Introduction
Esophageal cancer is one of the top ten most common
types of cancer in human beings, with operable esophageal cancer
patients accounting for only 20% of patients. The survival rate is
<10% after five years; the primary reason for the poor prognosis
of esophageal cancer patients is the aggressiveness of tumor
towards surrounding healthy tissue and the ease at which it
metastasizes to the lymph nodes. Although there have been numerous
studies on the molecular markers of esophageal cancer, the
underlying mechanisms of invasion and metastasis remain to be
determined. As an important member of the a disintegrin and
metalloproteinase (ADAM) family, ADAM17, termed tumor necrosis
factor-α converting enzyme (TACE), is the primary secretase
responsible for releasing the soluble form of tumor necrosis
factor-α from the plasma membrane and is the primary sheddase for
multiple epidermal growth factor receptor (EGFR) pro-ligands
(1,2). The function of the ADAMl7 protein is
the hydrolysis and release of precursor cell surface proteins. It
transversely activates cell surface molecules of the cell signaling
pathway in order to alter the signal transmission in the tumor
microenvironment, which associated with tumorigenesis and tumor
progression (3–5). ADAM17 is involved in proteolysis of
collagen V, VII and X, and gelatin, as well as the release of
certain integrins from the cell surface. This suggests that ADAM17
may affect tumor angiogenesis and the invasive activity of tumor
cells (1). Recent studies have
shown an increase in the expression and activity of ADAM17 in a
number of human tumor tissues, including breast cancer, lung
cancer, gastric cancer and liver cancer (6–9).
However, there are limited studies reporting the expression of
ADAM17 in esophageal cancer. An improved understanding of the
importance of ADAM17 and the downstream EGFR signaling pathway in
esophageal cancer invasion may aid in the development of
therapeutic strategies aimed at reducing the invasiveness of
esophageal cancer.
The current study used the reverse transcription
polymerase chain reaction (RT-PCR) technique and streptavidin
peroxidase conjugated (SP) immunohistochemistry to detect ADAMl7
mRNA and protein expression in esophageal squamous cell carcinoma
in order to explore its clinical pathological significance and
prognostic value in patients with esophageal squamous cell
carcinoma.
Materials and methods
Clinical pathological material
Between 2007 and 2010 in the Pathology Department of
the First People’s Hospital of Nantong, esophageal squamous cell
carcinoma and normal esophageal mucosa specimens were obtained from
50 patients and prepared for RT-PCR detection. The specimens
consisted of fresh tissues without necrosis; 30 min following the
extraction of the specimens they were placed into liquid nitrogen
and quickly transferred to a refrigerator where they were
maintained at −80°C prior to use. None of the patients received
pre-operative radiotherapy or chemotherapy, and the cases were
diagnosed by histopathological examination. The patients included
33 males and 17 females with ages ranging from 45 to 77 years, and
a median age of 61 years. The patients were spread among all phases
of tumor, node and metastasis (TNM) classification: phase I (n=5),
phase II (n=24), phase III (n=15) and phase IV (n=6).
From 2000 to 2002, esophageal squamous cell
carcinoma and normal esophageal mucosa specimens were obtained from
80 patients and subjected to SP immunohistochemistry. Patients
included 57 males and 23 females with ages ranging from 47 to 75
years, and a median age of 61 years. The patients were spread among
all phases of TNM classification: phase I (n=5), phase II (n=38),
phase III (n=31) and phase IV (n=6). No patients had a history of
preoperative radiotherapy or chemotherapy. Of the 80 esophageal
squamous cell carcinoma patients who received a follow-up letter,
16 cases were missing and 64 cases replied to the follow-up, giving
a follow-up rate of 80% (64/80). The follow-up time was >60
months. The study was approved by the ethics committee of the
Second Affiliated Hospital of Nantong University (Nantong, China).
Written informed consent was obtained from all participants.
ADAMl7 mRNA detection using RT-PCR
Total RNA extraction was carried out as follows: 50
mg tissue homogenates were collected, and the total tissue RNA was
extracted using TRIzol® (Invitrogen Life Technologies,
Carlsbad, CA, USA). The purity of a sample of the total RNA was
measured using an ultraviolet spectrophotometer (Beijing Purkinje
General Instrument Co., Ltd., Beijing, China).
ADAM17 mRNA primers were designed according to the
literature (1), and the size of
their product was 440 bp. The primer sequences were as follows:
forward, 5′-GCACAGGTAATAGCAGTGAGTGC-3′, and reverse,
5′-CACACAATGGACAAGAATGCTG-3′ for ADAM17 mRNA; and forward,
5′-TTCCAGCCTTCCTTCCTGG-3′, and reverse, 5′-TTGCGCTCAGGAGGAGCAAT-3′
for β-actin. ADAM17 mRNA and β-actin primers were synthesized by
Shanghai Biotechnology Company (Shanghai, China). The ADAM17 and
β-actin reaction were performed within a tube, and the conditions
for gene amplification were as follows: 95°C denaturation for 5
min, followed by 40 cycles of 94°C for 50 sec, 52°C for 1 min and
72°C for 1 min.
Semi-quantification of PCR results
PCR products were transferred onto a 1.5% agarose
gel for electrophoresis, the Eagle Eye™ II Image Analysis system
(Agilent Technologies Inc., Santa Clara, CA, USA) was used to scan
the bands of the amplified products. The ratio of the optical
density of ADAM17 to the optical density of β-actin in the same
tube was used to represent the ADAM17 mRNA expression.
ADAM17 and EGFR protein detection with SP
immunohistochemistry
All specimens were fixed in 10% neutral formalin,
paraffin-embedded and sliced (thickness, 4 μm). Hematoxylin and
eosin (HE)staining and immunohistochemical SP staining were then
performed. The primary anti-ADAM17 and EGFR antibodies were mouse
anti-human monoclonal antibodies purchased from Beijing Zhongshan
Golden Bridge Company (Beijing, China). According to the literature
(2), based on the percentage of
positive cells, the cells were divided into four groups, 0: no
positive cells, 1 point: <30% positive cells, 2 points: 30–70%
positive cells, 3 points: >70% positive cells. The cells were
subsequently divided into further groups according to the staining
intensity, 0 point: no staining, 1 point: positive cells were
stained pale yellow; 2 points: positive cells were stained yellow
and 3 points: positive cells were stained brown. The two combined
scores were taken as the final result: 0 point (−), 1 to 2 points
(+), 3 to 4 points (+ +), 5 to 6 points (+ + +). In statistics, the
(−) and (+) were classified as the negative group, (+ +) and (+ +
+) were classified as the positive group.
Statistical analysis
The Stata7.0 statistical package was used for all
statistical analysis. ADAM17 mRNA expression levels were expressed
as the mean ± standard deviation (χ̄ ± s). The Student’s t test was
used for comparison in different groups. The rates were compared
with a χ2 test. The univariate analysis of the follow-up
results was performed with a logrank test, and Kaplan-Meier
survival curves were created. Multivariate survival analysis was
performed using Cox proportional hazard model statistics. P<0.05
was considered to indicate a statistically significant
difference.
Results
ADAM17 mRNA expression of esophageal
squamous cell carcinoma
The length of the reference β-actin gene fragment
was 218 bp. The length of ADAM17 gene fragment was 440 bp. The
normal esophageal mucosa and esophageal squamous cell carcinoma had
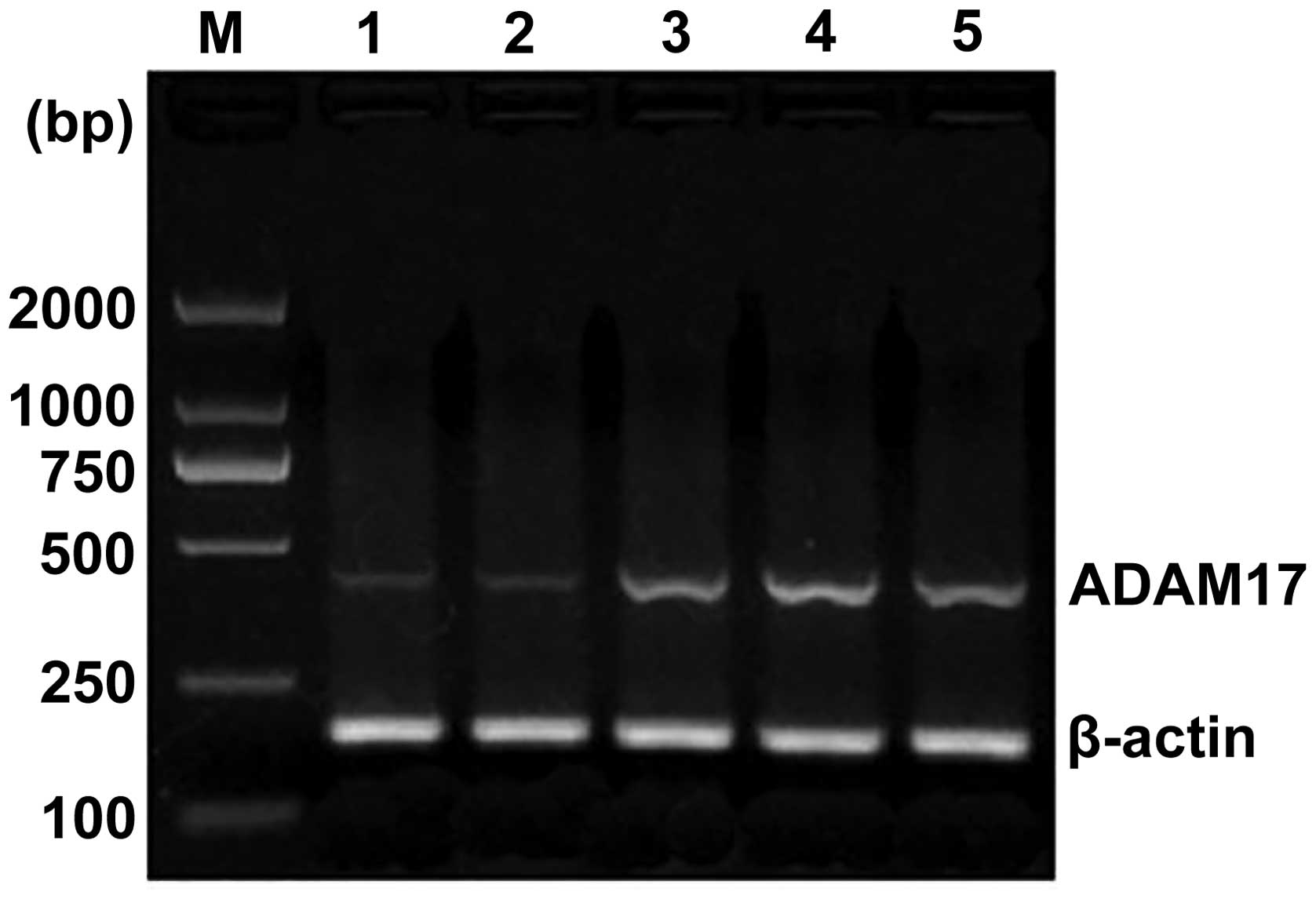
varying intensities of expression (Fig. 1). In the 50 cases of esophageal
squamous cell carcinoma, the ratio of ADAM17 mRNA expression to
that of β-actin was increased to 0.937±0.241 (Table I), compared with the ratio of
expressions in normal esophageal mucosa, which was 0.225 ± 0.077
(P<0.01). As shown in Table
II, in the 50 cases of esophageal squamous cell carcinoma,
ADAM17 mRNA expression was significantly higher in the lymph node
metastasis group than that of the lymph node-negative group
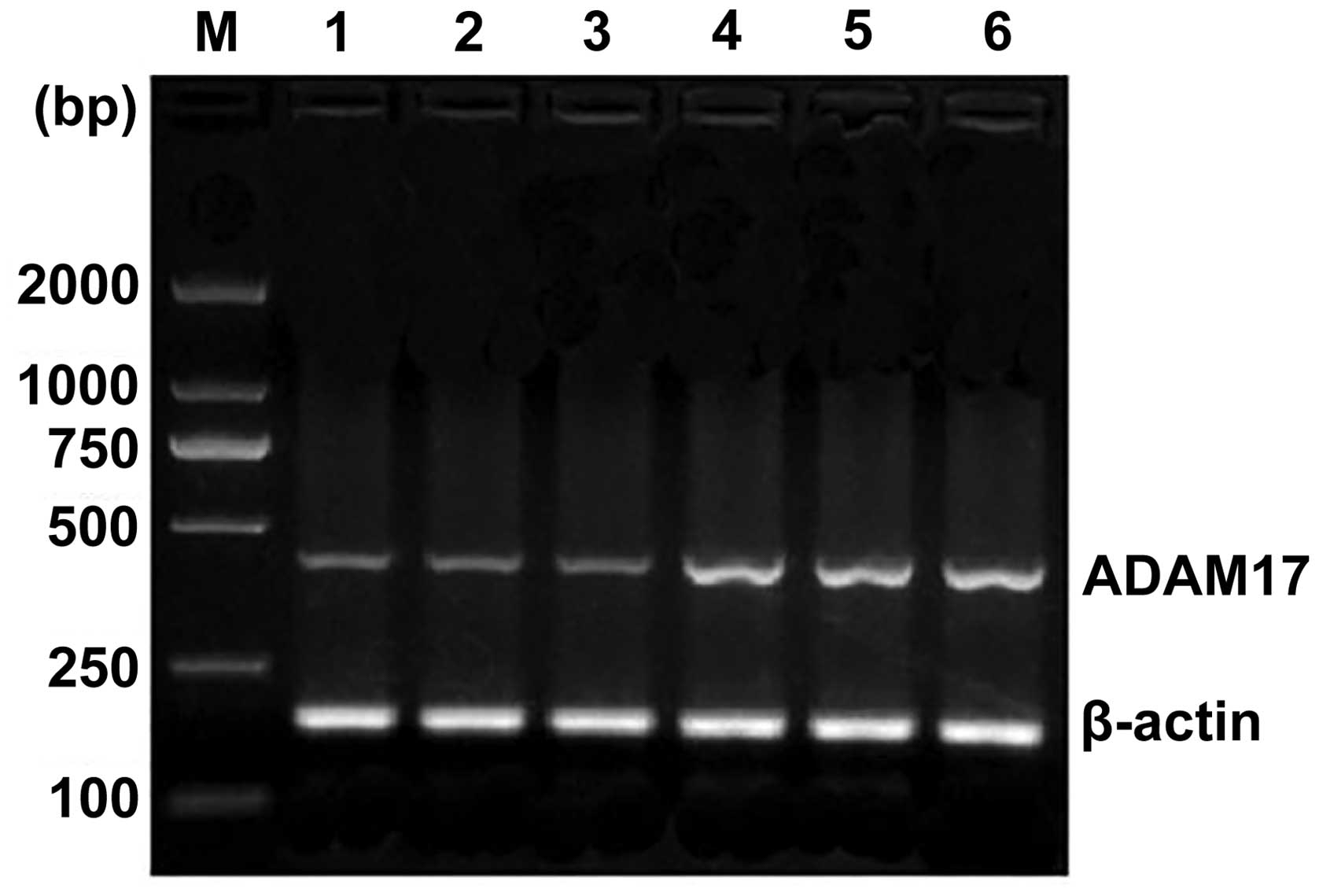
(P<0.01) (Fig. 2). In the TNM
stages, the ADAM17 mRNA expression of stage I + II patients was
significantly different to that of stage III + IV patients
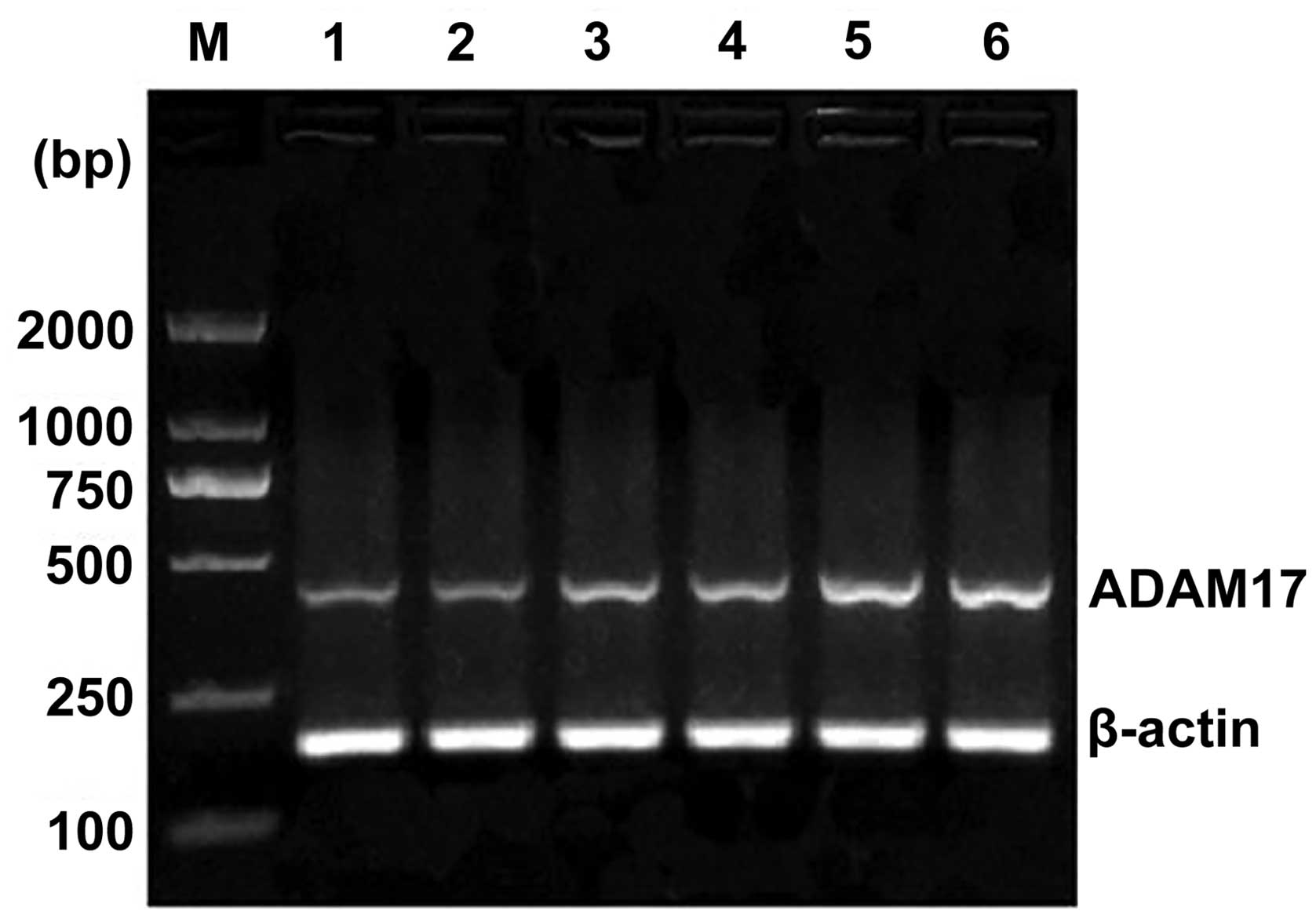
(P<0.05). Esophageal squamous cell histology revealed that the
levels of ADAM17 mRNA expression in stage I, II and III was
increased, however, no significant difference was determined
(P>0.05) (Fig. 3). In addition,
ADAM17 mRNA expression had no correlation with other
clinicopathological factors, including gender and age
(P>0.05).
 | Table IADAM17 mRNA and protein levels in
esophageal squamous cell carcinoma. |
Table I
ADAM17 mRNA and protein levels in
esophageal squamous cell carcinoma.
| RT-PCR | SP |
|---|
|
|
|
|---|
| Group | Cases (n) | ADAM17 | P-value | Cases (n) | Negative (%) | Positive (%) | P-value |
|---|
| Esophageal squamous
cell carcinoma | 50 | 0.937±0.241 | <0.001 | 80 | 27 (33.75) | 53 (66.25) | <0.001 |
| Normal
esophageal | 50 | 0.225±0.077 | | 80 | 75 (93.75) | 5 (6.25) | |
 | Table IIADAM17 mRNA and protein in esophageal
squamous cell carcinoma. |
Table II
ADAM17 mRNA and protein in esophageal
squamous cell carcinoma.
| RT-PCR | SP |
|---|
|
|
|
|---|
| Category | Cases (n) | ADAM17 | P-value | Cases (n) | Negative (%) | Positive (%) | P-value |
|---|
| Gender | | | 0.766 | | | | 0.518 |
| Male | 33 | 0.947±0.276 | | 57 | 18 (31.58) | 39 (68.42) | |
| Female | 17 | 0.923±0.252 | | 23 | 9 (39.13) | 14 (60.87) | |
| Age (years) | | | 0.369 | | | | 0.297 |
| ≤60 | 23 | 0.902±0.314 | | 35 | 14 (40.00) | 21 (60.00) | |
| >60 | 27 | 0.974±0.247 | | 45 | 13 (28.89) | 32 (71.11) | |
| Histological
grade | | | 0.148 | | | | 0.150 |
| I | 10 | 0.852±0.184 | | 15 | 8 (53.33) | 7 (46.67) | |
| II | 26 | 0.923±0.218 | | 43 | 14 (32.56) | 29 (67.44) | |
| III | 14 | 1.241±0.141 | | 22 | 5 (22.73) | 17 (77.27) | |
| Lymph node
metastasis | | | 0.000 | | | | 0.006 |
| Absence | 30 | 0.884±0.184 | | 45 | 21 (46.67) | 24 (53.33) | |
| Presence | 20 | 1.172±0.249 | | 35 | 6 (17.14) | 29 (82.86) | |
| TNM stagea | | | 0.011 | | | | 0.009 |
| I + II | 29 | 0.894±0.131 | | 43 | 20 (46.51) | 23 (53.49) | |
| III + IV | 21 | 1.024±0.215 | | 37 | 7 (18.92) | 30 (81.08) | |
ADAM17 protein levels in esophageal
squamous cell carcinoma
Following SP immunohistochemical staining, ADAM17
expression in esophageal squamous cell carcinoma was defined as
brown or brown-black particles located in the cell cytoplasm
(Fig. 4). Of the 80 cases of
esophageal squamous cell carcinoma (Table I), 53 cases demonstrated positive
ADAM17 protein expression (66.25%) and five cases had positive
expression in the corresponding normal tissues (6.25%), a
statistically significant difference (P<0.01). The percentage of
positive ADAM17 protein expression in the lymph node metastasis
group was significantly higher than that of the lymph node-negative
group (P<0.05). In TNM stages, the rate of positive ADAM17
protein expression in stage I + II patients was significantly
increased compared to that of III + IV patients (P<0.05). The
rate of positive ADAM17 protein expression increased with the
stages of esophageal histology, however, this change was not found
to be statistically significant (P>0.05). The ADAM17 protein
expression showed no correlation with other clinicopathological
factors, including gender and age (P>0.05, Table II). Of the 80 cases of esophageal
squamous cell carcinoma, 53 cases showed positive ADAM17
expression, 40 of which had positive EGFR expression, while of the
27 cases that were negative for ADAM17 expression, 9 cases had
positive EGFR expression. Statistical analysis showed that the EGFR
positive expression rate in the ADAM17 positive expression group
was significantly higher than the of the ADAM17 negative expression
group (P<0.01). Column correlation coefficient analysis showed
that ADAM17 expression and EGFR expression were positively
correlated (P<0.01, Table
III).
 | Table IIIADAM17 and EGFR expression in
esophageal squamous cell carcinoma compared. |
Table III
ADAM17 and EGFR expression in
esophageal squamous cell carcinoma compared.
| | EGFR | | | |
|---|
| |
| | | |
|---|
| ADAM17 | Cases (n) | Negative (%) | Positive (%) | P-value | Cramer’s V | γ |
|---|
| Negative | 27 | 18 (66.67) | 9 (33.33) | <0.001 | 0.409 | 0.720 |
| Positive | 53 | 13 (24.53) | 40 (75.47) | | | |
Prognostic significance of ADAM17 and
EGFR expression in esophageal squamous cell carcinoma
The follow-up data showed that the 1, 3 and 5-year
survival rates in esophageal squamous cell carcinoma were 85.94,
34.38 and 11%, respectively. Through the follow-up data of
esophageal squamous cell carcinoma patients, a univariate logrank
survey showed that ADAM17 and EGFR were prognostic factors
affecting esophageal squamous cell carcinoma (All P<0.05,
Table IV). Further ADAM17
Kaplan-Meier survival curves showed that the survival rate in the
ADAM17 positive group was significantly lower than those in ADAM17
negative group (P<0.01) (Fig.
5). The multivariate Cox proportional hazards model analysis
showed that ADAM17, EGFR, lymph node metastasis and TNM stage all
had independent prognostic significance (all P<0.05, Table V). This indicates that ADAM17 and
EGFR expression may increase the risk of esophageal cancer
mortality.
 | Table IVADAM17 and EGFR expression in
esophageal squamous cell carcinoma and patient survival data
(Logrank test). |
Table IV
ADAM17 and EGFR expression in
esophageal squamous cell carcinoma and patient survival data
(Logrank test).
| Category | Cases (n) | Average survival
time (month) | 95% CI | χ2 | P-value |
|---|
| ADAM17
expression |
| Negative | 27 | 64.444±5.191 | 54.271, 74.618 | 7.56 | 0.006 |
| Positive | 53 | 37.377±4.247 | 29.053, 45.702 | | |
| EGFR
expression |
| Negative | 31 | 59.349±4.664 | 50.208, 68.491 | 5.28 | 0.022 |
| Positive | 49 | 39.755±5.109 | 29.740, 49.770 | | |
 | Table VMultivariate survival analysis of
esophageal cancer (Cox proportional hazards model). |
Table V
Multivariate survival analysis of
esophageal cancer (Cox proportional hazards model).
| Clinicopathological
factors | Variable
coefficient | Standard error | Z-statistic | P-value | 95% CI |
|---|
| Age | −0.122 | 0.252 | −0.491 | 0.627 | −0.615, 0.371 |
| Gender | −0.311 | 0.285 | −1.094 | 0.275 | −0.869, 0.247 |
| Classification | 0.041 | 0.178 | 0.232 | 0.819 | −0.307, 0.389 |
| Lymph node
metastasis | 0.601 | 0.243 | 2.375 | 0.018 | 0.104, 1.097 |
| TNM staging | 0.526 | 0.226 | 2.085 | 0.037 | 0.031, 1.021 |
| EGFR | 0.585 | 0.260 | 2.254 | 0.024 | 0.076, 1.095 |
| ADAM17 | 0.724 | 0.271 | 2.683 | 0.007 | 0.194, 1.254 |
Discussion
China has a high incidence of esophageal cancer. The
majority of patients do not exhibit early symptoms, and a number of
them do not receive treatment in the late phase. In the early and
mid terms of esophageal cancer, the most common treatments are
surgery or radiotherapy and supplemented with chemotherapy
treatment. For late phase patients, chemotherapy was used as a
palliative treatment. With the progress in tumor molecular biology,
the tumors molecular targeted therapy acting on tumor cells had
target selectivity, efficiency, low toxicity and other advantages.
It achieved notable results in breast cancer and non-small cell
lung cancer therapy. Currently, looking for the effective molecular
targets in esophageal cancer treatment became a research hotspot of
esophageal cancer.
The ADAM family includes a set of transmembrane
secretory proteins composed of multiple domains. Their most clear
effect is the release of certain important biological ligands,
including tumor necrosis factor α, epidermal growth factor and
transforming growth factor α. Since these ligands involved in tumor
formation and progression, it can be inferred that the specific
molecular ADAMs associated with the release of these ligands are
involved in the malignant process (1,2).
ADAMl7 is an ADAM superfamily also known as TACE. Le et al
(3) found that in ADAM17 deficient
mice, T cells lost almost all of their ability to release tumor
necrosis factor-α. and that ADAM17 was likely to be physiological
shedding enzyme of tumor necrosis factor-α; the function of the
ADAMl7 protein was that it hydrolyzes and releases precursor cell
surface proteins. It transversely activates the cell surface
molecules of the cell signaling pathway to change the signal
transmission. The biological behaviors of tumor cells, including
tumor angiogenesis, extracellular matrix degradation, remodeling
and cell adhesion functions, reflect the biological behavior of
tumor cells and cellular signal transduction, and exhibited a close
association with ADAMl7 function (4,5).
In recent years, studies found the high expression
of ADAM17 in a variety of human tumors, it reflected the degree of
malignancy, promoted tumor invasion and metastasis process, it was
associated with prognosis of cancer in patients. McGowan et
al (6) reported the
association between ADAM17 and breast cancer in MRNA and protein
levels respectively. They found that ADAM17 mRNA expression in
breast cancer tissue was positively correlated with the number of
lymph node metastasis. It proved that the ADAM17 mRNA was involved
in breast carcinogenesis and progression, the high expression of
ADAM17 will increase the invasiveness and spreading ability of
MCF-7 breast cancer cells in vitro. ADAM17 can shed
amphiregulin, transforming growth factor α and other EGFR ligands,
it can also activate the EGFR to thereby improve the lung cancer
cell proliferation and cell motility capacity (7). ADAM17 expression increased in gastric
cancer cells. ADAM17 promoted cancer cell proliferation through
shedding EGFR ligands (8). Ding
et al (9) found that the
HCC ADAM17 mRNA expression was significantly higher than that in
the surrounding liver tissue. It was closely associated with degree
of differentiation in liver cancer suggesting that the ADAM17 was
associated with the development of liver cancer. Kornfeld et
al (10) reported that ADAM17
can activate the EGFR through the release of regulatory proteins in
two ways to thereby enhance the proliferation and invasion of head
and neck squamous cell carcinoma. Hinsley et al (11) confirmed that ADAM17-mediated
release of EGFR ligands triggered the head and neck cancer cell
migration and were involved in formation of metastatic squamous
cell carcinoma. ADAM17 and esophageal cancer studies were rarely
reported, Sakamoto, etc. (12)
used the RT-PCR and western blot analysis protein electrophoresis
methods, they detected the elevated ADAMTS16 protein expression in
esophageal tissues. In the medium TE5 esophageal cancer cell lines,
ADAMTS16 protein levels were detected. By blocking the ADAMTS16,
the growth and invasion of TE5 esophageal cancer cell can be
inhibited.
The present study used the RT-PCR and SP methods,
the results showing that ADAMl7 mRNA and protein exhibited varying
intensity expression of normal esophageal mucosa and esophageal
squamous cell carcinoma. However, ADAM17 mRNA and protein
expression in esophageal squamous cell carcinoma was significantly
higher than that in the normal esophageal mucosa. ADAMl7 mRNA and
protein overexpression suggested gene mutation occurs in the
process of esophageal carcinoma. ADAMl7 mRNA exhibited a certain
association with the occurrence and development of esophageal
squamous cell carcinoma. This provided a reference for the
diagnosis and differential diagnosis of benign and malignant
lesions in esophageal cancer.
The study also showed that ADAM17 mRNA expression
and protein expression positive rate increased with the I, II, III
stage of esophageal squamous cell histology. However, there was no
statistically significant difference. ADAMl7 mRNA expression and
protein positive expression rate in lymph node metastasis group of
esophageal squamous cell carcinoma were significantly higher than
that in the lymph node-negative group. With regard to TNM staging,
ADAM17 mRNA expression and protein expression rate in patients at
stages I and II were significantly different from those in patients
at stages III and IV. This suggested that ADAMl7 mRNA may be
associated with uncontrolled proliferation of esophageal squamous
cell cancer and it may be involved in the invasion, metastasis and
distant dissemination mechanisms of esophageal squamous cell
carcinoma. The follow-up data showed that the esophageal squamous
cell carcinoma ADAM17 expression was an important prognostic factor
affecting the esophageal squamous cell carcinoma, it had
independent prognostic significance as EGFR, lymph node metastasis
and TNM stage. This indicated that high ADAMl7 mRNA and protein
expression may become an marker for metastasis and prognosis in
esophageal squamous cell carcinoma.
Lorenzen et al (5) found that ADAMl7 can mediate the
release of specific EGFR ligands, the latter binding with EGF which
activates EGFR, resulting in the epithelial hyperplasia disorder
developing into cancer. Previous studies found that selective
inhibitors of ADAM17 protease can block the tumor cell EGFR pathway
(5,8,13).
Currently the EGFR signaling pathway is a known target of targeted
anticancer drugs (14). Certain
drugs which use EGFR as the therapeutic target, including
Erbitux®, are already on the market (15). The present study showed that ADAM17
expression had a positive correlation with EGFR expression
indicating that in patients with esophageal squamous cell
carcinoma, certain target EGFR drugs produce tolerance.
ADAMl7-mediated release of specific EGFR ligands can be altered for
clinical treatment. It can be envisaged that as a target for
targeted therapy, ADAM17 may be a good supplement for the existing
treatment of EGFR targeted therapy.
Acknowledgements
This study was supported by the Applied Research
Foundation of the Science and Technology Department, Nantong,
Jiangsu, China (no. K2010061).
References
|
1
|
Klein T and Bischoff R: Active
metalloproteases of the A Disintegrin and Metalloprotease (ADAM)
family: biological function and structure. J Proteome Res.
10:17–33. 2011. View Article : Google Scholar
|
|
2
|
Benarroch EE: ADAM proteins, their
ligands, and clinical implications. Neurology. 78:914–920. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Le Gall SM, Bobe P, Reiss K, et al: ADAMs
10 and 17 represent differentially regulated components of a
general shedding machinery for membrane proteins such as
transforming growth factor alpha, L-selectin, and tumor necrosis
factor alpha. Mol Biol Cell. 20:1785–1794. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saftig P and Reiss K: The “A Disintegrin
And Metalloproteases” ADAM10 and ADAM17: novel drug targets with
therapeutic potential? Eur J Cell Biol. 90:527–535. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lorenzen I, Trad A and Grozinger J:
Multimerisation of A disintegrin and metalloprotease protein-17
(ADAM17) is mediated by its EGF-like domain. Biochem Biophys Res
Commun. 415:330–336. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McGowan PM, Ryan BM, Hill AD, et al:
ADAM-17 expression in breast cancer correlates with variables of
tumor progression. Clin Cancer Res. 13:2335–2343. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baumgart A, Seidl S, Vlachou P, et al:
ADAM17 regulates epidermal growth factor receptor expression
through the activation of Notch1 in non-small cell lung cancer.
Cancer Res. 70:5368–5378. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ebi M, Kataoka H, Shimura T, et al:
TGFbeta induces proHB-EGF shedding and EGFR transactivation through
ADAM activation in gastric cancer cells. Biochem Biophys Res
Commun. 402:449–454. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ding X, Yang LY, Huang GW, Wang W and Lu
WQ: ADAM17 mRNA expression in isolation, large hepatocellular
carcinoma and its significance. Zhong Hua Gan Dan Wai Ke Za Zhi.
11:544–546. 2005.
|
|
10
|
Kornfeld JW, Meder S, Wohlberg M, et al:
Overexpression of TACE and TIMP3 mRNA in head and neck cancer:
association with tumour development and progression. Br J Cancer.
104:138–145. 2011. View Article : Google Scholar :
|
|
11
|
Hinsley EE, Hunt S, Hunter KD, Whawell SA
and Lambert DW: Endothelin-1 stimulates motility of head and neck
squamous carcinoma cells by promoting stromal-epithelial
interactions. Int J Cancer. 130:40–47. 2012. View Article : Google Scholar
|
|
12
|
Sakamoto N, Oue N, Noguchi T, et al:
Serial analysis of gene expression of esophageal squamous cell
carcinoma: ADAMTS16 is upregulated in esophageal squamous cell
carcinoma. Cancer Sci. 101:1038–1044. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Merchant NB, Voskresensky I, Rogers CM, et
al: TACE/ADAM-17: a component of the epidermal growth factor
receptor axis and a promising therapeutic target in colorectal
cancer. Clin Cancer Res. 14:1182–1191. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu YP and Xu L: Influence of epidermal
growth factor receptor targeted therapy related molecular
detection. Zhong Hua Zhong Liu Za Zhi. 33:81–83. 2011.
|
|
15
|
Han Y, Xu JM, Duan HQ, et al: The
treatment of EGFR gene mutation and the curative effect of
treatment of advanced non-small cell lung cancer and prognosis.
Zhong Hua Zhong Liu Za Zhi. 29:278–283. 2007.
|