Introduction
The development of the mouse female genitalia is
primarily completed through a postnatal tissue remodeling process
in which the blind-ending vaginal cavity opens to the skin in
accordance with the rapid increase of sex hormones in the
five-week-old female mouse internal environment (1). The postnatal tissue remodeling
process is largely dependent on massive mucosal apoptosis in the
distal section of the mouse vaginal cavity near the skin, and can
only be observed during the brief period of vaginal opening
(1). Transgenic mice lines
expressing the human anti-apoptotic protein, B-cell lymphoma 2
(Bcl-2), in their vaginal mucosa developed a closed vaginal
phenotype due to the failure of the vaginal epithelium to execute
apoptosis, indicating that vaginal mucosal apoptosis is crucial for
postnatal vaginal opening at approximately five weeks of age
(1). Thereafter, several knockout
mice studies revealed the involvement of proapoptotic Bcl-2 family
proteins (2,3) and other molecules in postnatal
vaginal tissue remodeling (4,5).
However, the exact mechanism by which extensive apoptosis is
induced in the vaginal epithelium in response to a rapid increase
in estrogen in the mouse internal environment at puberty remains to
be elucidated (1,6). In particular, little information is
available as to whether an apoptosis-inducing ligand is important
in the postnatal vaginal tissue remodeling process (1,6). The
present study found that imperforate vagina and hydrometrocolpos
occurred with a high incidence in mice lacking semaphorin 4D
(Sema4D), a member of the semaphorin family known to guide neuronal
axon extension during nervous system development (7,8).
Semaphorins can be divided into eight classes,
composing a family of soluble and transmembrane glycoproteins with
a phylogenetically conserved domain structure, and were originally
identified as chemorepellents for axon guidance in the developing
nervous system (9). Sema4D, also
termed CD100, is a class 4 transmembrane-type semaphorin that
induces repulsive cytoskeletal changes in growth cone collapse in
hippocampal neurons and retinal ganglial cells in culture (10). To trigger these alterations, Sema4D
binds to the transmembrane receptor plexin-B1, a member of the
plexin family (10). Sema4D and
plexin-B1 have a conserved sema domain of ~400 amino acids
characterized by a seven-bladed β-propeller fold in their
respective extracellular domains (9,11).
Sema4D as a ligand and plexin-B1 as a receptor mutually interact
via their respective sema domains as demonstrated by a previous
crystallographic study (12).
Sema4D binding to plexin-B1 induces clustering of plexin-B1
receptors and then accelerates the GTPase-activating protein (GAP)
activities granted by two GAP domains in the intracellular region
of plexin-B1 (13). The activities
of plexin-B1 GAP downregulate the activities of Ras family members,
including R-Ras and M-Ras, which in cultured neurons lowers an
integrin-mediated cell attachment to the extracellular matrix and
thus induces remodeling of growth cone or dendrite morphology
(13,14). Sema4D binding to plexin-B1 also
stimulates the guanine nucleotide exchange factor (GEF) activities
of PDZ-RhoGEF and leukemia-associated RhoGEF bound to the plexin-B1
C terminal PDZ-binding motif, which facilitates conversion of RhoA
from the GDP-bound form to the GTP-bound from (10). The increase in GTP-bound RhoA
augments actomyosin contractility through Rho kinase activation and
myosin light chain phosphorylation, which also facilitates
Sema4D-induced growth cone collapse in cultured hippocampal neurons
(8,10).
The high incidence of the closed vaginal phenotype
in Sema4D-deficient (Sema4D−/−) mice implies a degree of
impairment in vaginal mucosal apoptosis at the vaginal opening.
Previous studies have demonstrated that Sema4D is involved in the
apoptotic induction of neural precursor cells and oligodendrocytes
in a cultured model (15,16). However, it remains to be elucidated
whether Sema4D is crucially implicated in the apoptosis of the
postnatal vaginal tissue remodeling process. Thus, the present
study aimed to examine the possible involvement of the semaphorin
protein Sema4D in vaginal epithelial apoptosis in the postnatal
tissue remodeling process of the female mouse.
Materials and methods
Generation of Sema4D−/−
mice
Sema4D−/− mice were produced by gene
targeting (17). In brief, the
procedure for generating the mice was performed as follows. A
gene-targeting vector was designed to replace the 1.6-kb genomic
region, which contained the putative first exon covering the
initiation codon, with a neomycin-resistance gene. The
gene-targeting vector was transfected into E14.1 embryonic stem
(ES) cells by electroporation. Determination of the homologous
recombinants was confirmed by polymerase chain reaction (PCR) and
Southern blotting of G418- and ganciclovir-resistant clones. Mutant
ES cells with the homologous recombination were introduced into
mouse blastocysts and transferred into pseudopregnant mice to
generate chimeras. F1 heterozygous knockout mice were generated by
breeding the chimeras with BALB/c mice, and were then backcrossed
with BALB/c mice for 10 generations. Pairs of resultant
heterozygous mice were bred to gain homozygous knockout mice and
their wild-type (WT) littermates as controls. The mice were housed
in the Wakayama Medical University animal facilities and the animal
center at the Faculty of Pharmacy of Meijo University (Tempaku,
Nagoya, Japan). All researchers and experimenters conducted the
care and sacrifice of mice as well as other experimental protocols
in accordance with the guidelines promulgated by the Physiological
Society of Japan as well as the guidelines on animal
experimentation of Wakayama Medical University and Meijo
University. The Animal Ethics Review Committees of these
institutions approved the experimental protocol.
Genotype analysis
The genotypes of the mice were confirmed by PCR with
mouse tail DNA as the template and a Sema4D gene specific primer
set as previously reported (17).
Serum estradiol measurement
The estradiol levels in the serum of WT and
Sema4D−/− mice were measured using an enzyme immunoassay
kit (ERK R7005; Endocrine Technologies, Inc., San Francisco, CA,
USA) according to the manufacturer’s instructions.
Estradiol administration to induce
precocious vaginal opening
17β-estradiol (Sigma-Aldrich, St. Louis, MO, USA)
was dissolved in ethanol. The ethanolic solution of 17β-estradiol
diluted in corn oil (0.1 μg/kg body weight/day) was subcutaneously
injected into 12-day-old female WT and Sema4D−/− mice,
and the administrations were repeated daily for 5 days. Induction
of precocious vaginal opening was examined by visual inspection of
17-day-old estradiol-treated mice.
Immunohistochemistry and terminal
deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)
assay
Mice under anesthesia with pentobarbital sodium
(0.648 mg/10 g body weight via intraperitoneal injection;
Kyoritsuseiyaku Co., Tokyo, Japan) were subjected to transcardiac
perfusion of 4% paraformaldehyde. The vaginas were excised from the
mice and fixed overnight in 4% paraformaldehyde solution. The
vaginas were embedded longitudinally in paraffin and cut into 4-μm
serial sections. The sections were immunolabeled with anti-mouse
Sema4D (cat.no D142-3; Medical and Biological Laboratories Co.,
Ltd., Nagoya, Japan), anti-plexin-B1 (cat.no. sc-25642; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) and anti-cleaved
caspase-3 antibody (cat.no. #9664; Cell Signaling Technology,
Beverly, MA, USA). TUNEL assay was performed, as described
previously (18), using a Dead
End™ Fluorometric TUNEL system (Promega, Madison, WI, USA) and an
ApoTag Peroxidase In Situ Apoptosis Detection kit (Chemicon
International, Inc., Temecula, CA, USA) according to the
manufacturer’s instructions.
Western blot analysis
For Western blot analysis, tissue extracts were
prepared by homogenizing mouse vaginal tissue in T-PER Tissue
Protein Extraction Reagent (Thermo Scientific Inc., Waltham, MA,
USA) containing a protease inhibitor (α-complete; Roche Applied
Science, Penzberg, Germany) and a phosphatase inhibitor (PhosStop;
Roche Applied Science). Protein quantification of the tissue
extract was performed using the Bio-Rad Protein Assay (Bio-Rad,
Hercules, CA, USA). Each sample (15 μg) was prepared in a final
solution of 60 mM Tris-HCl (pH 6.8), 2% sodium dodecyl sulfate
(SDS), 10% glycerol, 0.1% bromophenol blue and 5%
β-mercaptoethanol. The sample solution was heated at 100°C for 5
min, electrophoresed through a 10% SDS-polyacrylamide gel and
transferred onto polyvinylidene fluoride membranes (Amersham
Pharmacia Biotech, Buckinghamshire, UK). Sema4D, plexin-B1 and
cleaved caspase-3 were detected with their respective antibodies
using an enhanced chemiluminescence or enhanced
chemiluminescence-plus western blot detection system in accordance
with the manufacturer’s instructions (Amersham Pharmacia Biotech).
The antibodies used were anti-CD100/Sema4D (cat.no. 610670; BD
Transduction Laboratories, Franklin Lakes, NJ, USA), anti-plexin-B1
(cat.no. sc-28372; Santa Cruz Biotechnology, Inc.) and anti-cleaved
caspase-3 (cat.no. #9664 Cell Signaling Technology).
Mouse vaginal epithelial cell
culture
Primary vaginal epithelial cell cultures derived
from Sema4D−/− mice were grown according to the
procedure developed previously (19). Four-week-old mouse vaginal tissue
was incubated with 1% collagenase solution (Wako Pure Chemical
Industries, Ltd., Osaka, Japan) at 37°C for 60 min to prepare an
epithelial sheet that was later cut into small pieces and dispersed
into individual cells by trypsin treatment. The dispersed vaginal
epithelial cells were grown on a 10-cm Primaria™-treated tissue
culture dish (BD, Tokyo, Japan) in Dulbecco’s modified Eagle’s
medium supplemented with 10% heat-inactivated fetal calf serum and
maintained at 37°C with a 5% CO2, 95% air atmosphere.
Following 5 days of culture, the cells were collected and seeded on
poly-L-lysine/laminin-coated coverslips with a density of
2×104 cells per 1 ml of culture medium. Recombinant
soluble mouse Sema4D fused to IgG1-Fc (20) was added to the culture at a
concentration of 2 μg/ml and after 36 h, the vaginal cell cultures
were fixed with 4% paraformaldehyde solution. The fixed cells were
subjected to TUNEL assay and anti-cleaved caspase-3
immunocytochemistry to examine apoptosis. To reduce the expression
of the plexin-B1 receptor on cultured vaginal epithelia cells via
gene knockdown, the vaginal epithelial cell cultures were
transduced at a multiplicity of infection of 2.5 with Mission Sigma
Lentiviral particles expressing short hairpin RNA (shRNA) directed
against mouse plexin-B1 mRNA (Clone ID: NM_172775.1-6159s1c1;
Sigma-Aldrich) and examined for Sema4D-induced apoptosis. The
vaginal epithelial cell culture transduced with non-target shRNA
(shRNA-NT) lentiviral particles (SHC002V; Sigma-Aldrich) was used
as a control. The knockdown of plexin-B1 mRNA was confirmed by
quantitative PCR using QuantiTect Primer assays according to the
manufacturer’s instructions (Mm_Plxnb1_1_SG QT00126483 and
Mm_B2m_2_SG QT01149547; Qiagen, Tokyo, Japan).
Statistical analysis
All data values are presented as the mean ± standard
error of the mean. Comparisons between WT and Sema4D−/−
mice were performed using Student’s t-test or one-way analysis of
variance followed by post-hoc analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
Sema4D−/− mice exhibit a
closed vaginal phenotype despite normal levels of estrogen
Sema4D−/− mice with the BALB/c genetic
background were generated by a homologous recombination method as
previously described (17). The
majority of the female Sema4D−/− mice showed lower
abdominal distention and swelling of the genital area, causing an
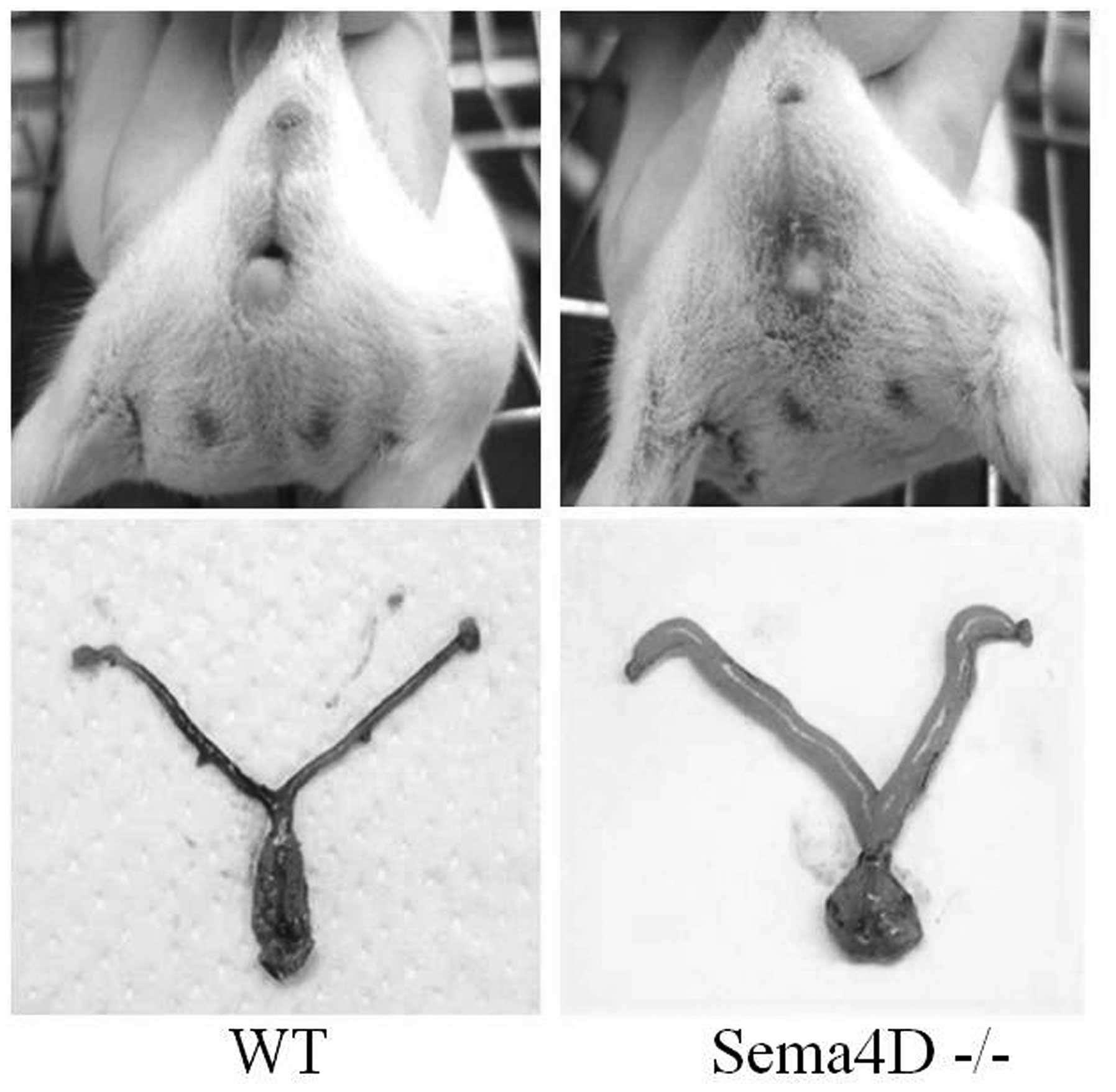
appearance similar to that of the male scrotum (Fig. 1). During inspection of the genital
areas, no vaginal openings to the skin were identified (Fig. 1). Anatomical dissections of these
mice revealed hydrometrocolpos, a condition where the absence of a
vaginal opening leads to lower abdominal and genital swelling
caused by the over-retention of secreted fluid in distended genital
tracts (Fig. 1). The incidence of
such imperforate vaginas was significantly higher in
Sema4D−/− mice compared with WT mice (WT: 0%, n=80;
heterozygous: 7.3%, n=288; Sema4D−/−: 59.5%, n=279;
χ2-test, P<0.05).
The mouse vaginal opening process is modulated by
the level of estrogen (1). An
enzyme immunoassay was thus conducted to evaluate the serum
estrogen level corresponding to the time of vaginal opening of
five-week-old WT and Sema4D−/− mice, and no significant
difference was identified between the two genotypes (WT: 19.89±6.80
pg/ml, n=5; Sema4D−/−: 23.20±3.92 pg/ml, n=5; Student’s
t-test, P>0.05). To further exclude the possibility of
insufficient estrogen secretion during the critical period of
vaginal opening, β-estradiol was injected into infant mice for five
consecutive days beginning at 12 days of age to induce precocious
vaginal opening in the 17-day-old specimens (1). Administration of β-estradiol to WT
mice induced premature vaginal opening in 17-day-old mice and
induce apoptosis in vaginal tissue, which was detected by TUNEL
assay and activated caspase-3 immunohistochemistry (Fig. 2). By contrast, administration of
β-estradiol to Sema4D−/− mice did not induce premature
vaginal opening, and the apoptotic level in Sema4D−/−
mouse vaginal tissue was significantly lower than that in the
vaginal tissue of WT mice treated with β-estradiol (Fig. 2).
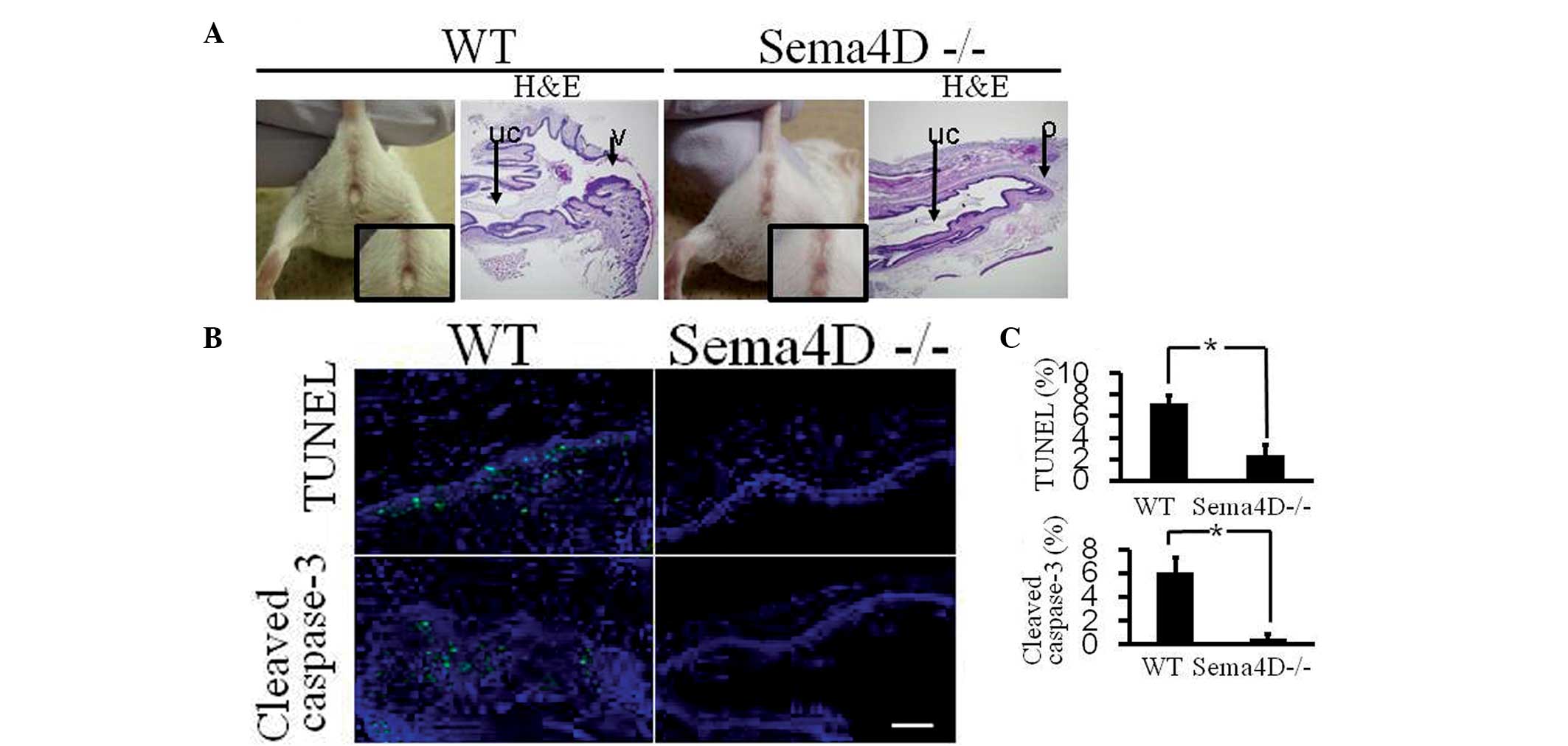 | Figure 2Precocious vaginal opening is not
induced by β-estradiol injection in Sema4D−/− mice. (A)
Daily subcutaneous injection of β-estradiol (0.1 μg/kg body weight)
into WT mice for five consecutive days (between 12 and 16-days-old)
induced precocious vaginal opening at 17 days old (vaginal opening:
four out of four mice). Identical injection into
Sema4D−/− mice did not induce precocious vaginal opening
at 17 days old, which was further indicated by histological
analysis (vaginal opening: zero out of four mice). (Magnification,
×40). uc, uterogenital canal; o, obstruction; v, external vaginal
entrance; H&E, hematoxylin and eosin staining. (B)
TUNEL-positive and cleaved caspase-3-positive apoptotic cells
(green) were significantly less numerous in the vaginal epithelium
of β-estradiol-injected Sema4D−/− mice than in
β-estradiol-injected WT mice. Cellular nuclei were visualized with
DAPI (blue). (Magnification, ×400). Scale bar=50 μm. (C) Graphs
show the rate of TUNEL- or cleaved caspase-3-positive cells,
respectively, among nucleated cells in vaginal epithelia. Each
column represents the mean ± standard error of the mean (WT, n=4;
Sema4D−/−, n=4). *P<0.05. Sema4D,
semaphorin 4D; WT, wild-type; TUNEL, terminal deoxynucleotidyl
transferase dUTP nick end labeling; DAPI,
4′,6-diamidino-2-phenylindole. |
Sema4D and plexin-B1 are localized to
mouse vaginal epithelia
In order to ascertain whether Sema4D mRNA is
expressed in the mouse vagina, reverse transcription PCR analyses
were performed with RNA from mouse uteri, vaginas and ovaries.
Sema4D mRNA was detected in the mouse vagina; however, the
transcripts were not amplified in any organs in the
Sema4D−/− mice (Fig.
3A). To confirm the existence of Sema4D protein in the mouse
vagina, western blotting was performed using protein extracts from
WT and Sema4D−/− vaginas (Fig. 3B). The analysis detected Sema4D
protein in WT vaginas, but not in Sema4D−/− vaginas
(Fig. 3B). Using the same blot,
the antibodies against plexin-B1, a Sema4D receptor, revealed the
existence of plexin-B1 in WT and Sema4D−/− vaginas
(Fig. 3B). To localize the
expression of Sema4D and plexin-B1 in the mouse vagina,
immunohistochemical analyses were performed on vaginal tissues from
WT and Sema4D−/− mice. The antibodies against Sema4D
detected Sema4D in the suprabasal layer of the vaginal epithelia in
WT mice (Fig. 3C), but not in
Sema4D−/− mice (Fig.
3D). However, the plexin-B1 antibodies detected plexin-B1
localization in WT (Fig. 3E) and
Sema4D−/− vaginal epithelia (Fig. 3F).
 | Figure 3Sema4D and plexin-B1 are expressed in
mouse vaginal epithelia. (A) Sema4D mRNA was detected by RT-PCR in
the uteri, vaginas and ovaries of WT mice, but not in those of
Sema4D−/− mice. Plexin-B1 mRNA was detected by RT-PCR in
WT and Sema4D−/− vaginas. +/+, WT mice; −/−,
Sema4D−/− mice. (B) Western blotting detected Sema4D in
WT vaginas, but not in Sema4D−/− vaginas. Plexin-B1
protein was detected in WT and Sema4D−/− vaginas. +/+,
WT mice; −/−, Sema4D−/− mice. (C) Immunohistochemical
analyses with anti-Sema4D antibodies detected Sema4D in the
suprabasal layer of the vaginal epithelia in WT mice (arrow). (D)
IHC did not detect Sema4D in Sema4D−/− vaginas. (E)
Plexin-B1 was detected in WT vaginal mucosa by immunohistochemical
analysis using plexin-B1-specific antibodies (arrow). (F) Plexin-B1
was also detected in Sema4D−/− vaginal mucosa by IHC
(arrow). Scale bar=50 μm. (Magnification of C-F, ×400). Sema4D,
semaphorin 4D; WT, wild-type; RT-PCR, reverse
transcription-polymerase chain reaction; IHC,
immunohistochemistry. |
Fewer apoptotic cells exist in
Sema4D−/− vaginal epithelia
TUNEL assay and cleaved caspase-3
immunohistochemistry was applied to detect apoptotic cells in
situ and examine apoptosis in the vaginal epithelia of
five-week-old WT and Sema4D−/− mice. Several
TUNEL-positive and cleaved caspase-3-positive cells were observed
in the WT vaginal epithelia (Fig.
4A). By contrast, there were fewer TUNEL-positive and cleaved
caspase-3-positive cells in the Sema4D−/− vaginal
epithelia (Fig. 4A). Statistical
analyses revealed significantly fewer TUNEL-positive and cleaved
caspase-3-positive cells in Sema4D−/− vaginal epithelia
compared with WT epithelia (Fig.
4B). Western blotting of cleaved caspase-3 confirmed the
significantly lower level of apoptosis in the Sema4D−/−
vaginal tissues compared with the WT tissues (Fig. 4C).
Sema4D induces apoptosis in cultured
vaginal epithelial cells derived from Sema4D−/− mice
To examine whether Sema4D induces apoptosis of
vaginal epithelial cells, recombinant Sema4D was added to primary
vaginal epithelial cells derived from Sema4D−/− mice.
After 36 h, Sema4D increased TUNEL-positive cells in culture
(Fig. 5A). Quantitative analysis
demonstrated that Sema4D induced a significant increase in the
percentage of TUNEL-positive vaginal epithelial cells (Fig. 5B). Immunocytochemistry with
antibodies against cleaved caspase-3 also demonstrated that vaginal
cells with activated caspase-3 were significantly more numerous in
Sema4D-treated culture as compared with the untreated culture
(Fig. 5B). Thus, Sema4D induced
apoptosis of Sema4D−/− vaginal epithelial cells in
culture.
Sema4D-induced apoptosis of vaginal
epithelial cells is mediated through plexin-B1
To investigate whether plexin-B1 is involved in the
Sema4D-induced apoptosis of vaginal epithelial cells in culture,
lentiviruses with shRNA directed to knockdown plexin-B1 were added
to cultured Sema4D−/− vaginal epithelial cells. Two days
after lentivirus application, the level of plexin-B1 mRNA was
significantly reduced in the vaginal cell culture treated with
shRNA against plexin-B1 (Fig. 6A).
Sema4D-induced apoptosis was examined on a TUNEL assay 36 h after
recombinant Sema4D was added to the culture with plexin-B1
knockdown. As a result, vaginal epithelial cells with plexin-B1
knockdown had significantly fewer TUNEL-positive cells compared
with cells harboring control shRNA (Fig. 6B and C). Thus, knockdown of
plexin-B1 in vaginal epithelial cells inhibited Sema4D-induced
apoptosis, indicating that Sema4D used plexin-B1-mediated cell
signaling to execute the apoptosis of vaginal epithelial cells.
 | Figure 6Sema4D-induced vaginal epithelial
cell apoptosis is mediated through plexin-B1. (A) Quantitative
reverse transcription-polymerase chain reaction confirmed the knock
down of plexin-B1 mRNA expression by the lentiviral vector
harboring plexin-B1 specific shRNA in cultured vaginal epithelial
cells derived from Sema4D−/− mice.
*P<0.05, Student’s t-test. (B) Sema4D-induced
TUNEL-positive cells were hardly detectable in cultured
Sema4D−/− vaginal epithelial cells infected with the
lentiviral vector harboring plexin-B1 specific shRNA. shRNA-NT +
Sema4D, control shRNA-NT-transduced Sema4D−/− vaginal
epithelial cell culture applied with recombinant Sema4D; Plexin-B1
shRNA + Sema4D, Plexin-B1 shRNA-transduced Sema4D−/−
epithelial cell culture applied with recombinant Sema4D. Arrow-head
indicates TUNEL-positive cells. Scale bar=10 μm. (C) Knockdown of
plexin-B1 expression using lentiviral vector harboring plexin-B1
specific shRNA significantly inhibited Sema4D-induced apoptosis in
mouse vaginal epithelial cells. The graph shows the ratio of
TUNEL-positive cells to DAPI-positive nucleated cells. shRNA-NT,
vaginal epithelial cell culture transduced with shRNA-NT;
shRNA-Plexin-B1, vaginal epithelial cell culture transduced with
plexin-B1 specific shRNA. Sema4D -, without Sema4D. Sema4D +,
applied with recombinant Sema4D. *P<0.05. Sema4D,
semaphorin 4D; DAPI, 4′,6-diamidino-2-phenylindole; TUNEL, terminal
deoxynucleotidyl transferase dUTP nick end labeling; shRNA, short
hairpin RNA; shRNA-NT, non-target shRNA. |
Discussion
The present study analyzed Sema4D−/−
BALB/c mice with a closed vaginal phenotype and revealed the
importance of Sema4D (a class 4 semaphorin) in the vaginal opening
process, a mouse postnatal tissue remodeling phenomenon (1). The present study also revealed the
apoptosis-inducing activity of Sema4D in cultured vaginal
epithelial cells and the integral role of plexin-B1 for the
completion of apoptosis. Thus, to the best of our knowledge, the
present study is the first to report the novel physiological role
of semaphorin, a known axon guidance molecule, in the mouse
postnatal vaginal opening process.
The mouse postnatal vaginal opening process
occurring at approximately five weeks old is largely dependent on
massive vaginal mucosal apoptosis, which is initiated by rapidly
elevated levels of estrogen in the body at the time of postnatal
vaginal tissue remodeling (1).
Since administration of β-estradiol to infant Sema4D−/−
mice did not induce either precocious vaginal opening or vaginal
mucosal apoptosis (Fig. 2), Sema4D
may be crucially implicated in apoptosis of the vaginal opening
process by acting downstream of estrogen during its elevation at
mouse puberty. The lower number of apoptotic cells observed in the
vaginal epithelia of five-week-old Sema4D−/− mice
(Fig. 4) suggests insufficient
apoptosis at the time of vaginal opening as the cause of
imperforate vagina in Sema4D−/− mice. The high incidence
of imperforate vagina and prominent decrease in vaginal epithelial
apoptosis during the vaginal opening period in Sema4D−/−
mice implies that Sema4D is able to induce apoptosis in vaginal
epithelial cells. The present study, by adding recombinant Sema4D
to Sema4D−/− vaginal epithelial cells in culture,
demonstrated the apoptosis-inducing ability of Sema4D (Fig. 5). Sema3A, a class 3 semaphorin, is
known to induce apoptosis in kidney podocytes (21). Sema4D, released from activated T
lymphocytes, induces apoptosis in neural progenitor cells and
immature oligodendrocytes (15). A
previous study suggested that Sema4D is able to regulate the
differentiation of oligodendrocytes by facilitating
oligodendrocytic apoptosis (16).
Furthermore, Sema3A was demonstrated to regulate Fas-mediated
apoptosis by promoting migration of the Fas molecule to lipid rafts
(22). Thus, during development,
semaphorins function not only as axon guidance molecules but also
as inducers of apoptosis. The present study revealed the
involvement of plexin-B1 in the Sema4D-induced apoptosis of vaginal
epithelial cells in culture (Fig.
6). Therefore, Sema4D may promote postnatal vaginal opening by
inducing massive vaginal epithelial apoptosis by binding to
plexin-B1 receptors on vaginal epithelial cells.
Imperforate vagina has not been observed in
Sema4D−/− C57BL/6 mice, although they are present in
Sema4D−/− BALB/c mice with a high incidence. In
Sema4D−/− C57BL/6 mice, there is a migratory defect of
luteinizing-hormone-releasing-hormone neuron precursor cells from
the olfactory placode to the hypothalamus during embryonic
development (23). Furthermore,
there is a significant reduction in the number of secondary ovarian
follicles in Sema4D−/− C57BL/6 mice ovaries (24). In the present study, no significant
difference was identified in the serum estrogen level between WT
and Sema4D−/− BALB/c mice at the time of vaginal
opening. The injection of β-estradiol into infant
Sema4D−/− mice suggested that the closed vaginal
phenotype was not caused by insufficient estrogen secretion in the
mutant mice (Fig. 2). Although
plexin-B1 was identified as a receptor for the induction of vaginal
epithelial cell apoptosis in the present study (Fig. 6), imperforate vagina has not been
reported in plexin-B1-deficient C57BL/6 mice (25,26).
This may reflect the phenotypic differences dependent on genetic
background, where vaginal epithelial cell apoptosis may be more
highly dependent on Sema4D/plexin-B1 signaling in BALB/c mice than
in other mouse strains. Future studies are required to investigate
whether imperforate vagina is also observed in plexin-B1-deficient
BALB/c mice.
In conclusion, the results from the present study
suggest that the vaginal opening caused through postnatal tissue
remodeling in BALB/c mice proceeds as a result of massive
epithelial cell apoptosis in the vaginal cavity signaled by Sema4D
and plexin-B1 when the mice are five weeks old.
Acknowledgements
The authors would like to thank the members of the
Department of Physiology, Meijo University for their discussion and
technical assistance. This study was primarily supported by a
Grant-in-Aid for Scientific Research from the Ministry of
Education, Culture, Sports, Science and Technology, Japan (no.
19590178).
References
|
1
|
Rodriguez I, Araki K, Khatib K, Martinou
JC and Vassalli P: Mouse vaginal opening is an apoptosis-dependent
process which can be prevented by overexpression of Bcl2. Dev Biol.
184:115–121. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hübner A, Cavanagh-Kyros J, Rincon M,
Flavell RA and Davis RJ: Functional cooperation of the proapoptotic
Bcl2 family proteins Bmf and Bim in vivo. Mol Cell Biol. 30:98–105.
2010. View Article : Google Scholar
|
|
3
|
Lindsten T, Ross AJ, King A, et al: The
combined functions of proapoptotic Bcl-2 family members Bak and Bax
are essential for normal development of multiple tissues. Mol Cell.
6:1389–1399. 2000. View Article : Google Scholar
|
|
4
|
Simpson KJ, Wati MR, Deans AJ, Lindeman GJ
and Brown MA: MMTV-trBrca1 mice display strain-dependent
abnormalities in vaginal development. Int J Dev Biol. 48:675–678.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cano-Gauci DF, Song HH, Yang H, et al:
Glypican-3-deficient mice exhibit developmental overgrowth and some
of the abnormalities typical of Simpson-Golabi-Behmel syndrome. J
Cell Biol. 146:255–264. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sundberg JP and Brown KS: Imperforate
vagina and mucometra in inbred laboratory mice. Lab Anim Sci.
44:380–382. 1994.PubMed/NCBI
|
|
7
|
Pasterkamp RJ: Getting neural circuits
into shape with semaphorins. Nat Rev Neurosci. 13:605–618. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kruger RP, Aurandt J and Guan KL:
Semaphorins command cells to move. Nat Rev Mol Cell Biol.
6:789–800. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Semaphorin Nomenclature Committee. Unified
nomenclature for the semaphorins/collapsins. Cell. 97:551–552.
1999. View Article : Google Scholar
|
|
10
|
Swiercz JM, Kuner R, Behrens J and
Offermanns S: Plexin-B1 directly interacts with PDZ-RhoGEF/LARG to
regulate RhoA and growth cone morphology. Neuron. 35:51–63. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakamura F, Kalb RG and Strittmatter SM:
Molecular basis of semaphorin-mediated axon guidance. J Neurobiol.
44:219–229. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Janssen BJ, Robinson RA, Pérez-Brangulí F,
et al: Structural basis of semaphorin-plexin signaling. Nature.
467:1118–1122. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oinuma I, Ishikawa Y, Katoh H and Negishi
M: The semaphorin 4D receptor plexin-B1 is a GTPase activating
protein for R-Ras. Science. 305:862–865. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saito Y, Oinuma I, Fujimoto S and Negishi
M: Plexin-B1 is a GTPase activating protein for M-Ras, remodeling
dendrite morphology. EMBO Rep. 10:614–621. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Giraudon P, Vincent P, Vuaillat C, et al:
Semaphorin CD100 from activated T lymphocytes induces process
extension collapse in oligodendrocytes and death of immature neural
cells. J Immunol. 172:1246–1255. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamaguchi W, Tamai R, Kageura M, Furuyama
T and Inagaki S: Sema4D as an inhibitory regulator in
oligodendrocyte development. Mol Cell Neurosci. 49:290–299. 2012.
View Article : Google Scholar
|
|
17
|
Shi W, Kumanogoh A, Watanabe C, et al: The
class IV semaphorin CD100 plays nonredundant roles in the immune
system: defective B and T cell activation in CD100-deficient mice.
Immunity. 13:633–642. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li L, Tanaka T, Yukawa K, Akira S and
Umesaki N: Irinotecan-induced ovarian follicular apoptosis is
attenuated by deleting the kinase domain of death-associated
protein kinase. Int J Oncol. 34:905–914. 2009.PubMed/NCBI
|
|
19
|
Iguchi T, Uchima FD, Ostrander PL and Bern
HA: Growth of normal mouse vaginal epithelial cells in and on
collagen gels. Proc Natl Acad Sci USA. 80:3743–3747. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kumanogoh A, Watanabe C, Lee I, et al:
Identification of CD72 as a lymphocyte receptor for the class IV
semaphorin CD100: a novel mechanism for regulating B cell
signaling. Immunity. 13:621–631. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guan F, Villegas G, Teichman J, Mundel P
and Tufro A: Autocrine class 3 semaphorin system regulates slit
diaphragm proteins and podocyte survival. Kidney Int. 69:1564–1569.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moretti S, Procopio A, Lazzarini R, et al:
Semaphorin3A signaling controls Fas (CD95)-mediated apoptosis by
promoting Fas translocation into lipid rafts. Blood. 111:2290–2299.
2008. View Article : Google Scholar
|
|
23
|
Giacobini P, Messina A, Morello F, et al:
Semaphorin 4D regulates gonadotropin hormone-releasing hormone-1
neuronal migration through PlexinB1-Met complex. J Cell Biol.
183:555–566. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dacquin R, Domenget C, Kumanogoh A,
Kikutani H, Jurdic P and Machuca-Gayet I: Control of bone marrow
resorption by semaphorin 4D is dependent on ovarian function. PLoS
One. 6:e266272011. View Article : Google Scholar
|
|
25
|
Hirschberg A, Deng S, Korostylev A, et al:
Gene deletion mutants reveal a role for semaphorin receptors of the
plexin-B family in mechanisms underlying corticogenesis. Mol Cell
Biol. 30:764–780. 2010. View Article : Google Scholar :
|
|
26
|
Fazzari P, Penachioni J, Gianola S, et al:
Plexin-B1 plays a redundant role during mouse development and in
tumour angiogenesis. BMC Dev Biol. 7:552007. View Article : Google Scholar : PubMed/NCBI
|