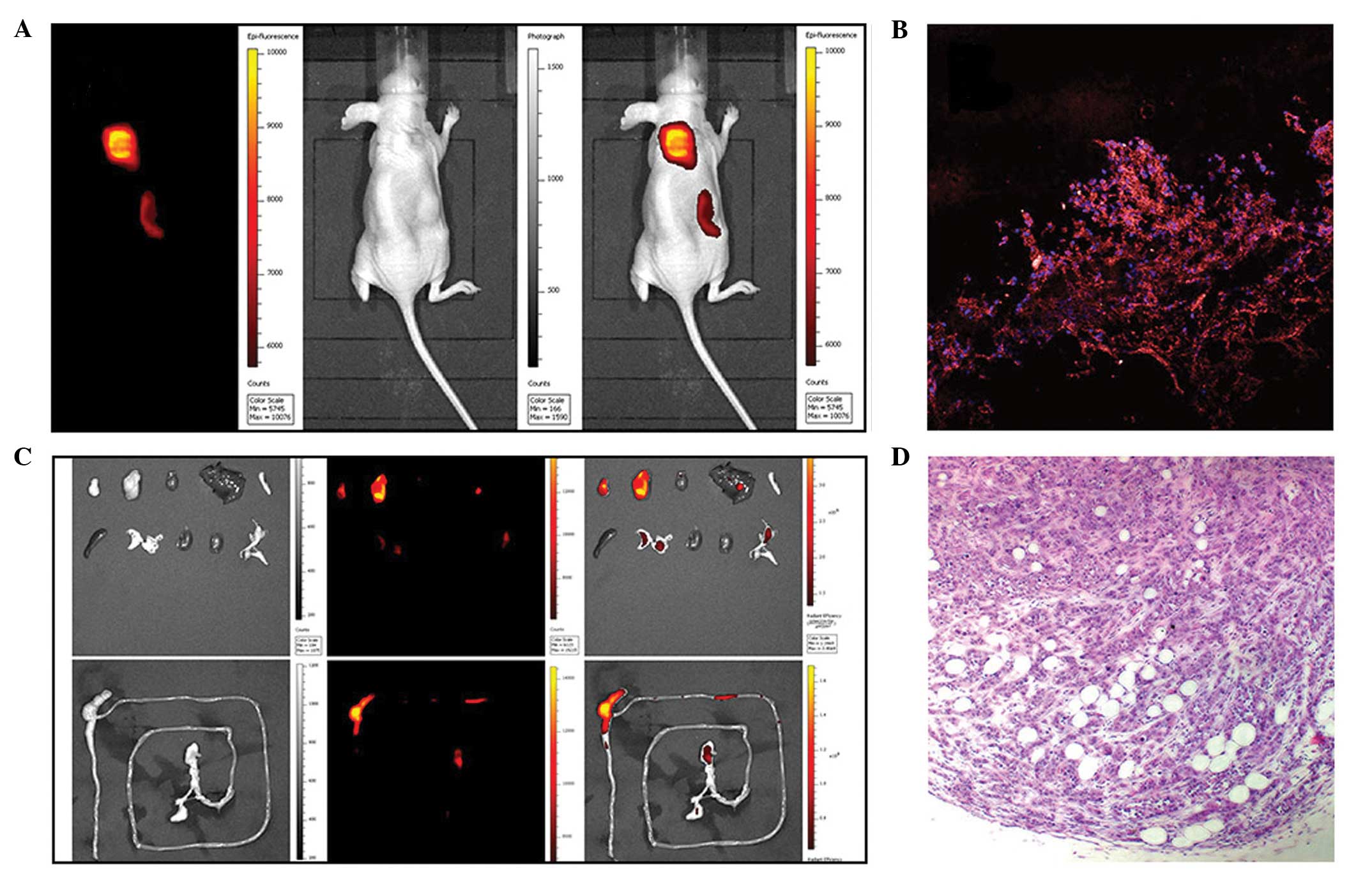 | Figure 6Near-infrared fluorescence (NIRF)
imaging of prostate cancer xenografts in a subcutaneous mice model
using IR-783. (A) Representative in vivo near infrared
fluorescence images of subcutaneous prostate cancer (from left to
right: NIRF image, bright light image and superimposition). Two
clear tumors were identified. (B) Frozen sections of the retrieved
xenografts were stained with DAPI and observed under a confocal
microscope (magnification, ×200). (C) Bio-distribution of IR-783 in
the organs of nude mice bearing prostate cancer. The top row of
images show the following from upper left to lower right: tumor,
tumor, heart, liver, pancreas, spleen, lung, kidney, kidney,
bladder. Bottom row: the whole intestine. (D) Tumors were confirmed
by hematoxylin and eosin staining (magnification, ×200). |
Discussion
Rapid progress has been made in the development of
luminescent nanoparticles, and a number of them have been evaluated
as potential contrast agents or delivery vehicles for molecular
imaging, owing to their abilities of fast screening and early
detection of cancer, which provide invaluable guidance in cancer
therapy. However, conventional dyes are susceptible to
photobleaching and rarely achieve a sufficient target-to-background
ratio for clinical use, particularly due to the pharmacokinetics
and potential toxicity associated with their concentration, surface
coating, and chemical composition. NIRF dyes have received
increasing attention in recent years for diagnostic imaging using
near infrared light radiation, which penetrates up to 10 cm deep in
certain tissues (20,26,27).
The majority of NIRF dyes lack a specific targeting property,
limiting their biomedical applications (28). In probe design using these dyes, a
crucial targeting element has to be considered. Frequently used
targeting moieties include antibodies, peptides, proteins,
aptamers, and small receptor ligands (29,30).
IR-783 and MHI-148 are two novel heptamethine indocyanine dyes that
have been identified that preferentially accumulate in cancer
tissues, hence displaying great advantages over the more commonly
used fluorescent dyes with bioimaging applications (12). These NIRF dyes display dual imaging
and targeting abilities in addition to a very low cytotoxicity.
The underlying mechanism of NIRF dye uptake remains
elusive, although the contribution of sodium independent OATPs in
dye uptake has previously been determined (31). In the present study, the roles of
OATPs in prostate cancer detection were assessed through in
vivo and in vitro experiments and the contribution of
OATP subtype OATP1B3 was evaluated. OATP1B3 has previously been
identified as an aberrantly expressed transporter in prostate
cancer and has additionally been implicated in the progression of
prostate cancer (8,22). The results of the current study
indicate that OATP1B3 may be the predominant transporter involved
in the dye uptake. These results are in good agreement with
previous descriptions concerning the role of OATP1B3 in prostate
cancer (14,32).
Cell imaging techniques for cancer diagnosis are
simple, cost-effective, and relatively sensitive techniques that
rely on the use of reporter genes and fluorescent dyes (33). The threshold sensitivity of
photoacoustic flow cytometry is estimated to be as high as one
cancer cell in a background of 107 normal blood cells (34). However, a fluorescence-based
approach is only suitable for short-term labeling applications,
while NIRF dyes allow for long-term tracking strategies with a
satisfactory accuracy for diagnosis (5). In the current study, the labeling
efficiency of NIRF dyes in all three types of prostate cancer cells
was found to be satisfactory. Utilizing the tumor cell-specific
behavior of these two dyes allowed for the detection of an accurate
quantification of live CTCs in prostate cancer patients (23). Additionally, their preferential
accumulation within prostate cancer xenografts allowed for
noninvasive monitoring of uptake kinetics, tumor growth and
therapeutic outcome.
NIRF imaging is a sensitive method of tagging a
target of interest and is reliable for the noninvasive imaging of
the microscopic and macroscopic levels, however, detailed
anatomical information cannot be obtained. Multifunctional NIRF
probes, which combine NIRF dyes with imaging modalities that
provide anatomical information, including MRI, CT, PET, SPECT and
photoacoustic imaging (PAI), are the next aim of the relatively
novel rising field of cancer-targeting NIRF imaging technology
(35–37). Whilst multifunctional NIRF probes
are still far away from clinical application, it is anticipated
that NIRF imaging technology will be unquestionably an integral
part of biomedical research in the near future.
Acknowledgements
Funding for this study was provided by the
Scientific Innovative Project of Shaanxi Province (grant no:
2012KTCL03-03).
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cai QY, Yu P, Besch-Williford C, et al:
Near-infrared fluorescence imaging of gastrin releasing peptide
receptor targeting in prostate cancer lymph node metastases.
Prostate. 73:842–854. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Osborne JR, Akhtar NH, Vallabhajosula S,
Anand A, Deh K and Tagawa ST: Prostate-specific membrane
antigen-based imaging. Urol Oncol. 31:144–154. 2013. View Article : Google Scholar
|
|
4
|
Yi X, Wang F, Qin W, Yang X and Yuan J:
Near-infrared fluorescent probes in cancer imaging and therapy: an
emerging field. Int J Nanomedicine. 9:1347–1365. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang X, Shao C, Wang R, et al: Optical
imaging of kidney cancer with novel near infrared heptamethine
carbocyanine fluorescent dyes. Journal Urol. 189:702–710. 2013.
View Article : Google Scholar
|
|
6
|
Svoboda M, Riha J, Wlcek K, Jaeger W and
Thalhammer T: Organic anion transporting polypeptides (OATPs):
regulation of expression and function. Curr Drug Metab. 12:139–153.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Obaidat A, Roth M and Hagenbuch B: The
expression and function of organic anion transporting polypeptides
in normal tissues and in cancer. Annu Rev Pharmacol Toxicol.
52:135–151. 2012. View Article : Google Scholar :
|
|
8
|
Hamada A, Sissung T, Price DK, et al:
Effect of SLCO1B3 haplotype on testosterone transport and clinical
outcome in caucasian patients with androgen-independent prostatic
cancer. Clin Cancer Res. 14:3312–3318. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shimizu Y, Temma T, Hara I, et al:
Micelle-based activatable probe for in vivo near-infrared optical
imaging of cancer biomolecules. Nanomedicine. 10:187–195. 2014.
View Article : Google Scholar
|
|
10
|
Chen Q, Wang C, Cheng L, He W, Cheng Z and
Liu Z: Protein modified upconversion nanoparticles for
imaging-guided combined photothermal and photodynamic therapy.
Biomaterials. 35:2915–2923. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang E, Luo S, Tan X and Shi C:
Mechanistic study of IR-780 dye as a potential tumor targeting and
drug delivery agent. Biomaterials. 35:771–778. 2014. View Article : Google Scholar
|
|
12
|
Yang X, Shi C, Tong R, et al: Near IR
heptamethine cyanine dye-mediated cancer imaging. Clin Cancer
Research. 16:2833–2844. 2010. View Article : Google Scholar
|
|
13
|
Ismair MG, Stieger B, Cattori V, et al:
Hepatic uptake of cholecystokinin octapeptide by organic
anion-transporting polypeptides OATP4 and OATP8 of rat and human
liver. Gastroenterology. 121:1185–1190. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Karlgren M, Vildhede A, Norinder U, et al:
Classification of inhibitors of hepatic organic anion transporting
polypeptides (OATPs): influence of protein expression on drug-drug
interactions. J Med Chem. 55:4740–4763. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gui C, Wahlgren B, Lushington GH and
Hagenbuch B: Identification, Ki determination and CoMFA analysis of
nuclear receptor ligands as competitive inhibitors of
OATP1B1-mediated estradiol-17beta-glucuronide transport. Pharmacol
Res. 60:50–56. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He W, Kularatne SA, Kalli KR, et al:
Quantitation of circulating tumor cells in blood samples from
ovarian and prostate cancer patients using tumor-specific
fluorescent ligands. International J Cancer. 123:1968–1973. 2008.
View Article : Google Scholar
|
|
17
|
Yi X, Zhang G and Yuan J: Renoprotective
role of fenoldopam pretreatment through hypoxia-inducible
factor-1alpha and heme oxygenase-1 expressions in rat kidney
transplantation. Transplant Proc. 45:517–522. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Valentim AM, Alves HC, Olsson IA and
Antunes LM: The effects of depth of isoflurane anesthesia on the
performance of mice in a simple spatial learning task. J Am Assoc
Lab Anim Sci. 47:16–19. 2008.PubMed/NCBI
|
|
19
|
Wu TT, Sikes RA, Cui Q, et al:
Establishing human prostate cancer cell xenografts in bone:
induction of osteoblastic reaction by prostate-specific
antigen-producing tumors in athymic and SCID/bg mice using LNCaP
and lineage-derived metastatic sublines. Int J Cancer. 77:887–894.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yuan A, Wu J, Tang X, Zhao L, Xu F and Hu
Y: Application of near-infrared dyes for tumor imaging,
photothermal, and photodynamic therapies. J Pharm Sci. 102:6–28.
2013. View Article : Google Scholar
|
|
21
|
Buxhofer-Ausch V, Secky L, Wlcek K, et al:
Tumor-specific expression of organic anion-transporting
polypeptides: transporters as novel targets for cancer therapy. J
Drug Deliv. Feb 3–2013.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wright JL, Kwon EM, Ostrander EA, et al:
Expression of SLCO transport genes in castration-resistant prostate
cancer and impact of genetic variation in SLCO1B3 and SLCO2B1 on
prostate cancer outcomes. Cancer Epidemiol Biomarkers Prev.
20:619–627. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shao C, Liao CP, Hu P, et al: Detection of
live circulating tumor cells by a class of near-infrared
heptamethine carbocyanine dyes in patients with localized and
metastatic prostate cancer. PloS On. 9:e889672014. View Article : Google Scholar
|
|
24
|
Tjensvoll K, Nordgård O and Smaaland R:
Circulating tumor cells in pancreatic cancer patients: Methods of
detection and clinical implications. Int J Cancer. 134:1–8. 2014.
View Article : Google Scholar
|
|
25
|
Thalgott M, Rack B, Maurer T, et al:
Detection of circulating tumor cells in different stages of
prostate cancer. J Cancer Res Clin Oncol. 139:755–763. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hellebust A and Richards-Kortum R:
Advances in molecular imaging: targeted optical contrast agents for
cancer diagnostics. Nanomedicine (Lond). 7:429–445. 2012.
View Article : Google Scholar
|
|
27
|
Fomina N, McFearin CL, Sermsakdi M,
Morachis JM and Almutairi A: Low power, biologically benign NIR
light triggers polymer disassembly. Macromolecules. 44:8590–8597.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Muselaers CH, Stillebroer AB, Rijpkema M,
et al: Optical imaging of renal cell carcinoma with anti-carbonic
anhydrase IX monoclonal antibody girentuximab. J Nucl Med.
55:1035–1040. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheng K and Cheng Z: Near infrared
receptor-targeted nanoprobes for early diagnosis of cancers. Curr
Med Chem. 19:4767–4785. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bai M and Bornhop DJ: Recent advances in
receptor-targeted fluorescent probes for in vivo cancer imaging.
Curr Med Chem. 19:4742–4758. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Letschert K, Faulstich H, Keller D and
Keppler D: Molecular characterization and inhibition of amanitin
uptake into human hepatocytes. Toxicol Sci. 91:140–149. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pressler H, Sissung TM, Venzon D, Price DK
and Figg WD: Expression of OATP family members in hormone-related
cancers: potential markers of progression. PloS One. 6:e203722011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shan L: Near-infrared fluorescence
1,1-dioctadecyl-3,3,3,3- tetramethylindotricarbocyanine iodide
(DiR)-labeled macrophages for cell imaging. Molecular Imaging and
Contrast Agent Database (MICAD) Bethesda (MD): 2004
|
|
34
|
Zharov VP, Galanzha EI, Shashkov EV,
Khlebtsov NG and Tuchin VV: In vivo photoacoustic flow cytometry
for monitoring of circulating single cancer cells and contrast
agents. Opt Lett. 31:3623–3625. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qiao XF, Zhou JC, Xiao JW, Wang YF, Sun LD
and Yan CH: Triple-functional core-shell structured upconversion
luminescent nanoparticles covalently grafted with photosensitizer
for luminescent, magnetic resonance imaging and photodynamic
therapy in vitro. Nanoscale. 4:4611–4623. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim JS, Kim YH, Kim JH, et al: Development
and in vivo imaging of a PET/MRI nanoprobe with enhanced NIR
fluorescence by dye encapsulation. Nanomedicine (Lond). 7:219–229.
2012. View Article : Google Scholar
|
|
37
|
Guo Y, Yuan H, Cho H, et al: High
efficiency diffusion molecular retention tumor targeting. PloS One.
8:e582902013. View Article : Google Scholar : PubMed/NCBI
|