Introduction
In recent years, there has been an increasing focus
on the cancer stem cell (CSC) hypothesis. This hypothesis
identifies a small subset of cancer cells that constitute the bulk
of self-sustaining cells with a unique capacity for self-renewal,
which cause the development of heterogeneous lineages during tumor
formation (1–5). Assays that examine CSC activity
require evaluation of the self-renewal and tumor propagation
abilities of the cells (3). An
increasing number of CSCs in solid tumors have been recognized
through sorting cancer cells in serum-free suspension culture
conditions and identifying the CSCs on the basis of differential
expression of surface markers combined with in vivo
propagation of tumorogenecity (6–9).
Breast cancer, the most common type of malignancy
among females, has an increasing incidence, with an annual growth
rate of 3% in China, and is the primary cause of cancer-associated
mortality among urban females (10). Tumorigenic breast cancer cells with
stem cell properties have been isolated and identified in breast
carcinoma lesions (11,12). Due to the limited number of cells
within the breast tumor reservoir and the location of the cells
within the tumor interstitium, breast CSCs are able to develop
resistance to drugs and evade chemotherapy, resulting in disease
relapse, even if the primary lesion has been eradicated (13,14).
Therefore, investigation of novel drug resistance mechanisms that
target stem cells is important to improve the current therapeutic
strategies for treating breast cancer.
Octamer-binding protein 4 (Oct4) and Nanog, two of
the transcriptional factors that exert key roles in the maintenance
of self-renewal and pluripotency in human embryonic stem cells,
have been recently observed to be expressed in numerous types of
cancer cell line and tissue, and have been associated with
aggressive tumors (15–19). Furthermore, downregulation of Oct4
and Nanog has been shown to promote stem cell differentiation and
inhibit tumor development (20–22).
A number of studies have revealed that Oct4 and Nanog are detected
at high levels in human breast cancer tissues, which indicates the
critical roles of Oct4 and Nanog in breast stem cell state
maintenance and escape from conventional chemotherapy (23,24).
However, the underlying molecular mechanism by which Oct4 and Nanog
mediate the drug resistance response to chemotherapy in breast CSCs
remains to be elucidated.
In the present study, breast CSCs were isolated from
MDA-MB-231 breast cancer cells using a serum-free suspension
culture, which characterizes the differential expression of cluster
of differentiation 44 (CD44) and CD24 on the CSC cell surface
combined with the capacity of CSCs to generate novel tumors when
injected into a congenetic animal model. Subsequently, the
differential expression of Oct4 and Nanog mRNA in the isolated
mammosphere MDA-MB-231 breast CSCs (defined as MDA-MB-231 stem
cells) and the MDA-MB-231 breast cancer cells was examined. The
critical relevance of Oct4 and Nanog with breast CSC therapeutic
response to chemotherapy was also investigated.
Materials and methods
Ethics
This study was approved by the Institutional Ethics
Committee of the First Affiliated Hospital of Xiamen University
(Xiamen, China) and was in compliance with national legislation and
the Declaration of Helsinki guidelines. All animal experiments were
approved by the Animal Care and Use Committee of Xiamen University.
Animal care was in accordance with the Regulations for the
Administration of Affairs Concerning Experimental Animals of Xiamen
University.
Cell lines and in vitro propagation of
human breast stem cells in serum-free culture
MDA-MB-231 human breast cancer cell lines were
provided by the Cancer Center of Xiamen Medical College (Xiamen,
China). The cells were cultured in differentiation conditions in
Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine
serum (FBS). After three days, when the cells covered 90% of the
plate, adherent cells were dissociated by incubation in 0.25%
trypsin-ethylenediaminetetraacetic acid solution for 1 min at 37°C.
MDA-MB-231 cells in the logarithmic growth phase were plated at
106, 105, 104 and 103
cells/ml in serum-free DMEM/F12 (1:1) medium containing 2% B27
(Gibco-BRL, Carlsbad, CA, USA), 20 ng/ml epidermal growth factor
(EGF; Sigma-Aldrich, St. Louis, MO, USA) and 20 ng/ml basic
fibroblast growth factor (bFGF; Sigma-Aldrich). The cells were
cultured in these serum-free conditions as non-adherent mammosphere
clusters. Differentiation was induced by culturing mammosphere
cells for 12 h in DMEM supplemented with 10% FBS.
Flow cytometry
The cells were washed twice with phosphate-buffered
saline (PBS) and then resuspended in the wash buffer
(106 cells/ml). Antibodies against CD44 (fluorescein
isothiocyanate-conjugated) and CD24 (phycoerythrin-conjugated)
obtained from eBioscience (San Diego, CA, USA), and the
corresponding isotype controls were added to the cell suspension,
and the cells were incubated at 4°C in the dark for 40 min.
Subsequently, the cells were washed twice in 4 ml PBS buffer and
then resuspended in 400 μl PBS buffer for flow cytometric analysis.
The stained cells were processed using flow cytometry (BD FACSAria™
II; BD Biosciences, Franklin Lakes, NJ, USA). The results were
analyzed using FlowJo v.7.6.5 software (TreeStar, Inc., Ashland,
OR, USA).
Paclitaxel inhibition
Cells in a single cell suspension state were seeded
in a 96-well plate at a density of 3×103 cells/ml in
serum-free DMEM. Subsequently, paclitaxel (Jiangsu Aosaikang
Pharmaceutical Co. Ltd., Nanjing, China) was added to the
suspension to bring the volume in each plate to 200 μl, and the
paclitaxel concentrations to 0.5, 1.0, 2.0, 4.0, 8.0, 16.0 and 32.0
μg/ml, respectively. The zero-adjusting well and control group were
then set-up, the former without cells and the latter without
paclitaxel. A volume of 20 μl CellTiter-Blue® reagent
(Promega Corporation, Madison, WI, USA) was added to every well
after 48 h incubation. After 4 h culture at 37°C, the optical
density (OD) of each well was then measured by a fluorescence
microplate reader (Beckman Institute, Urbana, IL, USA) at 570 nm.
The cell inhibition rate was defined as follows: Drug uptake
percentage = (OD of control group − OD of experimental group)/(OD
of control group)×100%. The data were obtained from three
independent experiments each performed in triplicate, in which the
median inhibitory concentration (IC50) was calculated
using ProHits analysis (http://prohitsms.com/Prohits_download/list.php).
Quantitative (q)PCR
Total RNA extraction from the MDA-MB-231 cells and
the MDA-MB-231 stem cells was conducted according to the
TRIzol® total RNA extraction kit manufacturer’s
instructions. Reverse transcription was performed from 1,000 ng
total RNA using the RevertAid First Strand cDNA Synthesis kit
according to the manufacturer’s instructions. The gene expression
levels relative to those of GAPDH were assessed using qPCR with the
ABI-7500 sequence detection system (Applied Biosystems, Inc.,
Carlsbad, CA, USA) and SYBR-Green chemistry (Shanghai Yingjun
Biotechnology Limited Company, Shanghai, China), as follows:
Initial denaturation at 95°C for 10 min, followed by 40 cycles of
denaturation at 95°C for 15 sec, and annealing and extension at
60°C for 1 min. The human GAPDH, Oct4 and Nanog primer sequences
(Sangon Biotech, Shanghai, China) employed are shown in Table I. The reactions were run in
triplicate and the generated products were analyzed with the sodium
dodecyl sulfate (SDS) software (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The data were evaluated as 2−ΔΔCt
values (Ct indicates the cycle threshold). The results are
expressed as the normalization ratio of the relative quantities of
the Oct4 and Nanog mRNAs to those of the control, and the fold
difference to the control was used for the comparison.
 | Table IPrimer sequences used in the
quantitative polymerase chain reaction experiments. |
Table I
Primer sequences used in the
quantitative polymerase chain reaction experiments.
| Gene | Primer sequence
(5′-3′) | Amplicon size
(bp) |
|---|
| Oct4 | Forward:
5′-AGCAAAACCCGGAGGAGT-3′
Reverse: 5′-CCACATCGGCCTGTGTATATC-3′ | 114 |
| Nanog | Forward:
5′-TGAACCTCAGCTACAAACAG-3′
Reverse: 5′-TGGTGGTAGGAAGAGTAAAG-3′ | 124 |
| GAPDH | Forward:
5′-GAAGGTGAAGGTCGGAGTC-3′
Reverse: 5′-GAAGATGGTGATGGGATTTC-3′ | 226 |
Western blot analysis
The MDA-MB-231 cells and the MDA-MB-231 stem cells
were collected and then lysed with radioimmunoprecipitation assay
buffer and the protein concentrations within the cells were
measured according to the RIPA lysate manufacturer’s instructions
(Applygen Technologies Inc., Beijing, China). Equivalent quantities
of protein for each sample were separated by SDS-PAGE, transferred
to PVDF membranes (Millipore, Billerica, MA, USA) and probed with
the following primary antibodies: Rabbit polyclonal antibody
against human OCT4 (1:8,000 dilution; cat no.: ab18976), mouse
monoclonal antibody against human Nanog (1:1,000 dilution; cat no.:
ab89500) and mouse monoclonal antibody against human GAPDH (1:3,000
dilution; cat no.: ab57062). All antibodies were obtained from
Abcam (Cambridge, MA, USA). The PVDF membranes were incubated
overnight at 4°C with the primary antibodies and then washed three
times. A secondary horseradish peroxidase-labeled goat polyclonal
antibody against rabbit (1:3,000 dilution; cat no.: ab97200) or a
goat polyclonal antibody against mouse (1:4,000 dilution; cat no.:
ab97265) were added and incubated for 2 h at room temperature.
Immunodetection was performed using an electrochemiluminescent
substrate (Pierce Biotechnology, Inc., Rockford, IL, USA) and the
Gel Doc XR type imaging system. The intensity of bands was
quantified using Image J software (Bio-Rad Laboratories). Three
independent experiments, each in triplicate, were conducted in
24-well plates.
Silencing through RNA interference
To inhibit Oct4 or Nanog expression in the
MDA-MB-231 stem cells, RNA interference silencing was performed
using RNAfectin Transfection Reagent (Tiangen, Beijing, China)
according to the manufacturer’s instructions. All double-stranded
siRNAs were designed and synthesized by Qiagen (Valencia, CA, USA).
The siRNA sequences are shown in Table II.
 | Table IIsiRNA sequences used for silencing in
the RNA interference experiments. |
Table II
siRNA sequences used for silencing in
the RNA interference experiments.
| siRNA target
gene | Primer sequence
(5′-3′) | Molecular
weight |
|---|
| Oct4 | Forward
5′-GGAUUAAGUUCUUCAUUCATT-3′ | 21 bp/4943.32 |
| Reverse
5′-UGAAUGAAGAACUUAAUCCCA-3′ | 21 bp/5540.69 |
| Nanog | Forward
5′-UGAUUGUUCCAGGAUUGGGTG-3′ | 21 bp/5257.49 |
| Reverse
5′-CACCCAATCCTGGAACAATCA-3′ | 21 bp/6407.11 |
The breast CSCs were initially plated in 24-well
plates at a density of 2×105 cells/well in DMEM medium,
and after 24 h were transfected with 7.5 ng/μl siRNA against Oct4,
Nanog or non-targeting siRNA. Cells that had not been transfected
served as controls. The cells were harvested 48 h after
transfection to calculate the mRNA and protein expression
levels.
In vivo injection of MDA-MB-231 stem
cells
A total of 60 four-week-old female NOD/SCID mice
with a mean body weight of 25±5 g were purchased from the
Experimental Animal Center of Xiamen University (Xiamen, China).
All mice were maintained in specific pathogen-free rooms at a
certain temperature and humidity, and were provided free access to
fresh water and food. A total of 40 of the NOD/SCID mice were
randomly divided into eight groups by drawing lots (n=5). The mice
were injected subcutaneously in the right back with 0.2 ml
MDA-MB-231 cells or MDA-MB-231 stem cells at concentrations of
106, 105, 104 and 103
cells/ml. The remaining 20 mice were divided into four experimental
groups (n=5). The mice were injected subcutaneously in the right
back with 0.2 ml MDA-MB-231 stem cells transduced with negative
interference RNA (RNAi), Oct4 RNAi and Nanog RNAi constructs at
concentrations of 106 cells/ml. Mice were injected
subcutaneously into the right back with 0.2 ml MDA-MB-231 stem
cells without siRNA transfection, as a control group. After four
weeks injection, all mice were sacrificed by cervical dislocation
and tumor nodules were confirmed by necropsy. All experiments were
approved by the Regional Ethical Committee for Animal
Experimentation at Xiamen University.
Statistical analyses
SPSS version 16.0 (SPSS, Inc., Chicago, IL, USA) was
used to analyze the data. All data are expressed as the mean values
or as the percentages of control values ± standard error of the
mean depending on the experiments performed. Comparisons between
two groups were calculated using Student’s t-test (two-tailed,
independent) and comparisons among more than two samples were
analyzed using one-way analysis of variance. P<0.05 was
considered to indicate a statistically significant difference.
Results
Isolation and identification of CSCs from
MDA-MB-231 breast cancer cell lines
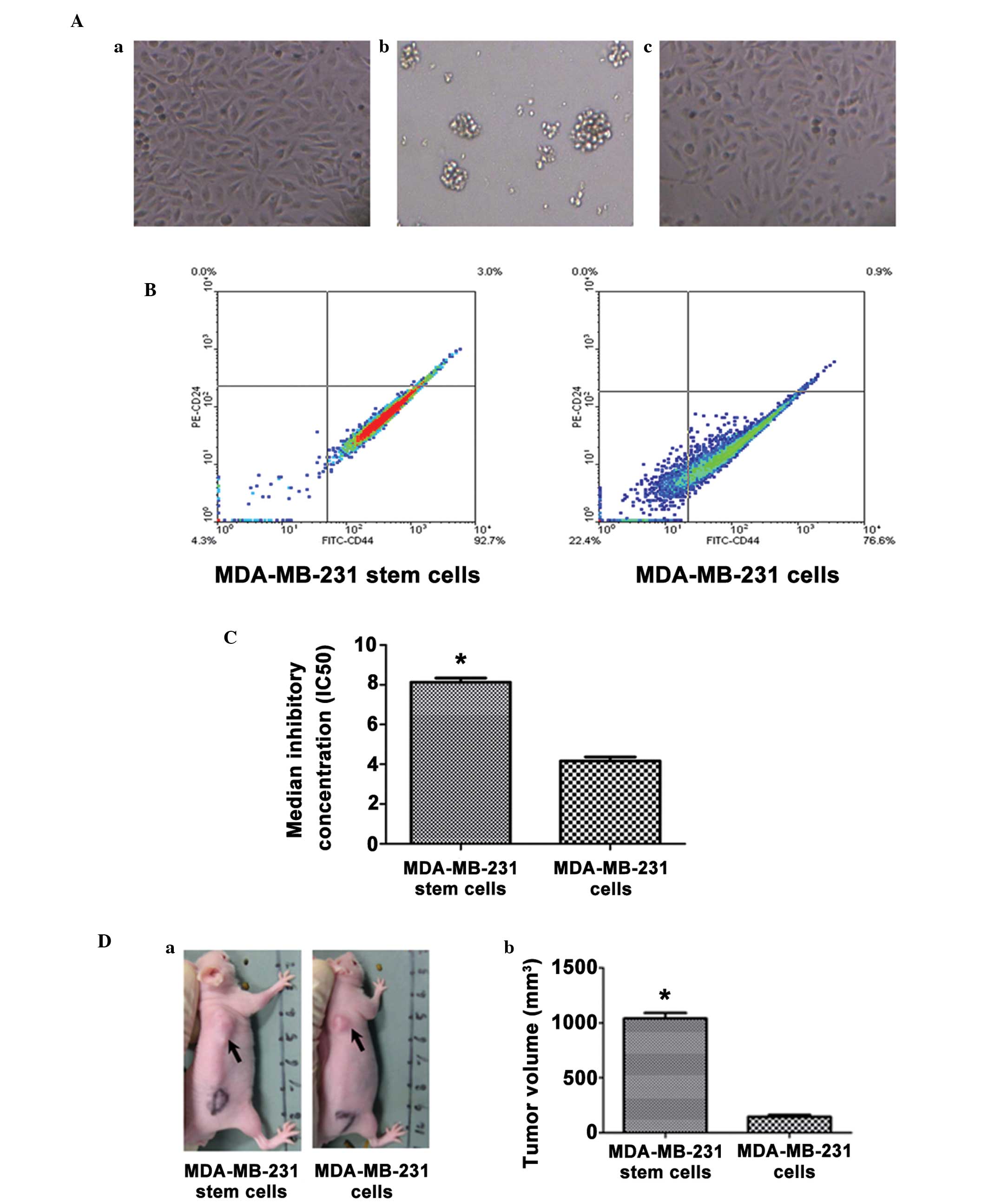
Differential growth patterns of
MDA-MB-231 breast CSCs
Human MDA-MB-231 breast cancer cells were plated
into 96-cell culture dishes in DMEM medium with 10% FBS and
cultured adherently (Fig. 1Aa).
After 48 h culture in serum-free medium supplemented with B27, EGF
and bFGF, the cells that were adherent to the dish died and left
behind spherical clusters formed in suspension, which were
subsequently defined as MDA-MB-231 stem cells (Fig. 1Ab). After 12 h culture in
differentiating medium with 10% FBS, the floating cells were able
to re-adhere and differentiate (Fig.
1Ac).
Elevated percentage of the
CD44+CD24-subpopulation in the mammosphere cell
population
As breast cancer progenitor cells have been
previously identified as CD44+CD24−/low
cells, the cellular expression of CD44 and CD24 was evaluated by
flow cytometry. The majority of the MDA-MB-231 stem cells (97.2%)
exhibited positive staining for CD44 and negative staining for
CD24, which was a significantly higher percentage than that of the
MDA-MB-231 cells (76.6%; Fig.
1B).
Isolated mammosphere cell resistance
to paclitaxel inhibition
The stem cell phenotype of the isolated mammosphere
cells was further verified by the high resistance of the cells to
chemotherapy. The result revealed cell inhibition curve of
MDA-MB-231 stem cells and MDA-MB-231 cells following exposure to
paclitaxel solution. The cell inhibition rate was dose-dependent.
In the MDA-MB-231 stem cells, 30 μg/ml paclitaxel was required to
reach a 100% cell inhibition rate; however, the MDA-MB-231 cells
only required 15 μg/ml paclitaxel to reach a 100% cell inhibition
rate. In the MDA-MB-231 stem cells, the paclitaxel IC50
value was almost two-fold higher than that of the MDA-MB-231 cells
(8.13±0.21 vs. 4.17±0.20 μg/ml; P<0.05; Fig. 1C).
High tumor-initiating capability of
isolated mammosphere cells
To compare the tumorigenicity of the MDA-MB-231 stem
cells and the MDA-MB-231 breast cancer cells, the two types of cell
were injected subcutaneously into NOD/SCID mice. After four weeks,
MDA-MB-231 cells gave rise to novel tumors when at least
0.2×106 cells per animal were injected; however, at
lower cell doses, no tumors developed. By contrast, the MDA-MB-231
stem cells formed tumors in five out of five, three out of five and
one out of five animals when 0.2×106, 0.2×105
and 0.2×104 cells/animal were injected, respectively
(Table III). When equal
quantities (0.2×106 cells/animal) of cells were
injected, the MDA-MB-231 stem cells formed significantly bigger
tumors than the MDA-MB-231 cells (1,040.00±49.80 vs. 146.20±16.48
mm3; P<0.05 Fig.
1D).
 | Table IIITumor-initiating capability of
isolated MDA-MB-231 stem cells and MDA-MB-231 cells. |
Table III
Tumor-initiating capability of
isolated MDA-MB-231 stem cells and MDA-MB-231 cells.
| Tumorigenicity |
|---|
|
|
|---|
| Cells/ animal | MDA-MB-231 stem
cells | MDA-MB-231
cells |
|---|
|
0.2×106 | 5/5 | 3/5 |
|
0.2×105 | 3/5 | 0/5 |
|
0.2×104 | 1/5 | 0/5 |
|
0.2×103 | 0/5 | 0/5 |
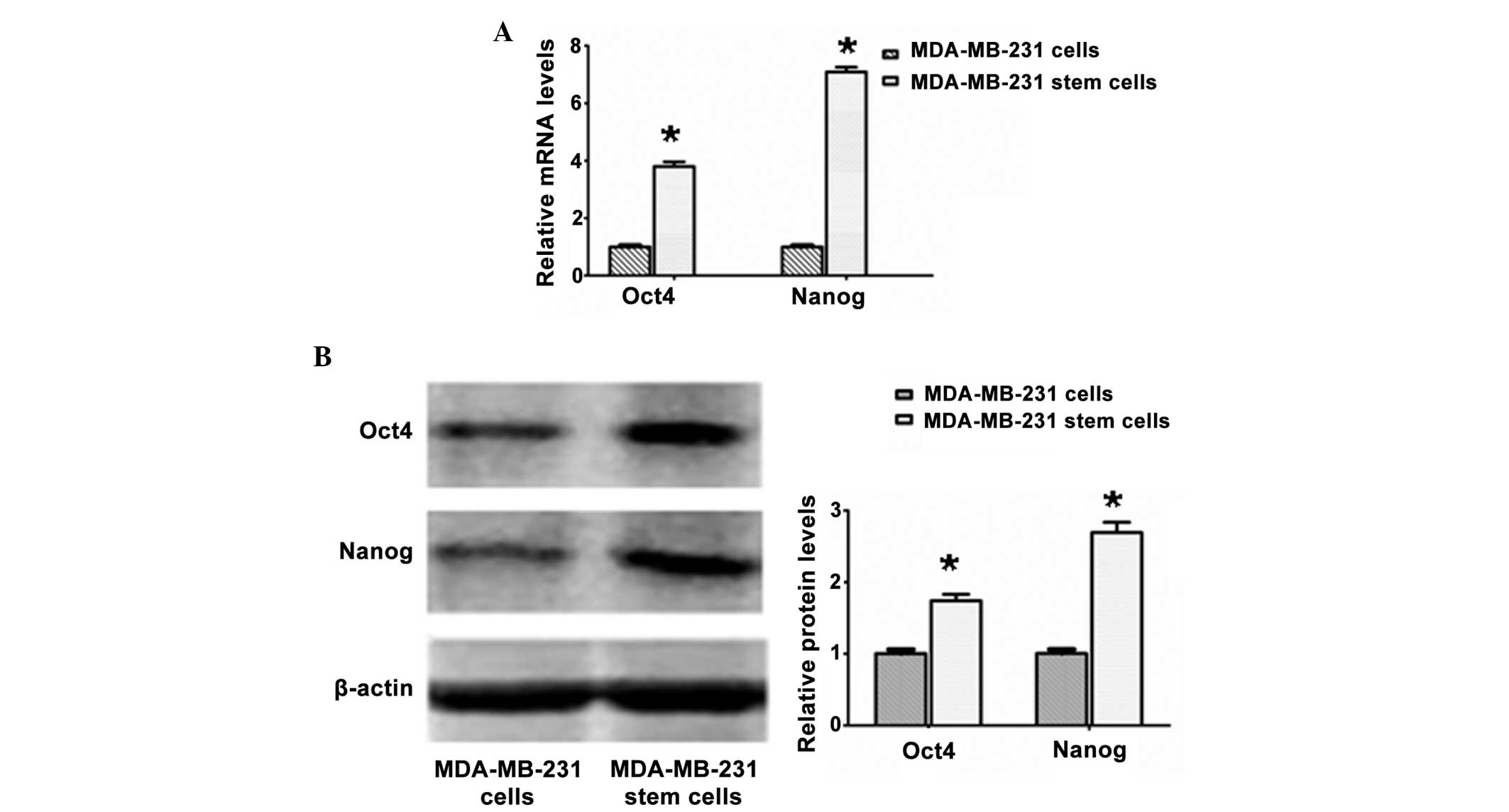
Higher expression levels of the Oct4 and
Nanog transcriptional factors in the MDA-MB-231 stem cells, as
compared with the MDA-MB-231 cells
The MDA-MB-231 stem cells exhibited significantly
higher relative mRNA and protein expression levels of the Oct4 and
Nanog putative stem cell markers than the MDA-MB-231 cells
(P<0.05), which was further confirmed by real-time PCR (Fig. 2A) and western blot analysis
(Fig. 2B).
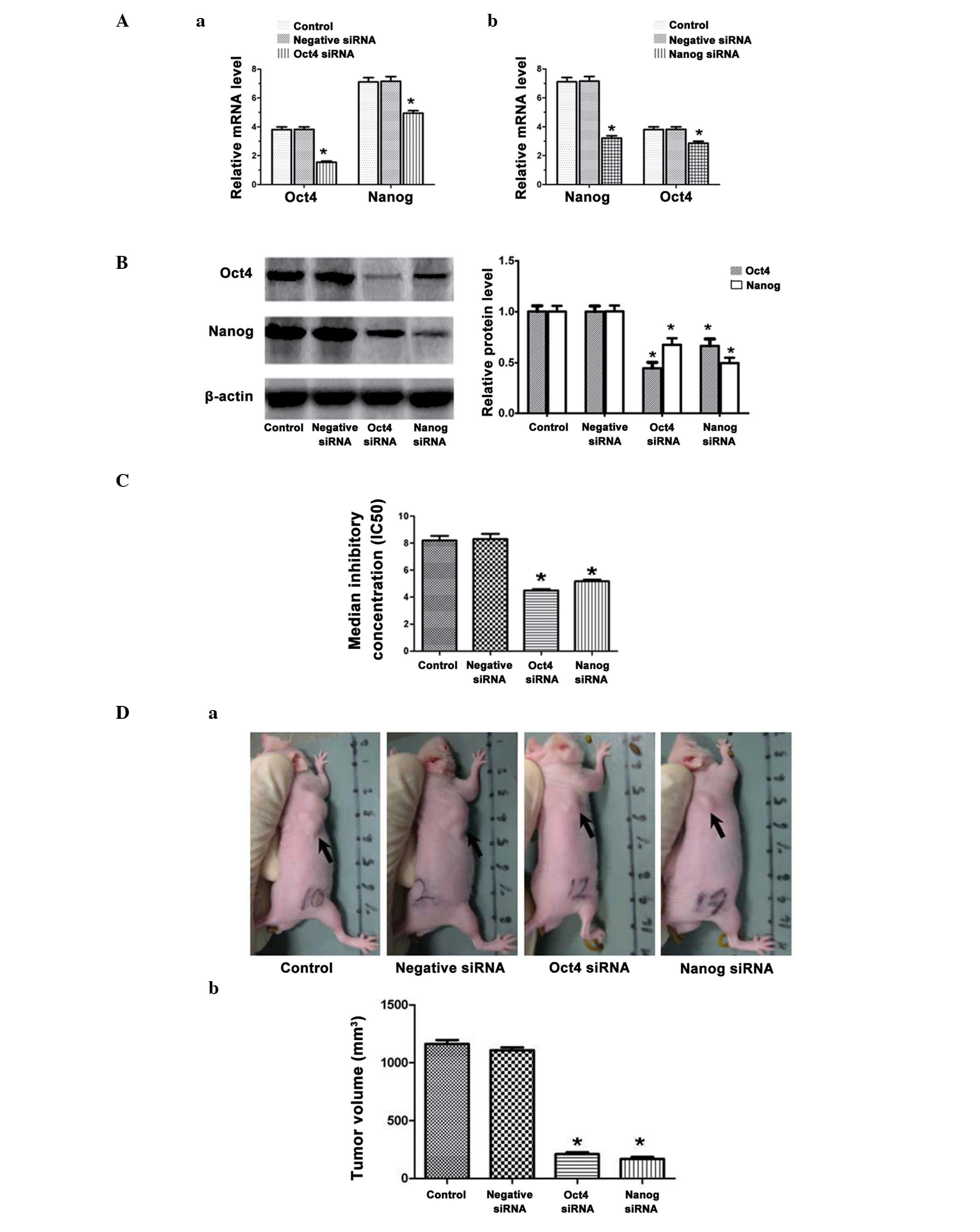
Reduced MDA-MB-231 stem cell drug
resistance to paclitaxel following downregulation of Oct4 and
Nanog
Expression levels of Oct4 and Nanog
mRNA are reduced when either Oct4 or Nanog is knocked-down
Oct4 and Nanog mRNA and protein downregulation
following transfection of MDA-MB-231 stem cells with the respective
RNAi molecules were analyzed by qPCR and western blot analysis,
respectively. shRNA transduction of Oct4 constructs not only
significantly reduced Oct4 mRNA but also significantly
downregulated Nanog transcripts in the MDA-MB-231 stem cells
(P<0.05; Fig. 3Aa). Similarly,
the MDA-MB-231 stem cells transfected with the Nanog RNAi
constructs exhibited significantly reduced the expression levels of
Nanog mRNA and significantly downregulated Oct4 mRNA (P<0.05;
Fig. 3Ab). The results were
consistent with the data concerning the respective protein
molecules in the western blot analysis (all P<0.05; Fig. 3B).
Reduced drug resistance and
tumor-initiating capability of mammoshere cells following
downregulation of Oct4 and Nanog
The MDA-MB-231 stem cells transfected with Oct4 or
Nanog RNAi constructs became more sensitive to paclitaxel
inhibition. The data revealed paclitaxel inhibition curves for
MDA-MB-231 stem cells or MDA-MB-231 stem cells transduced with
negative RNAi, Oct4 RNAi and Nanog RNAi constructs (Fig. 3C). In the MDA-MB-231 stem cells
transfected with the Oct4 RNAi constructs, the IC50
values were almost two-fold lower than those of cells transfected
with negative RNAi (4.49±0.10 vs. 8.30±0.39 μg/ml; P<0.05). The
MDA-MB-231 stem cells transfected with Nanog RNAi constructs also
exhibited reduced IC50 values, as compared with the
cells transfected with negative RNAi (5.17±0.12 vs. 8.30±0.39
μg/ml; Fig. 3C). Therefore, the
data demonstrated that downregulation of Oct4 or Nanog enhanced the
sensitivity of human breast CSCs to drug chemotherapy. Furthermore,
the tumorigenicity of the MDA-MB-231 stem cells transfected with
Oct4 RNAi or Nanog RNAi was reduced. When injecting equal
quantities of cells, the Oct4 RNAi and Nanog RNAi groups formed
significantly smaller tumors than either the negative RNAi or
control group (1,163.00±33.80 and 1,108.00±24.93 mm3 vs.
210.80±16.60 and 167.80±17.76 mm3; P<0.05; Fig. 3D).
Discussion
Breast cancer is currently the most frequently
occurring type of cancer and the primary cause of cancer-related
mortality in females worldwide (10). With increasing advances in the
investigation of CSCs, breast CSCs have been gradually determined
to be capable of self-renewal and maintaining tumor growth and
heterogeneity, as well as being rare, rendering these cells a
promising foundation for stem cell-based therapeutics (25–28).
Therefore, the identification of pure breast CSCs is key for the
development of targeted antitumor therapies. In the present study,
mammospheres were observed to form in serum-free medium in the
presence of B27, EGF and bFGF, and with sustained long-term
suspension culture. In medium with the addition of 10% FBS,
mammosphere cells are able to differentiate into numerous cell
types in vitro. The results from the present study support
the CSC hypothesis that stem cells in culture are characterized by
a self-renewing and proliferation ability upon appropriate
stimulation, as well as by an undifferentiated status and the
capacity to differentiate into heterogeneous mature cell types,
results comparable with those observed by Li et al (27). Even through breast CSCs have been
reported to be CD44+/CD24−/low cells, it is
not sufficient to define a stem cell solely on its surface markers
(8,9,29).
In the present study, mammosphere cells were demonstrated to
predominantly consist of the CD44+CD24−/low
subpopulation. However, MDA-MB-231 breast cancer cells also had
76.6% CD44+CD24−/low cells, indicating that
the CD44+CD24−/low subpopulation may
encompass stem cells with self-renewal and other cell types without
this property (29). Furthermore,
the isolated mammosphere cells were also revealed to be more
tumorigenic in vivo and more refractory to chemotherapy than
the original MDA-MB-231 breast cancer cells, which was consistent
with the results of previous studies (12,25,27).
These data revealed that the isolated mammosphere cells were true
breast CSCs, thus the cells were termed MDA-MB-231 stem cells.
In recent years, increasing evidence has emerged
that CSCs exert an important role in drug resistance, tumor relapse
and cancer metastasis in various types of cancer, including breast
cancer (13,14). Major factors that affect drug
sensitivity include drug-associated gene variation, the expression
of the ATP binding cassette family of membrane transport proteins
and the expression of antiapoptotic genes (30–33).
Oct4 and Nanog are core transcriptional factors within the
regulatory network required for the maintenance of self-renewal and
pluripotency in embryonic stem cells, and any upregulation or
downregulation induces divergent cell fates (22). Either Oct4 or Nanog depletion may
result in the differentiation of normal human pluripotent stem cell
cultures (34). Previous studies
have observed that Oct4 and Nanog are overexpressed among numerous
malignant solid tumor types that are immortal, undifferentiated and
invasive (19,23). Knockdown of the two factors may
inhibit tumor development and growth (20,22).
Thus, Oct4 and Nanog may serve as a regulatory code for the
response of breast CSCs to drug therapy. In concurrence with the
results of previous studies (23,24),
the MDA-MB-231 stem cells in the present study exhibited relatively
high expression levels of the Oct4 and Nanog. Furthermore, the
IC50 values were shown to be almost two-fold lower than
those of the controls when the MDA-MB-231 stem cells were
transfected with Oct4 RNAi constructs. MDA-MB-231 stem cells
transfected with Nanog RNAi constructs also exhibited reduced
IC50 values as compared with the controls, demonstrating
that downregulation of Oct4 or Nanog enhanced the sensitivity of
the human breast CSCs to drug chemotherapy. Furthermore, when
injecting equal quantities of cells into mice, the MDA-MB-231 stem
cells transfected with Oct4 RNAi or Nanog RNAi formed significantly
(P<0.05) smaller tumors than the negative RNAi or control group
cells, demonstrating that the downregulation of Oct4 or Nanog
reduced the tumorigenicity in breast CSCs. Therefore, the present
study indicated that Oct4 or Nanog-targeted therapy may be a
promising means of overcoming resistance to chemotherapy and
inhibiting tumor growth in breast cancer.
In conclusion, breast CSCs were isolated by
suspension culture in serum-free medium and human breast CSCs were
characterized with elevated percentages of the
CD44+CD24−/low subset, high tumorigenicity
and resistance to chemotherapy, which encompassed stem cell-like
properties. Furthermore, breast CSCs also expressed high levels of
the Oct4 and Nanog transcriptional factors. To the best of our
knowledge, the present study revealed for the first time the key
role of Oct4 and Nanog in chemotherapeutic resistance and tumor
growth in breast CSCs, which provides a possible novel insight into
stem cell-based target therapies in breast cancer.
Acknowledgements
This study was supported by the Key Projects of
Fujian Province Technology (grant no. 2010D026), the Medical
Innovations Topic in Fujian Province (grant no. 2012-CXB-29) and
the Projects of Xiamen scientific and technological plan (grant
nos. 3502Z20134011 and 3502Z20124018). This study was performed in
Xiamen University (Xiamen, China).
References
|
1
|
Odorico JS, Kaufman DS and Thomson JA:
Multilineage differentiation from human embryonic stem cell lines.
Stem Cells. 19:193–204. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Romano G: The role of adult stem cells in
carcinogenesis. Drug News Perspect. 18:555–559. 2005. View Article : Google Scholar
|
|
3
|
Clarke MF, Dick JE, Dirks PB, et al:
Cancer stem cells - perspectives on current status and future
directions: AACR Workshop on cancer stem cells. Cancer Res.
66:9339–9344. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo W, Lasky JL and Wu H: Cancer stem
cells. Pediatr Res. 59:59R–64R. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dalerba P, Cho RW and Clarke MF: Cancer
stem cells: Models and concepts. Annu Rev Med. 58:267–284. 2007.
View Article : Google Scholar
|
|
6
|
Gibbs CP, Kukekov VG, Reith JD, et al:
Stem-like cells in bone sarcomas: Implications for tumorigenesis.
Neoplasia. 7:967–976. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wilson H, Huelsmeyer M, Chun R, Young KM,
Friedrichs K and Argyle DJ: Isolation and characterisation of
cancer stem cells from canine osteosarcoma. Vet J. 175:69–75. 2008.
View Article : Google Scholar
|
|
8
|
Bhat-Nakshatri P, Appaiah H, Ballas C, et
al: SLUG/SNAI2 and tumor necrosis factor generate breast cells with
CD44+/CD24− phenotype. BMC Cancer.
10:4112010. View Article : Google Scholar
|
|
9
|
Sheridan C, Kishimoto H, Fuchs RK, et al:
CD44+/CD24− breast cancer cells exhibit
enhanced invasive properties: an early step necessary for
metastasis. Breast Cancer Res. 8:R592006. View Article : Google Scholar
|
|
10
|
DeSantis C, Siegel R, Bandi P and Jemal A:
Breast cancer statistics, 2011. CA Cancer J Clin. 61:409–418. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ponti D, Costa A, Zaffaroni N, et al:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Donnenberg VS and Donnenberg AD: Multiple
drug resistance in cancer revisited: The cancer stem cell
hypothesis. J Clin Pharmacol. 45:872–877. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Campbell PA, Perez-Iratxeta C,
Andrade-Navarro MA and Rudnicki MA: Oct4 targets regulatory nodes
to modulate stem cell function. PLoS One. 2:e5532007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tai MH, Chang CC, Kiupel M, Webster JD,
Olson LK and Trosko JE: Oct4 expression in adult human stem cells:
evidence in support of the stem cell theory of carcinogenesis.
Carcinogenesis. 26:495–502. 2005. View Article : Google Scholar
|
|
17
|
Mitsui K, Tokuzawa Y, Itoh H, et al: The
homeoprotein Nanog is required for maintenance of pluripotency in
mouse epiblast and ES cells. Cell. 113:631–642. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jeter CR, Badeaux M, Choy G, et al:
Functional evidence that the self-renewal gene NANOG regulates
human tumor development. Stem Cells. 27:993–1005. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang CC, Shieh GS, Wu P, Lin CC, Shiau AL
and Wu CL: Oct-3/4 expression reflects tumor progression and
regulates motility of bladder cancer cells. Cancer Res.
68:6281–6291. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Velkey JM and O’Shea KS: Oct4 RNA
interference induces trophectoderm differentiation in mouse
embryonic stem cells. Genesis. 37:18–24. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gidekel S, Pizov G, Bergman Y and Pikarsky
E: Oct-3/4 is a dose-dependent oncogenic fate determinant. Cancer
Cell. 4:361–370. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Boyer LA, Lee TI, Cole MF, et al: Core
transcriptional regulatory circuitry in human embryonic stem cells.
Cell. 122:947–956. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ezeh UI, Turek PJ, Reijo RA and Clark AT:
Human embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are
expressed in both seminoma and breast carcinoma. Cancer.
104:2255–2265. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu C, Lu Y, Wang B, et al: Clinical
implications of stem cell gene Oct-4 expression in breast cancer.
Ann Surg. 253:1165–1171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dontu G, Abdallah WM, Foley JM, et al: In
vitro propagation and transcriptional profiling of human mammary
stem/progenitor cells. Genes Dev. 17:1253–1270. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dontu G and Wicha MS: Survival of mammary
stem cells in suspension culture: Implications for stem cell
biology and neoplasia. J Mammary Gland Biol Neoplasia. 10:75–86.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li HZ, Yi TB and Wu ZY: Suspension culture
combined with anticancer regimens for screening breast cancer stem
cells. Med Hypotheses. 68:988–990. 2007. View Article : Google Scholar
|
|
28
|
Sell S: Stem cell origin of cancer and
differentiation therapy. Crit Rev Oncol Hematol. 51:1–28. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Abraham BK, Fritz P, McClellan M,
Hauptvogel P, Athelogou M and Brauch H: Prevalence of
CD44+/CD24−/low cells in breast cancer may
not be associated with clinical outcome but may favor distant
metastasis. Clin Cancer Res. 11:1154–1159. 2005.PubMed/NCBI
|
|
30
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schinkel AH and Jonker JW: Mammalian drug
efflux transporters of the ATP binding cassette (ABC) family: an
overview. Adv Drug Deliv Rev. 55:3–29. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Szakács G, Annereau JP, Lababidi S, et al:
Predicting drug sensitivity and resistance: profiling ABC
transporter genes in cancer cells. Cancer Cell. 6:129–137. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gottesman MM, Ludwig J, Xia D and Szakács
G: Defeating drug resistance in cancer. Discov Med. 6:18–23.
2006.
|
|
34
|
Ji J, Werbowetski-Ogilvie TE, Zhong B,
Hong SH and Bhatia M: Pluripotent transcription factors possess
distinct roles in normal versus transformed human stem cells. PLoS
One. 4:e80652009. View Article : Google Scholar : PubMed/NCBI
|