Introduction
The self-assembled monolayers (SAMs) technique
comprising alkanethiolates on gold is well established (1,2).
SAMs serve as a good model of the extracellular matrix (ECM) with
different terminal functional groups (3). These substrate-dependent differences
in protein adsorption have profound effects on cellular activities,
including cell adhesion, proliferation, migration and
differentiation (4–7). The SAMs on the gold surface provide
chemical functional group surfaces for cell behaviors. A number of
studies have focused on cell behavior in response to different
chemical groups using the SAM technique, including osteoblastic
cells (8), murine fibroblasts
(9) and mesenchymal stem cells
(10). SAMs greatly affect cancer
cells, including breast cancer (11) and hepatoma (12). Osteosarcoma is one of the most
common types of primary malignant skeletal tumor in children and
adolescents (13). It occurs
primarily around the distal femur, proximal tibia and humerus.
Other significant locations include the proximal femur, pelvis,
skull and jaw (14). The aim of
this study was to investigate the associations between chemical
functional groups and the behaviors of U-2OS human osteosarcoma
cells, including the phenotype, adhesion, proliferation and
apoptosis. The effects of chemical groups on U-2OS cells will aid
in improving the understanding of the regulatory mechanisms of
biomaterials on osteosarcoma cells and may provide a novel way to
use biomaterials to treat and prevent the recurrence of
osteosarcoma.
Materials and methods
Preparation of model surfaces on glass
coverslips
SAMs were prepared using a protocol modified from
our previous study (15). Briefly,
the Au surfaces (40 nm) were deposited on glass coverslips
following a Ti layer (40 nm), using an ANELVAL-400EK electron beam
evaporator (Canon Anelva Corp., Kanagawa, Japan). The silicon
wafers coated with Au (Zhongding Ltd., Yangzhou, China) were washed
with triple-distilled water in an ultrasonic bath for 10 min, and
immersed in 1 Mm 1-undecanethiol (-CH3; Sigma, St.
Louis, MO, USA), 11-amino-1-undecanethiol (-NH2; Sigma),
11-hydroxy-1-undecanethiol (-OH; Sigma), 12-mercaptododecanoic acid
(-COOH; Sigma) for 12 h (16).
Following the self-assembly process, the substrates were washed
with triple-distilled water and dried with nitrogen.
Characterization of substrates
Different groups with -CH3,
-NH2, -OH or -COOH surfaces were characterized by
contact angle measurements. Ambient air-water substrate contact
angle measurements (4 ml ultra-pure H2O) were performed
using a OCA20 contact angle system (Dataphysics, Filderstadt,
Germany) fitted with a digital camera, and analyzed using in-house
image analysis software.
Cell culture
The human osteosarcoma U-2OS cell line was obtained
from the American Type Culture Collection (Manassas, VA, USA).
U-2OS cells were cultured in RPMI-1640 (Hyclone, Logan, UT, USA)
containing 100 U/ml penicillin (Hyclone) and 100 mg/ml streptomycin
(Hyclone), supplemented with 10% fetal bovine serum (FBS;
Invitrogen Life Technologies, Carlsbad, CA, USA) at 37°C in 5%
CO2 and 95% air. U-2OS cells were maintained in 10% FBS
RPMI-1640 and passaged every 2 days.
Cell adhesion
U-2OS cells (5×103 cells/ml) were seeded
onto the different surfaces of the SAMs. Following a 3-, 6- and 9-h
culture, cell adhesion analyses were performed under an inverted
microscope (HBOSO/AC Hg lamp, Oberkochen, Germany; BX-51
microscope, Leica, Germany). Further adhesion analysis of the cells
cultured for 6 h was performed by scanning electron microscopy
(SEM; Hitachi, H50, Hitachi, Ltd., Tokyo, Japan).
The U-2OS cells attached to the surface of the SAMs
could be visualized clearly under the inverted phase contrast
microscope, so three different time points were selected to for
investigation. The cell morphology was examined, and the attached
cell numbers were counted in five random fields.
The morphology of the cells on the sample surfaces
was further examined by SEM. Following cultivation for 6 h, the
samples were removed from the culture plates and fixed with 3%
glutaraldehyde (Sigma-Aldrich, St. Louis, MO, USA) for 6 h.
Following this, they were washed three times with PBS (10 min each
time) and dehydrated sequentially in a series of ethanol (50, 70,
95 and 100%; each concentration twice for 10 min each time) and air
dried in a fume hood. Following sputter-coating with gold, samples
were examined by SEM, and the adhesion and spreading of the cells
in the different groups with modified substrates were observed by
SEM (Hitachi, H50).
Cell viability analysis
Cell viability was analyzed by live/dead cell
staining, MTT and lactate dehydrogenase (LDH) measurement. For
viability staining studies, cells were seeded in 24-well plates at
a concentration of 1×104 cells/ml at 37°C with 5%
CO2. Cells were grown to confluence. The gold substrates
were added to the 24-well plates prior to seeding and incubation
for 24 h. At the end of the incubation period, the media was
removed and the adherent cells were subjected to live/dead staining
following the manufacturer’s instructions (calcein AM; 17783-1MG;
Sigma-Aldrich). The viability of the different chemical groups on
the cells was conducted using the viability/cytotoxicity staining
method following the manufacturer’s instructions (Sigma-Aldrich).
Briefly, 1 μM calcein AM (17783-1MG Sigma-Aldrich) and 2 μM
ethidium homodimer-1 solutions (EthD-1, AnaSpec 83208, 1 mg,
AnaSpec, Fremont, CA, USA) were prepared in phosphate-buffered
saline. Following removal of the culture medium, the cells were
washed once in phosphate buffered saline (PBS; Sigma-Aldrich), 100
μl of 1 μM calcien AM and 2 μM ethidium homodimer-1 solution were
added and the cells were incubated for 30 min. Cell images were
captured using a fluorescence microscope (Olympus, BX51, Olympus
Corporation, Tokyo, Japan); the dyed red and green cells were
counted in five random fields.
Cell proliferation
The cell proliferative ability was tested using an
MTT assay (n=3 donors). The optical density (O.D.) value was tested
2, 4 and 6 days post-culture. At each point, 30 μl of MTT (5 mg/ml)
solution was added to 300 μl medium, and the cells incubated at
37°C for 4 h. The culture medium was removed and the cells washed
in PBS three times. The formazan reaction products were dissolved
in dimethylsulfoxide (DMSO; Sigma-Aldrich) for 10 min. The O.D. of
the formazan solution was measured on an ELISA plate reader
(Thermo, Multiskan G; Thermo Fisher Scientific, Waltham, MA, USA)
at 490 nm.
LDH is a cytoplasmic enzyme, often associated with
cell membrane damage and cell death (17). The LDH activity was measured
spectrophotometrically by assaying reduced nicotinamide adenine
dinucleotide oxidation at a wavelength of 340 nm during the
LDH-catalyzed reduction of pyruvate to lactate. Briefly, cells were
cultured with gold substrates in 24-well plates for 24 h. The
supernatant was then removed and centrifuged to eliminate the
non-adherent cell debris. Adherent cells were lysed with 0.5%
Triton X-100. Samples of each chemical group were then analyzed
spectroscopically.
Cell apoptosis and necrosis
Apoptosis is a form of programmed cell death that
occurs through the activation of intrinsic cell suicide machinery
(18). To analyze changes in
nuclear morphology, U-2OS cells were measured using Guava Nexin
Reagent (Millipore, Billerica, MA, USA). Following culture with
different chemical groups, the cells in contact with the substrates
were washed with PBS and centrifuged at 200 × g for 5 min. The cell
pellets were suspended in 100 μl RPMI-1640 medium supplemented with
1% FBS, then incubated with 100 μl of Annexin V-PE and 7-AAD
labeling solution for 20 min at room temperature. Cells were
analyzed on a Guava EasyCyte 5HT flow cytometer (Millipore) using a
488-nm excitation and a 575-nm bandpass filter for PE detection,
and a 546-nm excitation and a 647-nm filter for 7-AAD detection.
The data were analyzed using the Guava Nexin Software v2.2.2
(Millipore).
Statistical analysis
All statistics were performed with the Origin Pro
8.0 software package (OriginLab, Guangzhou, China). The data were
analyzed by one way analysis of variance using SPSS 13.0 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Physicochemical characterization of
different model surfaces
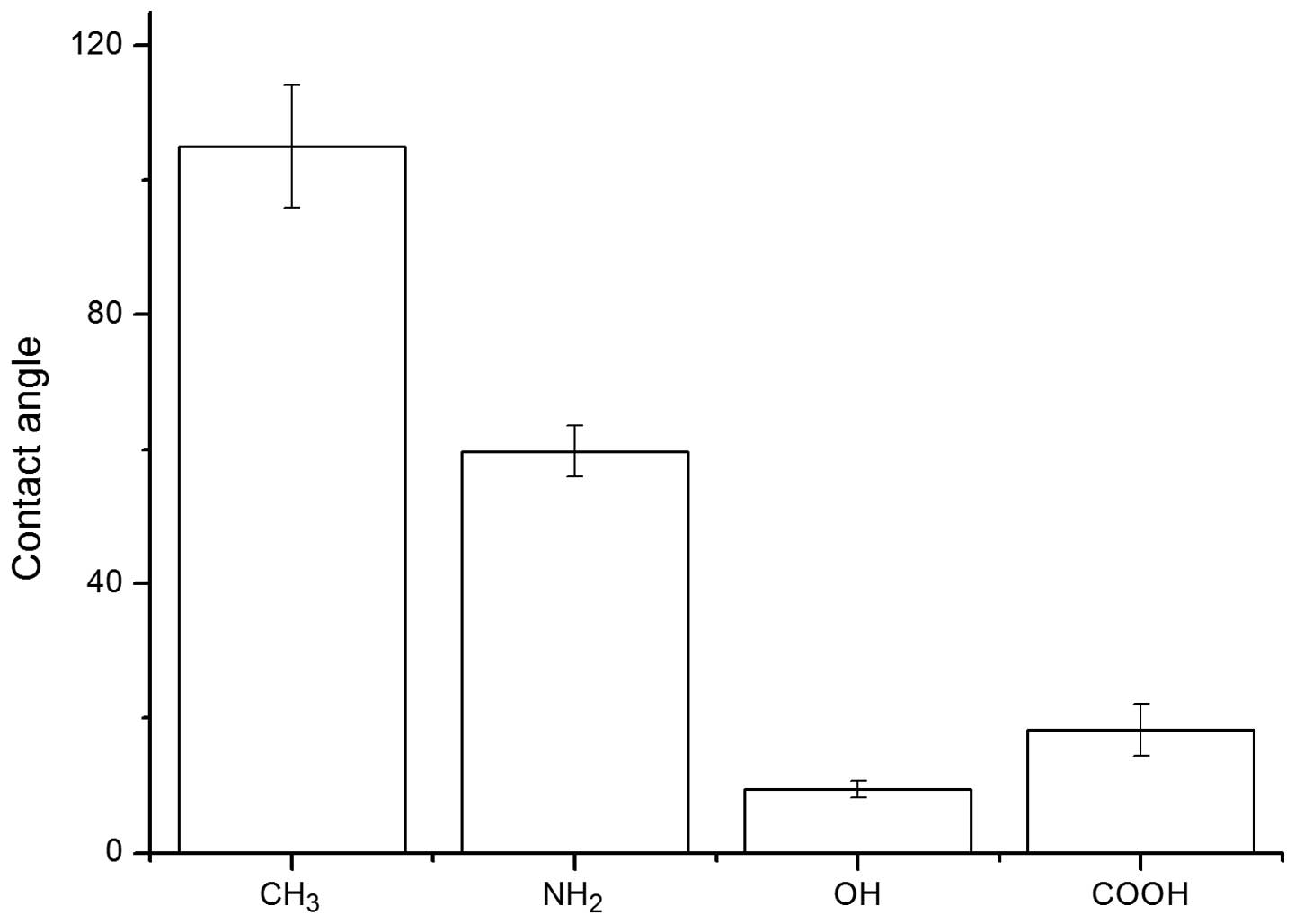
The results of contact angle measurements are shown
in Fig. 1. Among these test
surfaces, the -OH surface was the most hydrophilic, with a contact
angle of 9.5°±1.2°. The -COOH surface had a slightly higher contact
angle value of 18.3°±3.9°. The contact angle of the -NH2
surface was 59.7°±3.8°, although -NH2 was still
classified as hydrophilic. Due to its non-polar nature, the -CH3
surface had the highest contact angle of 105.0°±9.1°, which was the
most hydrophobic surface in these tests.
The adhesion of U-2OS cells
The adhesion of U-2OS cells to the chemical
group-modified substrates was investigated using microscopy and
SEM.
Cell adhesion numbers on the different chemical
surfaces after 3-, 6- and 9-h incubations are shown in Fig. 2. The adherent cell number of
-CH3 group remained at the initial level. In contrast,
the cell number on the -OH, -NH2 and -COOH surfaces was
dramatically increased compared with that on -CH3
surface. The cell number on the -NH2 surface was higher
than the other three groups during the incubation period. For the
-NH2, -OH, -COOH groups, the number of cells increased
accordingly. However, there were no clear differences between the
-NH2 and -COOH groups. In addition, the adhesion number
of the U-2OS cells followed the trend: -CH3 << -OH
< -COOH ≈ -NH2.
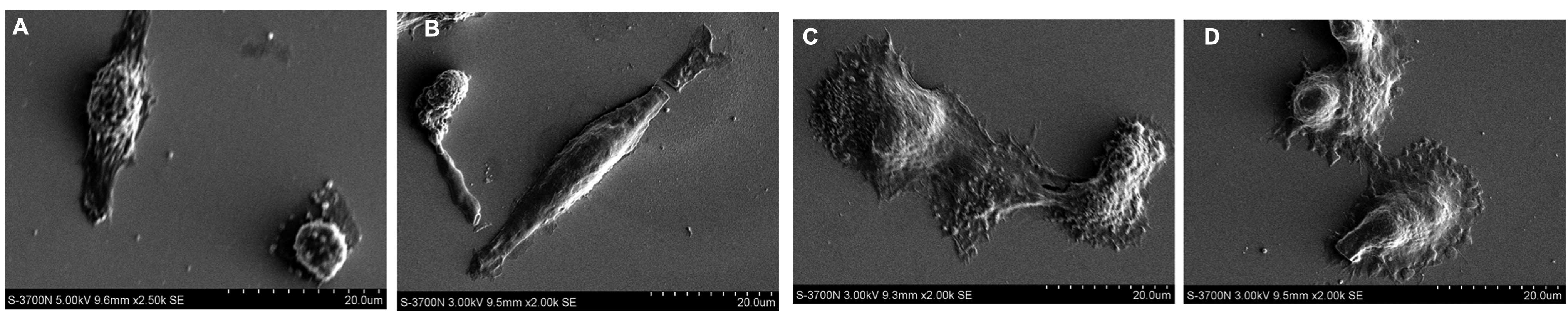
In order to further prove the different morphology
changes, SEM (Fig. 3) was used.
The results indicated that U-2OS cells cultured on the -OH and
-COOH functional groups exhibit polygonal and oval morphology,
those cultured on NH2 showed a spindle shape and cells
cultured on -CH3 group were smaller and in a spherical
shape, which was in accordance with the phase microscope
results.
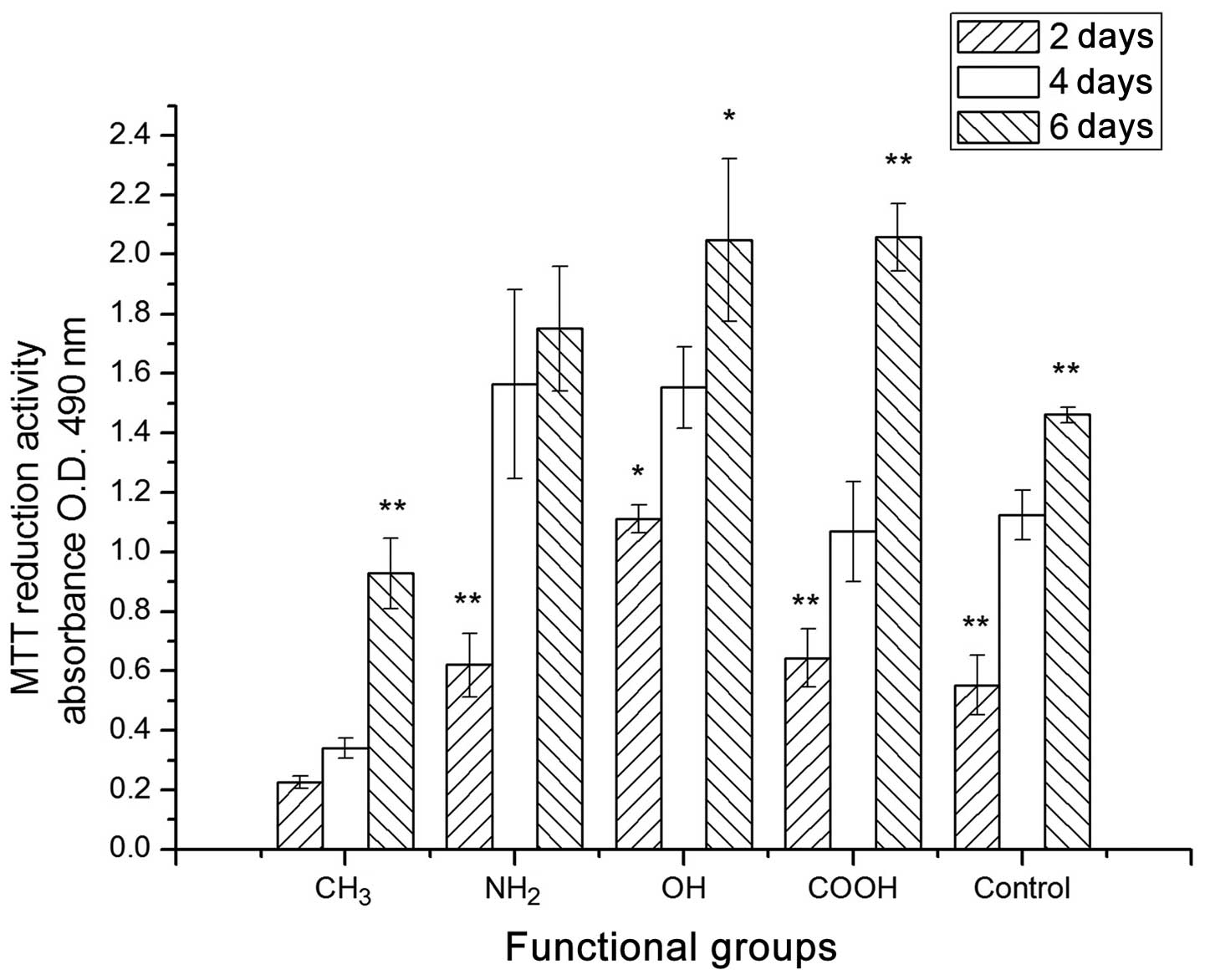
Cell viability
The MTT results for the U-2OS cells cultured on the
different chemical group-modified surfaces on days 2, 4 and 6 are
shown in Fig. 4. The U-2OS cells
on the different surfaces were uniformly seeded at the beginning of
the experiment. From day 2 to day 6, there was a significant
increase in the proliferation rate of U-2OS cells on the surfaces
with the -CH3, -NH2, -OH and -COOH groups.
However, the cells on the -CH3 surface had a low O.D.
level compared with that of the other functional groups from Day 2
to Day 6. The cells in the control showed a similar moderate
proliferation level to the -OH groups. It appears that the -COOH
and -NH2 groups had a promoting effect on the
proliferation of U-2OS cells in a longer culture period. However,
the -CH3 group had a negative effect on U-2OS
proliferation. Furthermore, the proliferation capacity of U-2OS on
the different surfaces followed the trend: -COOH > -OH ≥
-NH2 >> -CH3.
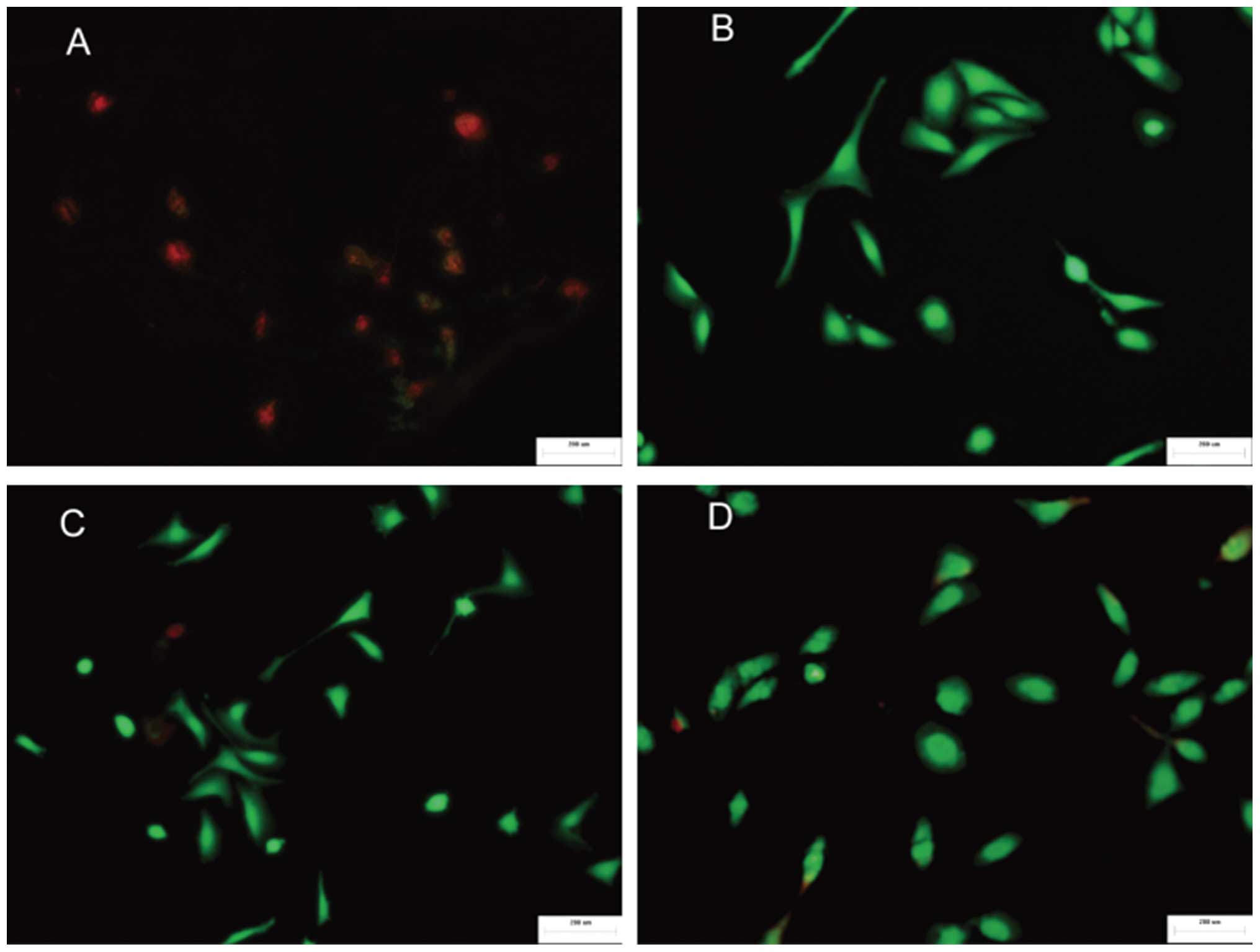
The effects of different chemical groups on cell
survival were also evaluated using live/dead viability and
cytotoxicity staining (Fig. 5). It
was determined that there were few cells on the -CH3
surface, which primarily consisted of dead cells. By contrast, on
the -COOH, -NH2 and -OH surfaces, there were a greater
number of viable cells in a markedly larger contact area. The cells
that were exposed to a larger area and the morphology were
consistent with the SEM results. The survival rates of cells on
different surfaces followed the trend: -NH2 > -OH ≥
-COOH >> -CH3. These results are supported
previous studies that found that the -NH2 functional
group diminishes cell toxicity while -CH3 exhibits cell
toxicity (11,12).
LDH
The present results indicated that the
-CH3 group may interrupt the continuity of the cell
membrane and subsequently lead to membrane breakdown and cytoplasm
leakage. To test this assumption, the release of the cytoplasmic
enzyme LDH by adherent cells was measured on chemical
group-modified substrates. Assays of the LDH activities revealed
the following trend: -CH3 > -OH ≥ -COOH >
-NH2, which were in agreement with the MTT results,
demonstrating the association between cell toxicity and the release
of LDH enzymes (Fig. 6).
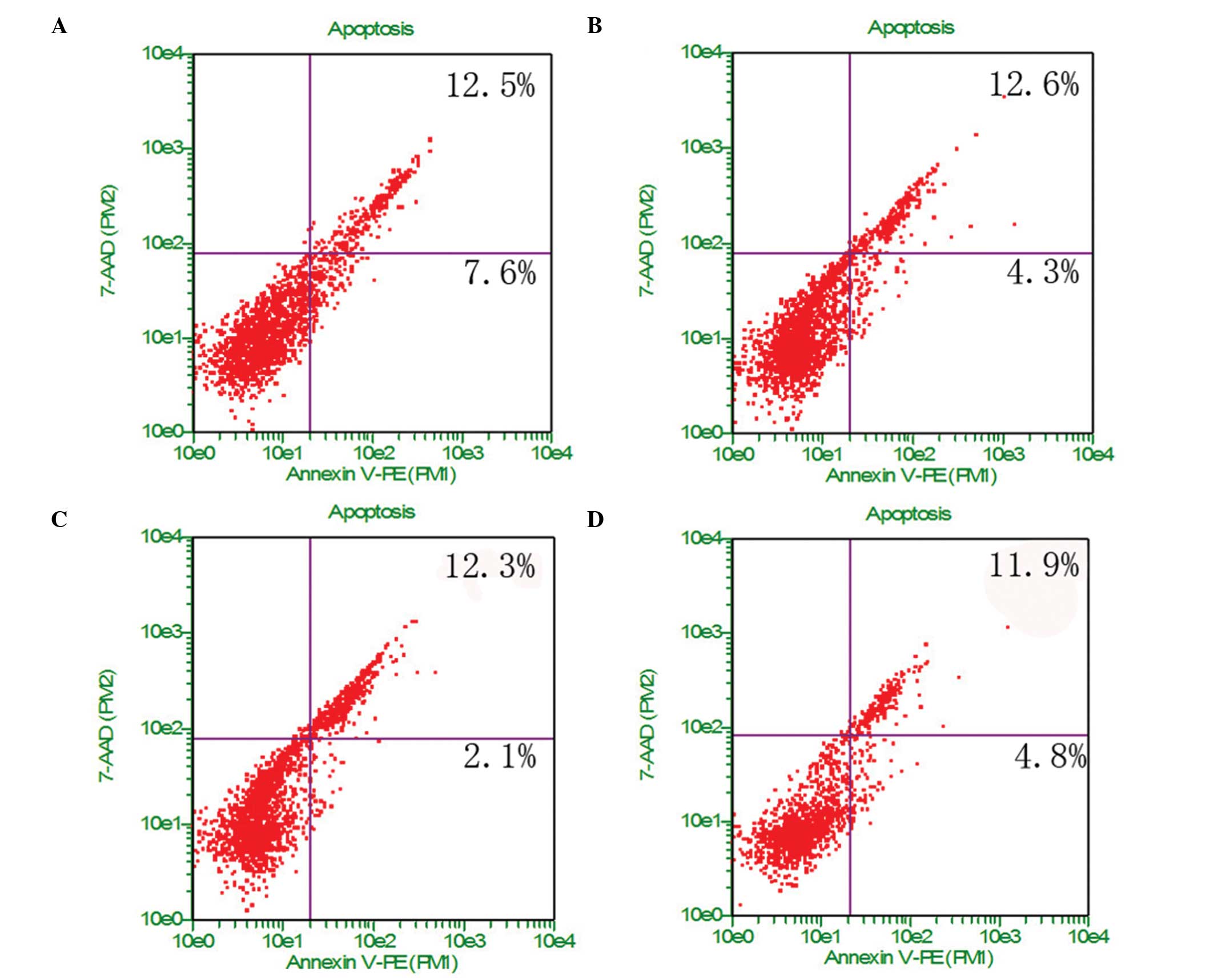
Cell apoptosis and necrosis
U-2OS cells in different chemical groups were
analyzed for apoptosis using Annexin V-PE and 7-AAD. Annexin V-PE
was used to detect phosphatidylserine (PS) on the external membrane
of apoptotic cells. 7-AAD, the cell impermeant dye, is used as an
indicator of cell membrane structural integrity. As shown in
Fig. 7, the -CH3 group
caused ~7.6% apoptosis and ~12.5% necrosis, whereas the
-NH2 group caused ~4.3% apoptosis and ~12.6% necrosis.
The apoptosis rate showed the following trend: -CH3 >
-COOH > -NH2 ≥ -OH. These results indicate that the
-NH2 surface exhibits improved cell biocompatibility,
and the -CH3 group may cause death by apoptosis and
early apoptosis.
Discussion
In the current study, -CH3, -OH,
-NH2 and -COOH were selected to construct SAMs on bare
gold surfaces, and these four types of chemical groups were used to
further study their effects on U-2OS cells. The functional groups
showed significant effects on cell morphology, adhesion,
proliferation and apoptosis. Adhesion and spreading of cells on
biomaterials via the ECM are integrin-mediated processes, and cells
use different adhesion mechanisms for the exploration of the
material’s surface. Surface chemistry has been reported to affect
cell interactions in a number of studies (9,12,13,19),
particularly cell morphology and adhesion. In this research, U-2OS
cells adhered and spread well on -OH, -NH2 and -COOH
terminal groups, which is in accordance with previous studies
(9,12,13).
However, the cells cultured on the -CH3 surface occupied
a small area and were spherical in shape, typical non-proliferating
cell characters, which is likely to exhibit cell toxicity and
promote cell apoptosis (15,16).
Cells cultured on -OH and -COOH terminal groups had a polygon
shape, while the cells on -NH2 surface exhibited spindle
and polygon morphologies. The cells cultured on these functional
groups increased from 3 to 24 h, and the density of the cells on
-OH and -NH2 surfaces was similar (Fig. 2D). Cellular activity is primarily
dependent on the surface of the materials, including roughness
(20), chemical composition
(21) and hydrophilicity (22). The -OH group is hydrophilic, while
-CH3 is hydrophobic when the charge is neutral. This
difference is the leading cause of different cell adhesion and
spreading.
Cell adhesion and morphology are tightly linked with
cell viability (19). Cells
cultured on -COOH and -NH2 markedly promote cell
proliferation, while the -CH3 functional group inhibits
cell proliferation, which is in accordance with previous studies
(12,13). The cell proliferation was inversely
correlated with cell toxicity (LDH and viability/cytotoxicity
staining). The integrity of the cell membrane is crucial to
maintain its viability, which means that the cells will undergo
necrosis if the membrane is broken (23). LDH release has been chosen to
determine the ratio of live to dead cells. In the current study,
the same method was used to check the toxicity of the chemical
functional group. It was determined that -CH3 causes the
largest amount of LDH release, indicating that -CH3 may
lead to the majority of cells to apoptosis and necrosis.
Osteosarcoma is a typical malignant tumor with
uncontrolled growth and metastasis. Apoptosis is a genetically
programmed cell death which occurs via the activation of intrinsi
cell suicide machinery (24). Cell
death in a tumor is commonly attributed to the induction of
apoptosis. The results of the present study showed that the
-CH3-cultured cells exhibited early apoptosis, reflected
in a shift from green to red cells in cytotoxicity staining.
Apoptosis assays in -CH3 functional groups are in
agreement with cell proliferation and biological behavior. The
results revealed that U-2OS cells showed a moderate proliferation
rate and little toxicity when cultured on -NH2 terminal
groups, while a strong proliferation rate when cultured on -COOH
group. Yan et al (11)
showed that MCF-7 cells cultured on -NH2 and -COOH
surfaces had the best biocompatibility, -OH had the weakest
viability and -CH3 did not affect cell viability and
migration, which was markedly different from the present results.
This difference suggests that -CH3 and -OH functional
groups have distinct roles in different cells.
In the present study, a lower ratio of cell
apoptosis existed in all of the cells cultured on different
chemical functional groups, but the greatest difference existed
between -CH3 and the other groups. The -CH3
group inhibits the proliferation of U-2OS cells and promotes cell
apoptosis, and it may give means to design novel therapeutic agents
or biomaterials to treat or prevent the recurrence of
osteosarcoma.
In conclusion, the results of this study have shown
that the type of chemical group is an important property of
biomaterials for the growth of osteosarcoma. -NH2 and
-COOH surfaces sustained visible cell adhesion and promoted cell
growth. Cells cultured on -OH surfaces exhibited similar effects on
proliferation but an increased ability to promote apoptosis and
death. In contrast, -CH3 surfaces showed anticancer
effects, inhibiting cell growth, causing poor cell adhesion and
increased levels of apoptosis and necrosis.
Acknowledgements
The authors thank Professor Zhang of the Herbarium
at Guangdong Institute of Microbiology (HMIGD) for the use of the
scanning electron microscope. This study was supported by grants
from the National Natural Science Foundation of China General
program (no. 81272057) and The Military Medical Science and
Technology Research of China during the ‘12th five-year plan’ (no.
81271957).
References
|
1
|
Kumar A, Biebuyck HA and Whitesides GM:
Patterning self-assembled monolayers: applications in materials
science. Langmuier. 10:1498–1511. 1994. View Article : Google Scholar
|
|
2
|
Ryan D, Parviz BA, Linder V, et al:
Patterning multiple aligned self-assembled monolayers using light.
Langmuir. 20:9080–8. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
García AJ, Vega MD and Boettiger D:
Modulation of cell proliferation and differentiation through
substrate-dependent changes in fibronectin conformation. Mol Biol
Cell. 10:785–798. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tziampazis E, Kohn J and Moghe PV:
PEG-variant biomaterials as selectively adhesive protein templates:
model surfaces for controlled cell adhesion and migration.
Biomaterials. 21:511–520. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bonfield TL, Colton E, Marchant RE and
Anderson JM: Cytokine and growth factor production by
monocytes/macrophages on protein preadsorbed polymers. J Biomed
Mater Res. 26:837–850. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shen M and Horbett TA: The effects of
surface chemistry and adsorbed proteins on monocyte/macrophage
adhesion to chemically modified polystyrene surfaces. J Biomed
Mater Res. 57:336–345. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Keselowsky BG, Collard DM and García AJ:
Surface chemistry modulates fibronectin conformation and directs
integrin binding and specificity to control cell adhesion. J Biomed
Mater Res. 66A:247–259. 2003. View Article : Google Scholar
|
|
8
|
Allen LT, Fox EJ, Blute I, et al:
Interaction of soft condensed materials with living cells:
phenotype/transcriptome correlations for the hydrophobic effect.
Proc Natl Acad Sci USA. 100:6331–6336. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Michael KE, Vernekar VN, Keselowsky BG,
Meredith JC, Latour RA and García AJ: Adsorption-induced
conformational changes in fibronectin due to interactions with
well-defined surface chemistries. Langmuir. 19:8033–8040. 2003.
View Article : Google Scholar
|
|
10
|
Curran JM, Chen R and Hunt JA: Controlling
the phenotype and function of mesenchymal stem cells in vitro by
adhesion to silane-modified clean glass surfaces. Biomaterials.
26:7057–7067. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yan H, Zhang S, He J, et al:
Self-assembled monolayers with different chemical group substrates
for the study of MCF-7 breast cancer cell line behavior. Biomed
Mater. 8:0350082013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu XL, Xu SJ, Shao JD, et al: Different
fate of cancer cells on several chemical functional groups. Surface
Coating Technol. 228(Suppl 1): S48–S54. 2013. View Article : Google Scholar
|
|
13
|
Picci P: Osteosarcoma (osteogenic
sarcoma). Orphanet J Rare Dis. 2:62007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Heare T, Hensley MA and Dell’Orfano S:
Bone tumors: osteosarcoma and Ewing’s sarcoma. Curr Opin Pediatr.
21:365–372. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li LH, Deng YH, He J, et al: Influence of
surface chemistry on the biological feature of giant cell tumor of
bone stromal cells in vitro. J Biomater Tissue Eng. 3:554–563.
2013. View Article : Google Scholar
|
|
16
|
Widrig CA, Alves CA and Porter MD:
Scanning tunneling microscopy of ethanethiolate and
n-octadecanethiolate monolayers spontaneously absorbed at gold
surfaces. J Am Chem Soc. 113:2805–2810. 1991. View Article : Google Scholar
|
|
17
|
Lappalainen K, Jääskeläinen I, Syrjänen K,
Urtti A and Syrjänen S: Comparison of cell proliferation and
toxicity assays using two cationic liposomes. Pharm Res.
11:1127–1131. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Green DR and Reed JC: Mitochondria and
apoptosis. Science. 281:1309–1312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Keselowsky BG, Collard DM and García AJ:
Integrin binding specificity regulates biomaterial surface
chemistry effects on cell differentiation. Proc Natl Acad Sci USA.
102:5953–5957. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lincks J, Boyan BD, Blanchard CR, et al:
Response of MG63 osteoblast-like cells to titanium and titanium
alloy is dependent on surface roughness and composition.
Biomaterials. 23:2219–2232. 1998. View Article : Google Scholar
|
|
21
|
Shin Y and Akao M: Tissue reactions to
various percutaneous materials with different surface properties
and structures. Artif Organs. 21:995–1001. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zambonin G and Grano M: Biomaterials in
orthopaedic surgery: effects of different hydroxyapatites and
demineralized bone matrix on proliferation rate and bone matrix
synthesis by human osteoblasts. Biomaterials. 16:397–402. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lobner D: Comparison of the LDH and MTT
for quantifying cell death: validity for neuronal apoptosis? J
Neurosci Methods. 96:147–152. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu J: Apoptosis and angiogenesis: two
promising tumor markers in breast cancer (review). Anticancer Res.
16(4B): 2233–2239. 1996.PubMed/NCBI
|