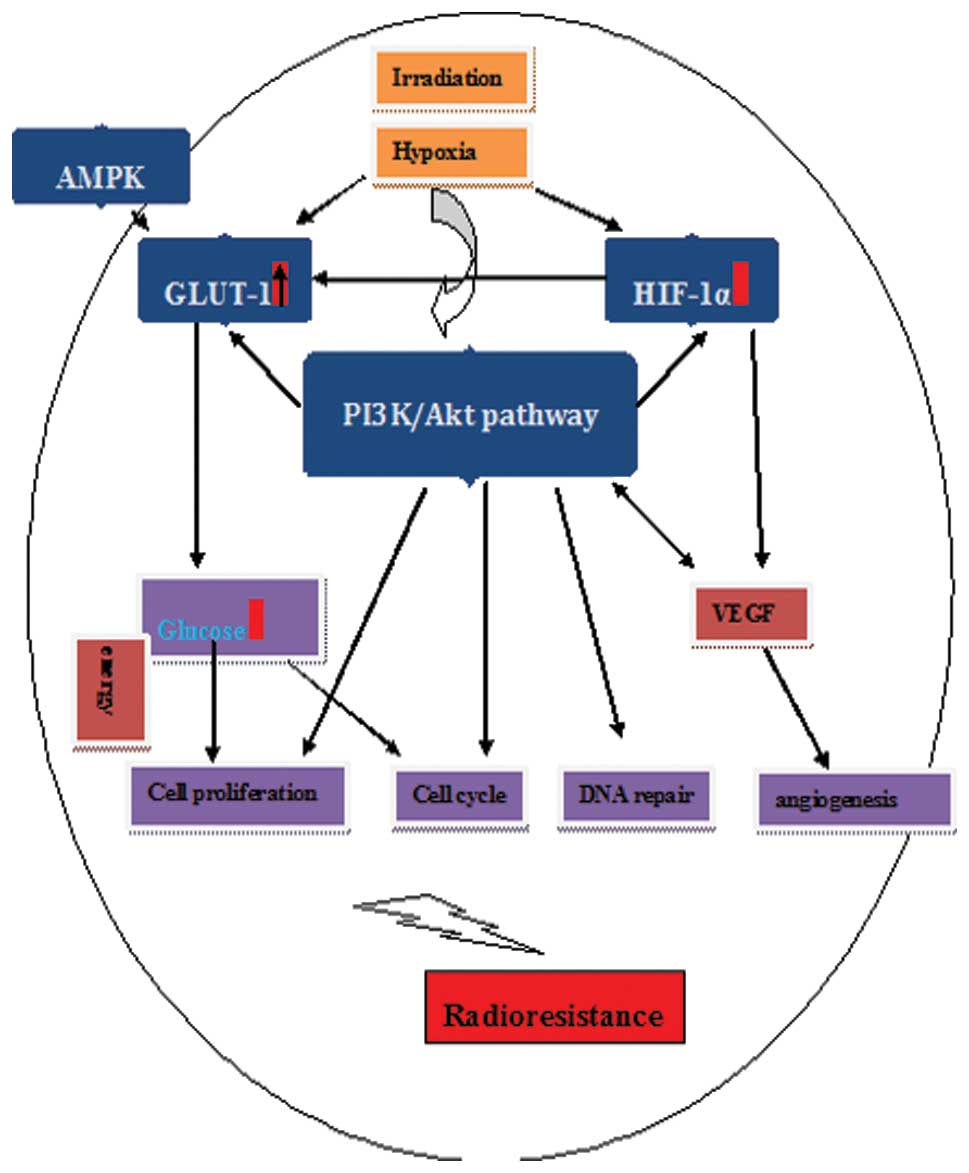
1. Introduction
Glucose is one of the primary energy sources
required to maintain the normal functioning of cells. The glucose
transporters (GLUTs) mediate glucose transport (1). Compared with their nonmalignant
counterparts, the metabolic rate of glucose is higher in malignant
cells. This phenomenon has been demonstrated using positron
emission tomography (PET) scanning with the glucose analog tracer,
18F-2-fluoro-2-deoxy-D-glucose (18F-FDG)
(2–4). Several mechanisms of
18F-FDG uptake that may explain the accelerated glucose
use in growing tumors and in transformed and malignant cells have
been proposed, including passive diffusion,
Na+-dependent glucose transport, the activation of
oncogenes, the phosphatidylinositol 3-kinase/protein kinase B
(PI3K/Akt) pathway and upregulation of facilitative GLUT (5–8).
GLUT5 is considered to be the primary mechanism for increasing
glucose influx into cells (5).
GLUTs are membrane proteins that facilitate the
transport of glucose across cellular membranes. Thirteen members of
the facilitative sugar transporter family are now recognized
(GLUT-1 to -12 and HMIT; gene name, SLC2A) (9). The human genes encoding these
proteins are named GLUT-l to -5 and GLUT-7 to -13; GLUT-6 and -14
are now known to be pseudogenes. Of the 14 isoforms, GLUT-1 appears
to be the most ubiquitously distributed (10). A number of studies have shown
increased GLUT-1 expression in various types of cancer (11–16),
including in head and neck cancer (5,17–20).
It has been reported that overexpression of GLUT-1 is associated
with lymph node metastasis and a poor prognosis in head and neck
cancers (17–20). Thus, GLUT-1 may be a potential
therapeutic target in malignant tumors (14,16,21–24).
Radiotherapy is important in treating advanced
cancers and in organ preservation strategies for cancers at an
earlier stage (25). However,
radioresistance of cancer cells affects treatment efficacy.
To date, a number of strategies have been introduced
in an attempt to increase radiosensitivity, including
hyperfractionation to overcome intrinsic radioresistance (26–28),
concurrent chemoradiotherapy (29,30)
and the use of certain radiosensitizers that enhance
radiosensitivity by improving the hypoxic status of tumors
(31,32). Although these efforts have
increased survival rates and regional control, certain issues have
been reported and the effects are less than ideal, including the
development of central radionecrosis as well as early or late
toxicity. Thus, more efficacious treatments with fewer side effects
are required in order to improve radiosensitivity.
Although a number of factors contributing to
radioresistance are understood, such as hypoxia, re-population and
DNA damage repair, other aspects remain unclear. A number of
studies have found that increased GLUT-1 expression is
significantly correlated with radioresistance (33–38).
Thus, the suppression of GLUT-1 expression as a novel therapeutic
target is a focus in research into increasing radiosensitvity of
malignant tumors (33,34,39).
However, abnormal expression of GLUT-1 in malignant tumors is not
the only cause of radioresistance. Other genes, including epidermal
growth factor receptor (EGFR) and NOTCH, may also be involved
(40,41). Abnormal expression of GLUT-1 and
its activity are regulated by a number of factors, including the
activation of oncogenes (13,42),
hypoxia via hypoxia-inducible factor (HIF)-1-dependent and
independent mechanisms (42,43),
and signaling pathways, such as mitogen-activated protein kinase
(MAPK) (44), and the PI3K/Akt
pathway (45–47). Recently, the PI3K/Akt pathway was
reported to be involved in the control of GLUT-1 trafficking and
activity (1,48,49).
It was also suggested that the PI3K/Akt pathway may regulate GLUT-1
localization in T cells (1,47).
The PI3K/Akt pathway is often found to be overactive
in a variety of tumor types and triggers a cascade of responses,
from cell growth and proliferation to increased cell survival and
motility, which drive tumor progression (40). Activation of the PI3K/Akt pathway
may be associated with radioresistance of cancer (25,50–52).
Thus, research has become increasingly focused on modifying the
expression of GLUT-1 and the PI3K/Akt pathway in order to increase
radiosensitivity.
Although GLUT-1 expression is a common feature in
patients with cancer, the prognostic value of this parameter, along
with the degree of FDG uptake, has not been evaluated with respect
to PI3K/Akt. The selection of GLUT-1 and Akt as targets is logical
considering their importance in cancer survival and resistance to
radiation and chemotherapy.
This review discusses the role of an interaction
between the elevated expression of GLUT-1 and activation of the
PI3K/Akt pathway in cancer radioresistance. It is proposed that
suppression of GLUT-1 expression and the PI3K/Akt pathway may be
therapeutic targets for carcinomas (Fig. 1).
2. Overexpression of GLUT-1 and
radioresistance
A number of studies have demonstrated that increased
GLUT-1 expression is associated with the development of
radioresistance in cancer. In the CPH 54A and CPH 54B lung cancer
cell lines, CPH 54A tumors are more radiosensitive than CPH 54B
tumors in vivo and in vitro. Pedersen et al
(36) found that GLUT-1 mRNA and
protein expression levels are higher in 54B than in 54A cells. They
also detected greater FDG uptake in 54B tumors, using PET scans,
and suggested that there appears to be a correlation between the
level of GLUT-1 and FDG uptake. Brophy et al (53) investigated GLUT-1 expression in 69
pretreatment biopsy samples from patients with rectal cancer. The
patients received preoperative chemoradiotherapy followed by
surgical resection. GLUT-1 negative tumors had a 70% probability of
a good response to chemoradiotherapy compared with a response rate
of 31% for GLUT-1 positive tumors. Korkeila et al (37) compared the expression of GLUT-1 in
53 operative samples from patients who had undergone a surgical
resection for rectal cancer and 78 preoperative biopsies of
patients with rectal cancer who had been treated by preoperative
radiotherapy. They found that negative or weak GLUT-1 expression
was linked to pronounced tumor regression. There was a tendency
towards improved disease-free survival following a long course of
radiotherapy when GLUT-1 staining intensity in the operative sample
was negative or weak (37).
Another study found that preoperative radiotherapy markedly
upregulated the expression of GLUT-1 (31). Saigusa et al (33) investigated whether GLUT-1
expression was associated with clinical outcome in 52 patients with
rectal cancer following preoperative chemoradiotherapy. They found
that elevated GLUT-1 gene expression was associated with a more
advanced stage of the disease, lymph node metastasis and distant
metastasis, and was an independent predictive factor for
recurrence-free and overall survival. In vitro, DLD1 and
LoVo colorectal cancer cell lines show high expression of GLUT-1
whereas the Caco-2 colorectal cancer cells have a lower level of
expression of GLUT-1 (33). The
relative gene expression levels of GLUT1 in DLD1 and LoVo cells
were found to be 30- and 14-fold that of Caco-2, respectively. It
was observed that DLD1 cells, which had the highest GLUT-1 gene
expression levels, were more resistant to irradiation than Caco-2
and LoVo cells. However, LoVo cells were more sensitive to
radiation than Caco-2 cells. One possible explanation for this may
be that radiosensitivity is dependent on Ki-67 expression, as LoVo
cells exhibited the highest MKI67 gene expression of the seven cell
lines examined. Following chemoradiotherapy, residual cancer
growths may contain cells with different characteristics, depending
on their location. GLUT-1 expression is predominantly found in the
central portion of such residual cancer masses (33). Finally, it was observed that the
growth of DLD1 and LoVo cells was inhibited by the glycolysis
inhibitor 3-BrPA to a greater extent than that of Caco-2 cells.
This suggested that the inhibition of glycolysis may be a potential
novel strategy for the treatment of patients with colorectal cancer
who express the KRAS mutation (33).
Few studies have investigated the association
between GLUT-1 and radioresistance in cancer (19,35).
A GLUT-1 labeling index (LI) was determined using
immunohistochemistry in 40 biopsies from patients with oral
squamous cell carcinoma (OSCC) prior to treatment (19). Clinical responders to radiation
showed a significantly lower expression of GLUT-1 when compared
with incomplete responders (P=0.009). A significant association
(P=0.023) was observed between the GLUT-1 LI and the resistance of
tumor cells. These results suggest that GLUT-1 expression could be
considered to be a marker of radioresistance in OSCC, in which high
GLUT-1 expression is associated with a poor radiation response and
vice versa (19). Doki et
al (35) found a high level of
expression of GLUT-1 in squamous cell carcinoma of the esophagus
following radiotherapy. In a previous study, it was shown that
GLUT-1 overexpression in vitro is associated with increased
cell proliferation and glucose uptake in Hep-2 laryngeal carcinoma
cells. Conversely, the suppression of GLUT-1 expression by
antisense oligodeoxynucleotides (AS-ODNs) may decrease glucose
uptake and inhibit the proliferation of Hep-2 cells (54). Recently, it was shown that
radioresistance in laryngeal carcinoma cells may be associated with
increased expression of GLUT-1 mRNA and protein. GLUT-1 AS-ODNs may
enhance the radiosensitivity of laryngeal carcinoma cells,
primarily by inhibiting the expression of GLUT-1 in vitro
and in vivo (55).
Possible mechanisms of GLUT-1-mediated
radioresistance Raised glucose metabolic rate
A higher glucose metabolic rate has been observed in
malignant tumor cells compared with non-malignant cells, even
during aerobic glycolysis. This phenomenon is referred to as the
Warburg effect (56,57) and was demonstrated using PET
scanning with the glucose analog tracer FDG (58). Transport of glucose across the
plasma membrane is the initial rate-limiting step in glucose
metabolism and it is mediated by facilitative glucose transporter
proteins (59). GLUT-1 is
important in glucose metabolism within malignant cells and may
contribute to the observed increase in FDG uptake. In addition,
GLUT-1 may be an intrinsic marker of hypoxia in malignant tumors
(14,16,21–24).
Elevated GLUT-1 expression may enable malignant tumors to increase
their energy expenditure leading to proliferation and
radioresistance of tumor cells.
Hypoxia
Hypoxic cells represent 10–50% of solid tumor cells.
Hypoxia is known to promote chemoradioresistance in carcinomas
(60,61). In addition, GLUT-1 is overexpressed
in hypoxic states. HIF-1α, a transcription factor associated with
the cellular response to hypoxia (62), upregulates the expression of
several hypoxia response genes, including GLUT-1 (64). A correlation has been demonstrated
between GLUT-1 and HIF-1α expression in laryngeal carcinoma
(65). It is suggested that GLUT-1
expression is associated with cancer radioresistance as a result of
upregulation by HIF-1α.
GLUT-1 expression increases cell
metabolism
Evans et al (66) showed that GLUT-1 overexpression
without a coordinated increase in HIF-1-regulated glycolytic
enzymes increased glucose uptake but not the glycolytic rate
(66). They found that increased
GLUT-1 expression resulted in chemoresistance by increasing cell
turnover. Thus, it is possible that a similar mechanism may be
involved in GLUT-1-mediated radioresistance. However, this requires
further investigation.
Involvement of cancer stem cells
CD133+ cancer stem cells may be important
in the development of cancer radioresistance (67,68).
Ke et al (67) reported
that GLUT-1 expression was higher in CD133+ than
CD133− cells in thyroid cancer following 131I
radiotherapy. Mai et al (69) showed that stem cells from
proliferating hemangiomas may produce GLUT-1. In a previous study,
our group found higher GLUT-1 mRNA and protein expression in
CD133+ Hep-2 laryngeal carcinoma cells than in
CD133− cells (70).
This also requires further investigation.
Mechanisms independent of hypoxia
A number of studies have shown that GLUT-1-mediated
chemoradioresistance is independent of hypoxia. Mayer et al
(44) found no correlation between
the expression of GLUT-1 and oxygenation variables. Evans et
al (66) showed that GLUT-1
overexpression was coordinated with increases in HIF-1-regulated
glycolytic enzymes, which increased glucose uptake, but not the
rate of glycolysis. GLUT-1 overexpression was correlated with
higher levels of phosphodiesterase in xenografts, which was related
to the metabolic turnover of phospholipids and involved in membrane
lipid degradation, indicating a mechanism by which GLUT-1 may be
involved in increased cell turnover (66). The regulation of GLUT-1 expression
is dependent not only on HIF-1-induced transcription but also on
the post-transcriptional steady-state of the GLUT-1 gene (71).
Changes in the cell cycle and
apoptosis
The cell cycle may be involved in cancer
radioresistance (72–74). G2/M phase arrest occurs
in a significant number of cancer cells following irradiation. A
previous study found that the percentage of cells that were
arrested in the G2/M phase increased in a dose-dependent
manner in response to radiation. This indicated that entry into
mitosis had been delayed by the administration of radiation.
G2/M arrest in the 12-Gy group was maximal, whilst the
expression of GLUT-1 mRNA and protein was higher than that in the
control groups (55).
Involvement of signaling pathways
AMPK and PI3K/Akt signaling pathways may regulate
the expression of GLUT-1.
3. Role of PI3K/Akt in radioresistance
Radiotherapy affects the expression of oncogenes and
tumor suppressor genes. This alters internal and external signal
transduction pathways of the cells, and affects the response of
tumor cells to radiotherapy (24).
Since 1995, the PI3K/Akt survival signal transduction pathways have
been shown to be involved in regulating the expression of a variety
of tumor biology markers (25,53,75).
PI3K is an important dimer enzyme that is involved in growth and
proliferation, and growth factor signal transduction pathways have
been found in recent years that may be activated primarily by a
combination of growth factors and receptors (75,76).
Akt is also termed PKB or Rac. PI3K is one of the important
downstream serine-threonine regulation kinases. A variety of
molecules may activate Akt, such as insulin, heat shock proteins
and tumor necrosis factor-α. Activated Akt is central to the
mediation of cell growth, survival and differentiation by the
PI3K/Akt signal transduction pathways. The biological effects of
the activation of this pathway include apoptosis, cell cycle
regulation and promotion of invasion, metastasis and angiogenesis
(75,76). The abnormal expression of certain
proteins, as well as abnormal increases in kinase activity in the
Akt cascade signal pathway have been identified in a number of
human malignancies. The PI3K/Akt pathway is associated with the
increased proliferation of tumor cells, and its activation is
closely correlated with a poor prognosis and resistance to cancer
radiotherapy.
Possible mechanisms of radiation resistance caused
by PI3K/Akt include hypoxia, intrinsic radiation resistance, and
external factors, such as tumor cell proliferation following
radiation therapy (25,75).
PI3K/Akt and hypoxia in radiation
resistance
The association between PI3K/Akt and the hypoxic
microenvironment of tumors is of interest in research into
radioresistance. Hypoxia often results in an increase in the
glucose metabolic rate of malignant cells. The abnormal expression
of GLUT-1 is known to be correlated with these factors. The supply
and consumption of oxygen in the majority of solid tumors are not
balanced, which results in tumor hypoxia. Cells that are
progressing through the cell cycle become hypoxic so that their
progression becomes delayed relative to well-oxygenated cells.
Slower progression of hypoxic than normoxic cells through
G2 leads to a temporary accumulation of hypoxic
G2 cells in poorly differentiated mammary adenocarcinoma
non-transgenic (NT) and anaplastic sarcoma F. Progression of
hypoxic cells through the cell cycle in each tumor type is delayed
as a result of the deprivation of oxygen and other nutrients
(78). Koritzinsky et al
(78) found that when cells that
had arrested in G1 during hypoxic conditions progressed
through S-phase following re-oxygenation, the speed with which they
progressed was similar to that of untreated cells. By contrast, the
cells that had arrested in S-phase during hypoxia progressed more
slowly through this S-phase following re-oxygenation. Groups of
cells that maintain proliferative capacity under hypoxic conditions
are a significant cause of treatment failure.
Hypoxia results in genomic instability and increased
instability in the malignant phenotype by stimulating invasion and
metastasis of tumors (79).
Hypoxia induces and promotes the mutation of key regulatory genes
(HIF-1, solute carrier family 2 and phosphatidylinositol-dependent
kinase-1) (80), leading to
increased resistance to therapy.
One of the key genes involved in the response to
hypoxia is HIF. HIF regulates the expression of >60 genes
involved in angiogenesis, anaerobic glycolysis and cell survival,
and the coordinated expression of these genes results in cellular
adaptation to acute and chronic hypoxia (81). Studies have shown that hypoxia of
head and neck squamous cell carcinoma is associated with poor local
control and overall survival (82,83).
The PI3K/Akt signal pathway is important in promoting an adaptive
response to low levels of oxygen in tumor cells.
Radiation increases HIF-1 activity, which has been
hypothesized to be involved in regulating the tumor response to
irradiation through a number of mechanisms (84). The PI3K/Akt pathway is involved in
HIF-1α protein expression. Activation of PI3K/Akt/mammalian target
of rapamycin (mTOR) leads to stimulation of de novo
synthesis and transcriptional activation of HIF-1α (85,86).
HIF-1α protects tumors from radiation damage directly and
indirectly. Inhibition of the PI3K/Akt pathway by wortmannin and
LY294002, and inhibition of HIF-1α by short interfering (si)RNA may
therefore enhance the efficacy of radiotherapy.
PI3K/Akt, reoxygenation and
neoangiogenesis in radiation resistance
Irradiation may lead to reoxygenation and
neoangiogenesis of cancer cells following radiotherapy. The
regulatory mechanism may occur via upregulation of VEGF. Inhibition
of neoangiogenesis results in normalization of the vasculature and
improved perfusion, leading to a reduction in tumor cell hypoxia
(50). The PI3K/Akt pathway may
induce the expression of VEGF via activation of HIF-1α (87). VEGF protects endothelial cells
against radiation by activating the PI3K/Akt pathway, leading to
enhanced expression of the antiapoptotic protein Bcl-2 (88). Antiangiogenic therapy may therefore
enhance the cytotoxic effects of radiotherapy (89,90).
Certain antiangiogenic drugs target the vasculature, directly or
indirectly, by disrupting VEGF. These include inhibitors of the
PI3K/Akt pathway. This may lead to increased blood flow and
oxygenation, thereby potentially increasing radiosensitivity
(91). A combination of low doses
of a PI3K inhibitor (LY294002) and cisplatin significantly enhanced
the therapeutic efficacy of radiation therapy by preferentially
targeting tumor blood vessels (89). However, the hypothesis that an
inhibitor of the PI3K/Akt pathway may also achieve prolonged
vascular normalization, and thereby enhance radiosensitivity,
requires further investigation (92).
PI3K/Akt and the cell cycle in radiation
resistance
Radiation may activate p53-dependent or independent
cell cycle G1 and G2 arrest (93). The PI3K/Akt pathway acts to
overcome p53-independent cell cycle arrest via activation of cyclin
D and inactivation of the cell cycle-dependent kinase inhibitor p27
(94). Activation of the Akt/PKB
pathway is able to override the G2/M phase cell cycle
arrest that occurs as a result of irradiation-induced DNA damage
(95). Phosphatase and tensin
homolog (PTEN), a tumor-suppressor gene, antagonizes the PI3K/AKT
signaling pathway that is involved in promoting escape from
cell-cycle arrest. Park et al (94) found that PTEN may be essential in
cancer cell radiosensitivity by using LY294002 or PTEN-specific
siRNA to block PI3K/Akt signaling in non-small-cell lung cancer
cells (NSCLC).
PI3K/Akt and DNA repair in radiation
resistance
Irradiation may cause DNA damage, including
single-strand breaks, double-strand breaks (DSBs), base excision
and glucose damage. Enhanced DNA repair activity tends to be
resistant to radiotherapy. DNA-dependent protein kinase catalytic
subunit (DNA-PKcs) and ataxia telangiectasia-mutated are two
members of the PI3K family that repair DNA DSBs (97–99).
Inhibition of PI3 kinases using a pharmacological approach may
improve the response of cancer cells to radiotherapy. Nimotuzumab
inhibits the radiation-induced activation of DNA-PKcs by blocking
the PI3K/AKT pathway (99).
Inhibition of the PI3K/Akt cell survival signaling pathway and
DNA-PKcs may contribute to the wortmannin-induced radiosensitivity
observed in NSCLC cells (100).
Azad et al (101) found
that BEZ235, a novel inhibitor of DNA-PK and PI3K/mTOR, abrogates
radiation-induced DSB repair, resulting in cellular
radiosensitization and growth delay in irradiated NSCLC
xenografts.
PI3K/Akt, epidermal growth factor
receptor (EGFR) and cell proliferation in radioresistance
Activated Akt promotes cell proliferation and
inhibits apoptosis. Radiation-induced Akt activation may modulate
the radioresistance of human cancer cells (102). Certain serum factors, including
integrin-β1, and growth factor receptors, including EGFR, may also
be involved (103,104). Minjgee et al (103) showed that there was increased
basal Akt phosphorylation as well as augmented output from the
PI3K/Akt pathway following EGF stimulation in cell lines with
higher levels of ErbB1 and integrin-β1 expression. Akt
phosphorylation may be related to adhesion and migration, which are
regulated by integrin signaling. Inhibition of AKT, EGFR and
integrin-β1 may thus improve radioresistance (104).
4. Association between GLUT-1 and
PI3K/Akt
The abnormal expression of GLUT-1 is correlated with
multiple signal transduction pathways, including the PI3K/Akt
signaling pathway, which is known to be important in the regulation
of GLUT-1 expression. Several studies have confirmed that the
PI3K/Akt pathway and GLUT-1 expression affect glucose metabolism
(1,47–49).
Hematopoietic cells and T lymphocytes depend on
GLUT-1 as the primary source of intracellular glucose, while growth
factors, such as interleukin (IL)-3, IL-7 or CD28 provide important
signals for GLUT-1 synthesis and glucose uptake in these cells
(47,105–107). Cell growth factors regulate
GLUT-1 predominantly through PI3K and its downstream effector Akt.
This leads to activation of mTOR and glycogen synthetase-3 (GSK-3),
as well as other methods of controlling the activation,
recirculation and internalization of GLUT-1.
In addition to the regulation of GLUT-1 expression
at the cell surface, Akt also controls the activity of GLUT-1 via
activation of mTOR (106). In
hematopoietic cells and T cells that have been transfected with the
GLUT-1 gene, an increase in glucose metabolism results in increased
levels of phosphorylation of GSK-3α,β (108). It has been reported that Akt
phosphorylates 21/9 serine of GSK-3 directly, thus inhibiting the
activity of GSK-3 kinase (48). As
a substrate of Akt, GSK-3 can also regulate the transmission of
GLUT-1 by improving the recycling of integrin (109). Continuous activation of Akt
expression increases the ability of lymphocytes to absorb and
utilize glucose (48,107,110), improves the glycolysis of T
lymphocytes (107) and may lead
to the development of autoimmune disorders and lymphoma.
Suppression of PI3K can prevent the activation of lymphocytes,
increase glucose metabolism following stimulation by cytokines and
reduce the ability of leukemic cells to absorb glucose (1,110–112).
PI3K pathways also affect insulin-induced glucose
transport in fatty cells (113,114). Apigenin downregulates the
expression of GLUT-1 mRNA and protein in CD18 and S2-013 pancreatic
cancer cell lines, and inhibits the PI3K/Akt channel (49,115). It has been found that inhibition
of the PI3K/Akt pathway may induce a decrease in GLUT-1 mRNA
(112,116).
Research has shown that cell growth factors promote
the transmission and activation of GLUT-1 in hematopoietic cells
and T lymphocytes via the PI3K/Akt pathway (48,105–107), and that activated Akt is
sufficient to maintain GLUT-1 and glucose uptake on the surface of
cells in the absence of cytokines (107,117). A previous study found that
expression of GLUT-1, p-Akt, and PI3K protein in adenoid cystic
carcinoma (ACC) was higher than that in inflammatory lesions or
benign tumors (P<0.001). The percentage of cells expressing
these proteins for GLUT-1, PI3K and p-Akt protein in ACC were 38.1
(16/42), 38.1 (16/42) and 50.0% (21/42), respectively. Significant
correlations between GLUT-1 and PI3K expression (r=0.394, p=0.01),
between GLUT-1 and p-Akt expression (r=0.528, P<0.001), and
between p-Akt and PI3K expression (r=0.528, P<0.001) were also
observed. In this study, a multivariate analysis showed that p-Akt
was a significant predictor of recurrence and that GLUT-1
expression was associated with T stage (according to the TNM
classification) and distant metastasis of ACC (118). In a ceruminous adenoma of the
external auditory canal, it was also shown by immunohistochemistry
that tumor cells were positive for GLUT-1, HIF-1, PI3K and p-Akt
(119). In U87MG glioblastoma
cells, inhibition of the PI3K pathway by LY294002 may decrease the
expression of GLUT-1 mRNA, VEGF mRNA, and HIF-1α mRNA (116).
mTOR is a downstream target of PI3K. Radhakrishnan
et al (112) found that
GLUT-1 was linked to the mTOR pathway and that GLUT-1 may be useful
as a biomarker of mTOR status in head and neck cancers. mTOR
inhibition may activate an AKT feedback loop in tumors sensitive to
rapamycin treatment. In acute lymphoblastic leukemia, IL-17
upregulates the expression of GLUT-1 via PI3K activation (120,121). In endometrial carcinoma cells,
GLUT-1, pAkt and pmTOR were found to be strongly expressed and the
mTOR inhibitor, rapamycin, induced apoptotic cell death (122). However, in breast cancer cells,
rapamycin and sorafenib downregulated GLUT-1 expression and glucose
uptake to similar extents, whereas the dual PI3K/mTORC1-C2
inhibitor NVP-BEZ-235 did not have the same effect. This suggested
that sorafenib-mediated activation of AMPK, rather than the
PI3K/Akt pathway, initially stimulated glucose uptake by increasing
GLUT-1 protein expression (123).
It is a novel idea to target GLUT-1 and AKT
expression with the aim of improving the radiosensitivity of
cancers. Other signaling pathways are involved in cancer
radioresistance, not all of which regulate or interact with GLUT-1,
and which may indeed be independent of the glucose/AKT pathway. The
stress-activated protein kinase/c-Jun NH(2)-terminal kinase pathway
has been found to be involved in the radioresistance of
nasopharyngeal carcinoma (124).
The RAF kinase/mitogen activated protein kinase/extracellular
signal-regulated kinase (ERK) pathways are also important in the
radiation resistance of squamous cell cancers, and kinase
suppressor of RAS 1 AS-ODN may act a radiosensitizer for treating
Ras-dependent human malignancies (125). It has been observed in clinical
trials that inhibition of PI3K (126) and GLUT-1 (127) increase the expression of other
oncogenes, such as that of pERK1/2 or pEGFR, and induce the
persistent phosphorylation of ribosomal protein S6. ERK1/2
activates the p90 ribosomal S6 kinase (128), which subsequently phosphorylates
S6 at Ser235/236, independently of PI3K/mTOR signaling, and
increases tumor resistance to radiation therapy (129).
5. Conclusion
Activation of the PI3K/Akt pathway, and the
transcription and expression of GLUT-1 (promoted by PI3K/Akt) are
closely associated with glucose uptake, energy consumption, cell
proliferation and the malignant transformation of tumor cells.
GLUT-1 activation by PI3K/Akt is an important metabolic regulator
of tumor cells. Overexpression of molecules in this pathway is
associated with a poor prognosis and resistance to
radiotherapy.
Radiation resistance of tumor cells, which develops
during the course of radiotherapy, necessitates the development of
novel therapies to combat this problem. The radiosensitivity of
tumor cells is key to treatment efficacy and is associated with
their inherent sensitivity prior to irradiation as well as
adaptations developed to deal with injury following irradiation.
Intrinsic radiosensitivity is determined genetically and by
disorders involving tumor suppressor genes, while the response of
cells to injury is induced by protein modifications and ultimately
by relevant alterations in signal transduction pathways.
Preclinical data have shown that enhancing
radiosensitivity by inhibiting PI3K/Akt is possible. LY294002 and
wortmannin, which target the p110 catalytic subunit of PI3K,
provide powerful preclinical tools with which to investigate the
cellular consequences of inhibiting this pathway (94,100). RAD-001, a rapamycin analog, is a
potent radiosensitizer that acts via mTOR-dependent enhancement of
radiation-induced autophagy and the induction of apoptosis in
vascular endothelial cells (130,131). In a phase III trial, CCI-779,
another mTOR inhibitor, showed a significant improvement in
progression-free survival (5.5 compared with 3.1 months) and in
overall survival in patients with metastatic renal cell carcinoma
(132). However, no data on
enhancing radiosensitivity by combining inhibition of PI3K/Akt with
that of GLUT-1 expression in carcinomas are available to date. A
number of studies have shown that activation of the PI3K/Akt
signaling pathway and abnormal expression of GLUT-1 are associated
with tumor progression, a poor prognosis and the development of
resistance to chemotherapy and radiotherapy. The ability of a
malignancy to resist radiation-induced damage is associated with
PI3K/Akt and the overexpression and activation of GLUT-1, which is
one of the key regulators of radiotherapy sensitivity. Targeted
therapy directed to the PI3K/Akt pathway and GLUT-1 may disrupt the
development of radiation resistance and enhance radiosensitivity,
thus increasing the survival rates of cancer. Targeting GLUT-1 with
antisense oligonucleotides, and the PI3K/Akt pathway with
wortmannin and LY294002, in an attempt to increase radiosensitivity
in laryngeal carcinoma will be the next focus for our group. The
prospect of targeted therapies aimed at these molecules currently
holds promise for the treatment of a variety of types of
cancer.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81172562 and 81372903), the
Science and Technology Department of Zhejiang Province, China
(grant no. 2009C33026), the Health Department of Zhejiang Province
(grant nos. 2010KYA062 and 2009B042)and the Department of Education
of Zhejiang Province, China (grant no. Y201121184).
References
|
1
|
Jacobs SR, Herman CE, Maciver NJ, et al:
Glucose uptake is limiting in T cell activation and requires
CD28-mediated Akt-dependent and independent pathways. J Immunol.
180:4476–4486. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kubicek GJ, Champ C, Fogh S, et al:
FDG-PET staging and importance of lymph node SUV in head and neck
cancer. Head Neck Oncol. 2:192010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peng NJ, Liou WS, Liu RS, Hu C, Tsay DG
and Liu CB: Early detection of recurrent ovarian cancer in patients
with low-level increases in serum CA-125 levels by
2-[F-18]fluoro-2-deoxy-D-glucose-positron emission
tomography/computed tomography. Cancer Biother Radiopharm.
26:175–181. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Masui T, Doi R, Ito T, et al: Diagnostic
value of (18)F -fluorodeoxyglucose positron emission tomography for
pancreatic neuroendocrine tumors with reference to the World Health
Organization classification. Oncol Lett. 1:155–159. 2010.PubMed/NCBI
|
|
5
|
Li LF, Zhou SH, Zhao K, et al: Clinical
significance of FDG single-photon emission computed tomography:
Computed tomography in the diagnosis of head and neck cancers and
study of its mechanism. Cancer Biother Radiopharm. 23:701–714.
2008. View Article : Google Scholar
|
|
6
|
Esen Akkas B, Gökaslan D, Güner L and
Ilgin Karabacak N: FDG uptake in brown adipose tissue-a brief
report on brown fat with FDG uptake mechanisms and quantitative
analysis using dual-time-point FDG PET/CT. Rev Esp Med Nucl.
30:14–18. 2011. View Article : Google Scholar
|
|
7
|
Ko BH, Paik JY, Jung KH and Lee KH:
17beta-estradiol augments 18F-FDG uptake and glycolysis of T47D
breast cancer cells via membrane-initiated rapid PI3K-Akt
activation. J Nucl Med. 51:1740–1747. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Prante O, Maschauer S, Fremont V, et al:
Regulation of uptake of 18F-FDG by a follicular human thyroid
cancer cell line with mutation-activated K-ras. J Nucl Med.
50:1364–1370. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wood IS and Trayhurn P: Glucose
transporters (Glut and SGLT): expanded families of sugar transport
proteins. Br J Nutr. 89:3–9. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun L, Zeng X, Yan C, et al: Crystal
structure of a bacterial homologue of glucose transporters GLUT1–4.
Nature. 490:361–366. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lastraioli E, Bencini L, Bianchini E, et
al: hERG1 channels and Glut-1 as independent prognostic indicators
of worse outcome in stage I and II colorectal cancer: A pilot
study. Transl Oncol. 5:105–112. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tong SY, Lee JM, Ki KD, et al: Correlation
between FDG uptake by PET/CT and the expressions of glucose
transporter type 1 and hexokinase II in cervical cancer. Int J
Gynecol Cancer. 22:654–658. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sasaki H, Shitara M, Yokota K, et al:
Overexpression of GLUT1 correlates with Kras mutations in lung
carcinomas. Mol Med Rep. 5:599–602. 2012.
|
|
14
|
Liu TQ, Fan J, Zhou L and Zheng SS:
Effects of suppressing glucose transporter-1 by an antisense
oligodeoxynucleotide on the growth of human hepatocellular
carcinoma cells. Hepatobiliary Pancreat Dis Int. 10:72–77. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Melstrom LG, Salabat MR, Ding XZ, et al:
Apigenin down-regulates the hypoxia response genes: HIF-1α, GLUT-1,
and VEGF in human pancreatic cancer cells. J Surg Res. 167:173–181.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fan J, Zhou JQ, Yu GR and Lu DD: Glucose
transporter protein 1-targeted RNA interference inhibits growth and
invasion of the osteosarcoma cell line MG63 in vitro. Cancer
Biother Radiopharm. 25:521–527. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rademakers SE, Lok J, van der Kogel AJ,
Bussink J and Kaanders JH: Metabolic markers in relation to
hypoxia; staining patterns and colocalization of pimonidazole,
HIF-1α, CAIX, LDH-5, GLUT-1, MCT1 and MCT4. BMC Cancer. 11:1672011.
View Article : Google Scholar
|
|
18
|
Eckert AW, Lautner MH, Taubert H, Schubert
J and Bilkenroth U: Expression of Glut-1 is a prognostic marker for
oral squamous cell carcinoma patients. Oncol Rep. 20:1381–1385.
2008.PubMed/NCBI
|
|
19
|
Kunkel M, Reichert TE, Benz P, et al:
Overexpression of Glut-1 and increased glucose metabolism in tumors
are associated with a poor prognosis in patients with oral squamous
cell carcinoma. Cancer. 97:1015–1024. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou S, Wang S, Wu Q, Fan J and Wang Q:
Expression of glucose transporter-1 and -3 in the head and neck
carcinoma - the correlation of the expression with the biological
behaviors. ORL J Otorhinolaryngol Relat Spec. 70:189–194. 2008.
View Article : Google Scholar
|
|
21
|
Choi JW, Yoon DJ, Lee HW, Han DP and Ahn
YH: Antisense GLUT1 RNA suppresses the transforming phenotypes of
NIH 3T3 cells transformed by N-Ras. Yonsei Med J. 36:480–486. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chan JY, Kong SK, Choy YM, Lee CY and Fung
KP: Inhibition of glucose transporter gene expression by antisense
nucleic acids in HL-60 leukemia cells. Life Sci. 65:63–70. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ito S, Nemoto T, Satoh S, Sekihara H,
Seyama Y and Kubota S: Human rhabdomyosarcoma cells retain
insulin-regulated glucose transport activity through glucose
transporter 1. Arch Biochem Biophys. 373:72–82. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Noguchi Y, Saito A, Miyagi Y, et al:
Suppression of facilitative glucose transporter 1 mRNA can suppress
tumor growth. Cancer Lett. 154:175–182. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bussink J, van der Kogel AJ and Kaanders
JH: Activation of the PI3-K/AKT pathway and implications for
radioresistance mechanisms in head and neck cancer. Lancet Oncol.
9:288–296. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stuschke M and Thames HD: Fractionation
sensitivities and dose-control relations of head and neck
carcinomas: analysis of the randomized hyperfractionation trials.
Radiother Oncol. 51:113–121. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Denekamp J, Daşu A, Waites A and Littbrand
B: Hyperfractionation as an effective way of overcoming
radioresistance. Int J Radiat Oncol Biol Phys. 42:705–709. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hingorani M, Colley WP, Dixit S and Beavis
AM: Hypofractionated radiotherapy for glioblastoma: strategy for
poor-risk patients or hope for the future? Br J Radiol.
85:e770–e781. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bollschweiler E, Hölscher AH and Metzger
R: Histologic tumor type and the rate of complete response after
neoadjuvant therapy for esophageal cancer. Future Oncol. 6:25–35.
2010. View Article : Google Scholar
|
|
30
|
Sho M, Akahori T, Tanaka T, et al:
Pathological and clinical impact of neoadjuvant chemoradiotherapy
using full-dose gemcitabine and concurrent radiation for resectable
pancreatic cancer. J Hepatobiliary Pancreat Sci. 20:197–205. 2013.
View Article : Google Scholar
|
|
31
|
Yamazaki H, Nakamura S, Nishimura T, et
al: Hypofractionated stereotactic radiotherapy with the hypoxic
sensitizer AK-2123 (sanazole) for reirradiation of brain
metastases: a preliminary feasibility report. Anticancer Res.
33:1773–1776. 2013.PubMed/NCBI
|
|
32
|
Chen FH, Chiang CS, Wang CC, et al:
Vasculatures in tumors growing from preirradiated tissues: formed
by vasculogenesis and resistant to radiation and antiangiogenic
therapy. Int J Radiat Oncol Biol Phys. 80:1512–1521. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Saigusa S, Toiyama Y, Tanaka K, et al:
Prognostic significance of glucose transporter-1 (GLUT1) gene
expression in rectal cancer after preoperative chemoradiotherapy.
Surg Today. 42:460–469. 2012. View Article : Google Scholar
|
|
34
|
Kunkel M, Moergel M, Stockinger M, et al:
Overexpression of GLUT-1 is associated with resistance to
radiotherapy and adverse prognosis in squamous cell carcinoma of
the oral cavity. Oral Oncol. 43:796–803. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Doki Y, Takachi K, Ishikawa O, et al:
Reduced tumor vessel density and high expression of glucose
transporter 1 suggest tumor hypoxia of squamous cell carcinoma of
the esophagus surviving after radiotherapy. Surgery. 137:536–544.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pedersen MW, Holm S, Lund EL, Højgaard L
and Kristjansen PE: Coregulation of glucose uptake and vascular
endothelial growth factor (VEGF) in two small-cell lung cancer
(SCLC) sublines in vivo and in vitro. Neoplasia. 3:80–87. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Korkeila E, Jaakkola PM, Syrjänen K,
Pyrhönen S and Sundström J: Pronounced tumour regression after
radiotherapy is associated with negative/weak glucose transporter-1
expression in rectal cancer. Anticancer Res. 31:311–315.
2011.PubMed/NCBI
|
|
38
|
Korkeila EA, Sundström J, Pyrhönen S and
Syrjänen K: Carbonic anhydrase IX, hypoxia-inducible factor-1α,
ezrin and glucose transporter-1 as predictors of disease outcome in
rectal cancer: multivariate Cox survival models following data
reduction by principal component analysis of the
clinicopathological predctors. Anticancer Res. 31:4529–4535.
2011.PubMed/NCBI
|
|
39
|
Luo XM, Zhou SH and Fan J: Glucose
transporter-1 as a new therapeutic target in laryngeal carcinoma. J
Int Med Res. 38:1885–1892. 2010. View Article : Google Scholar
|
|
40
|
Bai J, Guo XG and Bai XP: Epidermal growth
factor receptor-related DNA repair and radiation-resistance
regulatory mechanisms: a mini-review. Asian Pac J Cancer Prev.
13:4879–4881. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Theys J, Yahyanejad S, Habets R, et al:
High NOTCH activity induces radiation resistance in non small cell
lung cancer. Radiother Oncol. 108:440–445. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yun J, Rago C, Cheong I, et al: Glucose
deprivation contributes to the development of KRAS pathway
mutations in tumor cells. Science. 325:1555–1559. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yasuda M, Miyazawa M, Fujita M, et al:
Expression of hypoxia inducible factor-1alpha (HIF-1alpha) and
glucose transporter-1 (GLUT-1) in ovarian adenocarcinomas:
difference in hypoxic status depending on histological character.
Oncol Rep. 19:111–116. 2008.
|
|
44
|
Mayer A, Höckel M, Wree A and Vaupel P:
Microregional expression of glucose transporter-1 and oxygenation
status: lack of correlation in locally advanced cervical cancers.
Clin Cancer Res. 11:2768–2773. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ding XZ, Fehsenfeld DM, Murphy LO, Permert
J and Adrian TE: Physiological concentrations of insulin augment
pancreatic cancer cell proliferation and glucose utilization by
activating MAP kinase, PI3 kinase and enhancing GLUT-1 expression.
Pancreas. 21:310–320. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sommermann TG, O’Neill K, Plas DR and
Cahir-McFarland E: IKKβ and NF-κB transcription govern lymphoma
cell survival through AKT-induced plasma membrane trafficking of
GLUT1. Cancer Res. 71:7291–7300. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wofford JA, Wieman HL, Jacobs SR, Zhao Y
and Rathmell JC: IL-7 promotes Glut1 trafficking and glucose uptake
via STAT5-mediated activation of Akt to support T-cell survival.
Blood. 111:2101–2111. 2008. View Article : Google Scholar
|
|
48
|
Wieman HL, Wofford JA and Rathmell JC:
Cytokine stimulation promotes glucose uptake via
phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and
trafficking. Mol Biol Cell. 18:1437–1446. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Melstrom LG, Salabat MR, Ding XZ, et al:
Apigenin inhibits the GLUT-1 glucose transporter and the
phosphoinositide 3-Kinase/Akt pathway in human pancreatic cancer
cells. Pancreas. 37:426–431. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Schuurbiers OC, Kaanders JH, van der
Heijden HF, Dekhuijzen RP, Oyen WJ and Bussink J: The
PI3-K/AKT-pathway and radiation resistance mechanisms in non-small
cell lung cancer. J Thorac Oncol. 4:761–767. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Söderlund K, Pérez-Tenorio G and Stål O:
Activation of the phosphatidylinositol 3-kinase/Akt pathway
prevents radiation-induced apoptosis in breast cancer cells. Int J
Oncol. 26:25–32. 2005.
|
|
52
|
Florczak U, Toulany M, Kehlbach R and
Peter Rodemann H: 2-Methoxyestradiol-induced radiosensitization is
independent of SOD but depends on inhibition of Akt and DNA-PKcs
activities. Radiother Oncol. 92:334–338. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Brophy S, Sheehan KM, McNamara DA, Deasy
J, Bouchier-Hayes DJ and Kay EW: GLUT-1 expression and response to
chemoradiotherapy in rectal cancer. Int J Cancer. 125:2778–2782.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhou SH, Fan J, Chen XM, Cheng KJ and Wang
SQ: Inhibition of cell proliferation and glucose uptake in human
laryngeal carcinoma cells by antisense oligonucleotides against
glucose transporter-1. Head Neck. 31:1624–1633. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yan SX, Luo XM, Zhou SH, et al: Effect of
antisense oligodeoxynucleotides glucose transporter-1 on
enhancement of radiosensitivity of laryngeal carcinoma. Int J Med
Sci. 10:1375–1386. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Upadhyay M, Samal J, Kandpal M, Singh OV
and Vivekanandan P: The Warburg effect: insights from the past
decade. Pharmacol Ther. 137:318–330. 2013. View Article : Google Scholar
|
|
57
|
Bensinger SJ and Christofk HR: New aspects
of the Warburg effect in cancer cell biology. Semin Cell Dev Biol.
23:352–361. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hirschhaeuser F, Sattler UG and
Mueller-Klieser W: Lactate: a metabolic key player in cancer.
Cancer Res. 71:6921–6925. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Krzeslak A, Wojcik-Krowiranda K, Forma E,
et al: Expression of GLUT1 and GLUT3 glucose transporters in
endometrial and breast cancers. Pathol Oncol Res. 18:721–728. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sheehan JP, Shaffrey ME, Gupta B, Larner
J, Rich JN and Park DM: Improving the radiosensitivity of
radioresistant and hypoxic glioblastoma. Future Oncol. 6:1591–1601.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Overgaard J: Hypoxic modification of
radiotherapy in squamous cell carcinoma of the head and neck - a
systematic review and meta-analysis. Radiother Oncol. 100:22–32.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Shimanishi M, Ogi K, Sogabe Y, et al:
Silencing of GLUT-1 inhibits sensitization of oral cancer cells to
cisplatin during hypoxia. J Oral Pathol Med. 42:382–388. 2013.
View Article : Google Scholar
|
|
63
|
Pez F, Dayan F, Durivault J, et al: The
HIF-1-inducible lysyl oxidase activates HIF-1 via the Akt pathway
in a positive regulation loop and synergizes with HIF-1 in
promoting tumor cell growth. Cancer Res. 71:1647–1657. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yasuda M, Miyazawa M, Fujita M, et al:
Expression of hypoxia inducible factor-1alpha (HIF-1alpha) and
glucose transporter-1 (GLUT-1) in ovarian adenocarcinomas:
difference in hypoxic status depending on histological character.
Oncol Rep. 19:111–116. 2008.
|
|
65
|
Wu XH, Chen SP, Mao JY, Ji XX, Yao HT and
Zhou SH: Expression and significance of hypoxia-inducible factor-1α
and glucose transporter-1 in laryngeal carcinoma. Oncol Lett.
5:261–266. 2013.
|
|
66
|
Evans A, Bates V, Troy H, et al: Glut-1 as
a therapeutic target: increased chemoresistance and
HIF-1-independent link with cell turnover is revealed through
COMPARE analysis and metabolomic studies. Cancer Chemother
Pharmacol. 61:377–393. 2008. View Article : Google Scholar
|
|
67
|
Ke CC, Liu RS, Yang AH, et al:
CD133-expressing thyroid cancer cells are undifferentiated,
radioresistant and survive radioiodide therapy. Eur J Nucl Med Mol
Imaging. 40:61–71. 2013. View Article : Google Scholar
|
|
68
|
Piao LS, Hur W, Kim TK, et al:
CD133+ liver cancer stem cells modulate radioresistance
in human hepatocellular carcinoma. Cancer Lett. 315:129–137. 2012.
View Article : Google Scholar
|
|
69
|
Mai HM, Zheng JW, Wang YA, et al: CD133
selected stem cells from proliferating infantile hemangioma and
establishment of an in vivo mice model of hemangioma. Chin Med J
(Engl). 126:88–94. 2013.
|
|
70
|
Chen XH, Bao YY, Zhou SH, Wang QY, Wei Y
and Fan J: Glucose transporter-1 expression in CD133+
laryngeal carcinoma Hep-2 cells. Mol Med Rep. 8:1695–1700.
2013.PubMed/NCBI
|
|
71
|
Stein I, Neeman M, Shweiki D, Itin A and
Keshet E: Stabilization of vascular endothelial growth factor mRNA
by hypoxia and hypoglycemia and coregulation with other
ischemia-induced genes. Mol Cell Biol. 15:5363–5368.
1995.PubMed/NCBI
|
|
72
|
Gogineni VR, Nalla AK, Gupta R, Dinh DH,
Klopfenstein JD and Rao JS: Chk2-mediated G2/M cell cycle arrest
maintains radiation resistance in malignant meningioma cells.
Cancer Lett. 313:64–75. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Hematulin A, Meethang S, Ingkaninan K and
Sagan D: Derris scandens Benth extract potentiates radioresistance
of Hep-2 laryngeal cancer cells. Asian Pac J Cancer Prev.
13:1289–1295. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Young EF, Smilenov LB, Lieberman HB and
Hall EJ: Combined haploinsufficiency and genetic control of the
G2/M checkpoint in irradiated cells. Radiat Res. 177:743–750. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zhou Q, Lui VW and Yeo W: Targeting the
PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Future Oncol.
7:1149–1167. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Keck S, Glencer AC and Rugo HS: Everolimus
and its role in hormone-resistant and trastuzumab-resistant
metastatic breast cancer. Future Oncol. 8:1383–1396. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Webster L, Hodgkiss RJ and Wilson GD: Cell
cycle distribution of hypoxia and progression of hypoxic tumour
cells in vivo. Br J Cancer. 77:227–234. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Koritzinsky M, Wouters BG, Amellem O and
Pettersen EO: Cell cycle progression and radiation survival
following prolonged hypoxia and re-oxygenation. Int J Radiat Biol.
77:319–328. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kumareswaran R, Ludkovski O, Meng A, Sykes
J, Pintilie M and Bristow RG: Chronic hypoxia compromises repair of
DNA double-strand breaks to drive genetic instability. J Cell Sci.
125:189–199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Toustrup K, Sørensen BS, Nordsmark M, et
al: Development of a hypoxia gene expression classifier with
predictive impact for hypoxic modification of radiotherapy in head
and neck cancer. Cancer Res. 71:5923–5931. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Koumenis C: ER stress, hypoxia tolerance
and tumor progression. Curr Mol Med. 6:55–69. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Kitagawa N, Kondo S, Wakisaka N, et al:
Expression of seven-in-absentia homologue 1 and hypoxia-inducible
factor 1 alpha: novel prognostic factors of nasopharyngeal
carcinoma. Cancer Lett. 331:52–57. 2013. View Article : Google Scholar
|
|
83
|
Pentheroudakis G, Nicolaou I, Kotoula V,
et al: Prognostic utility of angiogenesis and hypoxia effectors in
patients with operable squamous cell cancer of the larynx. Oral
Oncol. 48:709–716. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Moeller BJ, Cao Y, Li CY and Dewhirst MW:
Radiation activates HIF-1 to regulate vascular radiosensitivity in
tumors: role of reoxygenation, free radicals, and stress granules.
Cancer Cell. 5:429–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Kim WY, Oh SH, Woo JK, Hong WK and Lee HY:
Targeting heat shock protein 90 overrides the resistance of lung
cancer cells by blocking radiation-induced stabilization of
hypoxia-inducible factor-1alpha. Cancer Res. 69:1624–1632. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Lee SM, Lee CT, Kim YW, Han SK, Shim YS
and Yoo CG: Hypoxia confers protection against apoptosis via
PI3K/Akt and ERK pathways in lung cancer cells. Cancer Lett.
242:231–238. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Edwards E, Geng L, Tan J, Onishko H,
Donnelly E and Hallahan DE: Phosphatidylinositol 3-kinase/Akt
signaling in the response of vascular endothelium to ionizing
radiation. Cancer Res. 62:4671–4677. 2002.PubMed/NCBI
|
|
88
|
Kumar P, Miller AI and Polverini PJ: p38
MAPK mediates gamma-irradiation-induced endothelial cell apoptosis
and vascular endothelial growth factor protects endothelial cells
through phosphoinositide 3-kinase-Akt-Bcl-2 pathway. J Biol Chem.
279:43352–43360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Kumar P, Benedict R, Urzua F, Fischbach C,
Mooney D and Polverini P: Combination treatment significantly
enhances the efficacy of antitumor therapy by preferentially
targeting angiogenesis. Lab Invest. 85:756–767. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Wu J, Chen C and Zhao KN:
Phosphatidylinositol 3-kinase signaling as a therapeutic target for
cervical cancer. Curr Cancer Drug Targets. 13:143–156. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Shinohara ET and Maity A: Increasing
sensitivity to radiotherapy and chemotherapy by using novel
biological agents that alter the tumor microenvironment. Curr Mol
Med. 9:1034–1045. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Fokas E, McKenna WG and Muschel RJ: The
impact of tumor microenvironment on cancer treatment and its
modulation by direct and indirect antivascular strategies. Cancer
Metastasis Rev. 31:823–842. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhan M and Han ZC: Phosphatidylinositide
3-kinase/AKT in radiation responses. Histol Histopathol.
19:915–923. 2004.PubMed/NCBI
|
|
94
|
Park JK, Jung HY, Park SH, et al:
Combination of PTEN and gamma-ionizing radiation enhances cell
death and G(2)/M arrest through regulation of AKT activity and p21
induction in non-small-cell lung cancer cells. Int J Radiat Oncol
Biol Phys. 70:1552–1560. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Kandel ES, Skeen J, Majewski N, et al:
Activation of Akt/protein kinase B overcomes a G(2)/m cell cycle
checkpoint induced by DNA damage. Mol Cell Biol. 22:7831–7841.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Mukherjee B, Tomimatsu N, Amancherla K,
Camacho CV, Pichamoorthy N and Burma S: The dual PI3K/mTOR
inhibitor NVP-BEZ235 is a potent inhibitor of ATM- and
DNA-PKCs-mediated DNA damage responses. Neoplasia. 14:34–43.
2012.PubMed/NCBI
|
|
97
|
Yang J, Xu X, Hao Y, et al: Expression of
DNA-PKcs and BRCA1 as prognostic indicators in nasopharyngeal
carcinoma following intensity-modulated radiation therapy. Oncol
Lett. 5:1199–1204. 2013.PubMed/NCBI
|
|
98
|
Millet P, Granotier C, Etienne O and
Boussin FD: Radiation-induced upregulation of telomerase activity
escapes PI3-kinase inhibition in two malignant glioma cell lines.
Int J Oncol. 43:375–382. 2013.PubMed/NCBI
|
|
99
|
Qu YY, Hu SL, Xu XY, et al: Nimotuzumab
enhances the radiosensitivity of cancer cells in vitro by
inhibiting radiation-induced DNA damage repair. PLoS One.
8:e707272013. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Zhang T, Cui GB, Zhang J, et al:
Inhibition of PI3 kinases enhances the sensitivity of non-small
cell lung cancer cells to ionizing radiation. Oncol Rep.
24:1683–1689. 2010.PubMed/NCBI
|
|
101
|
Azad A, Jackson S, Cullinane C, et al:
Inhibition of DNA-dependent protein kinase induces accelerated
senescence in irradiated human cancer cells. Mol Cancer Res.
9:1696–1707. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Li HF, Kim JS and Waldman T:
Radiation-induced Akt activation modulates radioresistance in human
glioblastoma cells. Radiat Oncol. 4:432009. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Minjgee M, Toulany M, Kehlbach R, Giehl K
and Rodemann HP: K-RAS(V12) induces autocrine production of EGFR
ligands and mediates radioresistance through EGFR-dependent Akt
signaling and activation of DNA-PKcs. Int J Radiat Oncol Biol Phys.
81:1506–1514. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Petrás M, Lajtos T, Friedländer E, et al:
Molecular interactions of ErbB1 (EGFR) and integrin-β1 in
astrocytoma frozen sections predict clinical outcome and correlate
with Akt-mediated in vitro radioresistance. Neuro Oncol.
15:1027–1040. 2013. View Article : Google Scholar
|
|
105
|
Gautier EL, Westerterp M, Bhagwat N, et
al: HDL and Glut1 inhibition reverse a hypermetabolic state in
mouse models of myeloproliferative disorders. J Exp Med.
210:339–353. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Swainson L, Kinet S, Mongellaz C,
Sourisseau M, Henriques T and Taylor N: IL-7-induced proliferation
of recent thymic emigrants requires activation of the PI3K pathway.
Blood. 109:1034–1042. 2007. View Article : Google Scholar
|
|
107
|
Rathmell JC, Fox CJ, Plas DR, Hammerman
PS, Cinalli RM and Thompson CB: Akt-directed glucose metabolism can
prevent Bax conformation change and promote growth
factor-independent survival. Mol Cell Biol. 23:7315–7328. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Zhao Y, Altman BJ, Coloff JL, et al:
Glycogen synthase kinase 3alpha and 3beta mediate a
glucose-sensitive antiapoptotic signaling pathway to stabilize
Mcl-1. Mol Cell Biol. 27:4328–4339. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Roberts MS, Woods AJ, Dale TC, Van Der
Sluijs P and Norman JC: Protein kinase B/Akt acts via glycogen
synthase kinase 3 to regulate recycling of alpha v beta 3 and alpha
5 beta 1 integrins. Mol Cell Biol. 24:1505–1515. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Doughty CA, Bleiman BF, Wagner DJ, et al:
Antigen receptor-mediated changes in glucose metabolism in B
lymphocytes: role of phosphatidylinositol 3-kinase signaling in the
glycolytic control of growth. Blood. 107:4458–4465. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Marko AJ, Miller RA, Kelman A and
Frauwirth KA: Induction of glucose metabolism in stimulated T
lymphocytes is regulated by mitogen-activated protein kinase
signaling. PLoS One. 5:e154252010. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Radhakrishnan P, Baraneedharan U,
Veluchamy S, et al: Inhibition of rapamycin-induced AKT activation
elicits differential antitumor response in head and neck cancers.
Cancer Res. 73:1118–1127. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Samih N, Hovsepian S, Aouani A, Lombardo D
and Fayet G: Glut-1 translocation in FRTL-5 thyroid cells: role of
phosphatidylinositol 3-kinase and N-glycosylation. Endrocrinology.
141:4146–4155. 2000. View Article : Google Scholar
|
|
114
|
Clarke JF, Young PW, Yonezawa K, Kasuga M
and Holman GD: Inhibition of the translocation of GLUT1 and GLUT4
in 3T3-L1 cells by the phosphatidylinositol 3-kinase inhibitor,
wortmannin. Biochem J. 300:631–635. 1994.PubMed/NCBI
|
|
115
|
Golkar L, Salabat MR, Ding XZ, et al:
Apigenin inhibits pancreatic cancer cell proliferation via
down-regulation of the GLUT-1 glucose transporter through the
phosphoinositide 3-kinase (PI3K)/Akt signaling pathway (Abstract).
Pancreas. 33:4642006. View Article : Google Scholar
|
|
116
|
Pore N, Jiang Z, Shu HK, Bernhard E, Kao
GD and Maity A: Akt1 activation can augment hypoxia-inducible
factor-1alpha expression by increasing protein translation through
a mammalian target of rapamycin-independent pathway. Mol Cancer
Res. 4:471–479. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Plas DR, Talapatra S, Edinger AL, Rathmell
JC and Thompson CB: Akt and Bcl-xL promote growth
factor-independent survival through distinct effects on
mitochondrial physiology. J Biol Chem. 276:12041–12048. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Fang J, Bao YY, Zhou SH, et al: Recurrent
prognostic factors and expression of GLUT-1, PI3K and p-Akt in
adenoid cystic carcinomas of the head and neck: Clinicopathological
features and biomarkers of adenoid cystic carcinoma. Oncol Lett.
4:1234–1240. 2012.PubMed/NCBI
|
|
119
|
Shen WQ, Cheng KJ, Bao YY, Zhou SH and Yao
HT: Expression of Glut-1, HIF-1α, PI3K and p-Akt in a case of
ceruminous adenoma. Head Neck Oncol. 4:182012. View Article : Google Scholar
|
|
120
|
Silva A, Gírio A, Cebola I, Santos CI,
Antunes F and Barata JT: Intracellular reactive oxygen species are
essential for PI3K/Akt/mTOR-dependent IL-7-mediated viability of
T-cell acute lymphoblastic leukemia cells. Leukemia. 25:960–967.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Barata JT, Silva A, Brandao JG, Nadler LM,
Cardoso AA and Boussiotis VA: Activation of PI3K is indispensable
for interleukin 7-mediated viability, proliferation, glucose use,
and growth of T cell acute lymphoblastic leukemia cells. J Exp Med.
200:659–669. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Wahl H, Daudi S, Kshirsagar M, et al:
Expression of metabolically targeted biomarkers in endometrial
carcinoma. Gynecol Oncol. 116:21–27. 2010. View Article : Google Scholar
|
|
123
|
Fumarola C, Caffarra C, La Monica S, et
al: Effects of sorafenib on energy metabolism in breast cancer
cells: role of AMPK-mTORC1 signaling. Breast Cancer Res Treat.
141:67–78. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Ou J, Luan W, Deng J, Sa R and Liang H: αV
integrin induces multicellular radioresistance in human
nasopharyngeal carcinoma via activating SAPK/JNK pathway. Plos One.
7:e387372012. View Article : Google Scholar
|
|
125
|
Xiao H, Zhang Q, Shen J, Bindokas V and
Xing HR: Pharmacologic inactivation of kinase suppressor of Ras1
sensitizes epidermal growth factor receptor and oncogenic
Ras-dependent tumors to ionizing radiation treatment. Mol Cancer
Ther. 9:2724–2736. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Li P, Zhang Q, Torossian A, et al:
Simultaneous inhibition of EGFR and PI3K enhances radiosensitivity
in human breast cancer. Int J Radiat Oncol Biol Phys. 83:e391–e397.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Tan C, de Noronha RG, Roecker AJ, et al:
Identification of a novel small-molecule inhibitor of the
hypoxia-inducible factor 1 pathway. Cancer Res. 65:605–612.
2005.PubMed/NCBI
|
|
128
|
Romeo Y and Roux PP: Paving the way for
targeting RSK in cancer. Expert Opin Ther Targets. 15:5–9. 2011.
View Article : Google Scholar
|
|
129
|
Cataldi A, di Giacomo V, Rapino M,
Genovesi D and Rana RA: Cyclic nucleotide Response Element Binding
protein (CREB) activation promotes survival signal in human K562
erythroleukemia cells exposed to ionising radiation/etoposide
combined treatment. J Radiat Res. 47:113–120. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Moretti L, Yang ES, Kim KW and Lu B:
Autophagy signaling in cancer and its potential as novel target to
improve anticancer therapy. Drug Resist Updat. 10:135–143. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Shinohara ET, Cao C, Niermann K, et al:
Enhanced radiation damage of tumor vasculature by mTOR inhibitors.
Oncogene. 24:5414–5422. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Hudes G, Carducci M, Tomczak P, et al:
Temsirolimus, interferon alpha, or both for advanced renal-cell
carcinoma. N Engl J Med. 356:2271–2281. 2007. View Article : Google Scholar : PubMed/NCBI
|