Introduction
It has been confirmed that red peppers demonstrate
anti-obesity, anti-hypertension, anti-diabetes and
anti-inflammatory functions (1).
This activity infers that the peppers contain certain ingredients
that play a crucial role in these processes. Following years of
study, researchers have demonstrated that one of the most effective
ingredients is capsaicin, which has a potential function in
ameliorating insulin resistance (2). The receptor of capsaicin is transient
receptor potential vanilloid subfamily member 1 (TRPV1) (3). By activating TRPV1, capsaicin
participates in the mediation of noxious stress response (4,5).
TRPV1 is a universal expression gene with abundant expression in
adipocytes, β-cells, skeletal muscles and hepatocytes (6). Notably, capsaicin intake promotes
energy consumption and fat metabolism, which has potential
anti-obesity effects (7). This
action may be a result of the effect of decreasing insulin
resistance by capsaicin (8–10).
In addition, capsaicin participates in type 2 diabetes. Several
studies reveal that capsaicin has a modest effect in type 2
diabetes (11,12). Capsaicin has also demonstrated both
beneficial and harmful effects on human health (4). In addition, capsaicin may act as a
carcinogen and chemopreventive depending on different factors
(13). Simultaneously, another
component, non-pungent capsinoids, as an analog of capsaicin, may
also activate TRPV1 (14). This
molecule may exert some of the same effects as capsaicin, but
without the harmful effects on human health. Thus, capsinoids
demonstrate a potential medicinal value and are worthy of further
study.
Adipocytokines secreted by fat tissue play an
essential role in controlling the accumulation of fat in organisms,
which is beneficial for health (15). Therefore, the upregulation of
adipocytokine secretion to promote energy expenditure promises a
potential way of preventing body fat accumulation (16). It has been reported that capsaicin
stimulates energy expenditure via activation of adipocytokine
secretion (2). In animals,
capsaicin activates adipocytokine secretion, which induces
catecholamine and uncoupling protein expression in brown adipose
tissue (2). Thus, capsaicin
induces energy expenditure by promoting oxygen consumption and core
temperature. AMP-activated protein kinase (AMPK) is a key factor in
this process, and is activated by lack of cellular energy. Once
AMPK is activated, the glucose uptake and fatty acid oxidation in
organisms are increased to produce more adenosine triphosphate
(17). Although the
capsaicin-inducing metabolism of adipocyte has been reported, the
effects of non-pungent capsinoids on the metabolism of adipocytes
have not been unveiled yet. This could provide potential
anti-obesity, anti-hypertension and anti-diabetes activity and
offer a new therapeutic strategy.
The aim of the present study was to elucidate the
changes in cellular levels following capsinoid treatment in
adiposity in vivo and in vitro, as well as the
changes in HMG-CoA reductase, CPT-1, FAT/CD36 and GLUT4 expression
that occur during the process.
Materials and methods
Mouse models of obesity
The high-fat diet (HFD) D12451 and low-fat diet
(LFD) D12450 by Research Diets, Inc. (New Brunswick, NJ, USA) were
formulated according to previous studies (18). Diets of HFD+5% capsinoids and
LFD+5% capsinoids were also prepared. Five-week-old mice were fed
with the diets for 12 weeks to induce obesity. Following this, the
mice were weighed and the expression of HMG-CoA reductase, CPT-1,
FAT/CD36 and GLUT4 was analyzed by semi-quantitative reverse
transcription-polymerase chain reaction (RT-PCR) and western
blotting. The study was approved by the ethics committee of Central
South University, Changsha, China.
Cell culture
The preadipocyte 3T3-L1 cells were first induced
into adipose cells according to the previous study (19) and then divided into four groups: a
non-treatment group, a low capsinoid treatment group (with
10−10 M capsinoids added), a medial capsinoid treatment
group (with 10−8 M capsinoids added), and a high
capsinoid treatment group (with 10−6 M capsinoids
added). The addition of capsinoids was performed 24 h after the
adipose cells had formed, as confirmed by microscopy. The cells
were harvested 16 h after the addition of capsinoids. Following
this, oil red O staining microscopic analysis was performed, and
the expression of HMG-CoA reductase, CPT-1, FAT/CD36 and GLUT4 was
analyzed by semi-quantitative RT-PCR and western blotting.
Oil red O staining
The frozen tissue sections were prepared at a
thickness of 8 mm. After fixing in formalin, the sections were
washed in 60% isopropanol and then stained with freshly prepared
oil red O working solution for 15 mins. Following rinsing with 60%
isopropanol, the sections were analyzed under the microscope. Oil
red O staining was also used to detect lipogenesis in cells.
Semi-quantitative RT-PCR
The adipose tissues and cells were used to extract
the total RNAs using TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA). Next, the RNAs were reverse transcribed into
first-strand cDNA by SuperScript II reverse transcriptase (Life
Technologies, Gaithersburg, MD, USA) from 1 μg RNA. The primers
were designed according to the sequences in GenBank (Table I). Semi-quantitative RT-PCR was
performed on an Applied Biosystems 7500 real-time PCR system (Life
Technologies). The reaction conditions were 1 cycle at 94°C for 4
min; 40 cycles at 94°C for 30 sec, 60°C for 30 sec and 72°C for 30
sec. The specifics of the primers were determined by dissociation
curve. Following the reaction, the data were analyzed using SDS 1.3
software on the Applied Biosystems 7500 real-time PCR system.
 | Table IPrimers used in this study. |
Table I
Primers used in this study.
| Primer name | Sequences (5′ to
3′) |
|---|
| HMG-CoA
reductase-forward |
5′-TTCTGGCAGTCAGTGGGAACT-3′ |
| HMG-CoA
reductase-reverse |
5′-TCCTCGTCCTTCGATCCAA-3′ |
| CPT-1-forward |
5′-GGACAGAGACTGTGCGTTCCT-3′ |
| CPT-1-reverse |
5′-GCGATATCCAACAGTGCTTGA3′ |
| FAT/CD36-forward |
5′-GATGACGTGGCAAAGAACAG-3′ |
| FAT/CD36-reverse |
5′-TCCTCGGGGTCCTGAGTTAT-3′ |
| GLUT4-forward |
5′-CTTCATCATTGGCATGGGTTT-3′ |
| GLUT4-reverse |
5′-AGGACCGCAAATAGAAGGAAGA-3′ |
Western blotting
The tissues and cells were lysed in RIPA buffer and
then centrifuged at 15,000 rpm for 15 min to obtain the
supernatant. Subsequently, the extracted protein samples were
resolved by 12% SDS-PAGE and transferred onto PVDF membranes
(Amersham Biosciences, Piscataway, NJ, USA). After blocking in 4%
skimmed milk for 10 min, the protein samples were probed by primary
antibodies.
The primary antibodies of HMG-CoA reductase
(ab98018), CPT-1 (ab128568), FAT/CD36 (ab78054) and GLUT4 (ab654)
were purchased from Abcam (Cambridge, MA, USA. Then, secondary
antibodies conjugated with horseradish peroxidase were incubated
with the membranes. The signals were detected using the
chemiluminescence system SuperSignal West Pico Chemiluminescent
Substrate (Pierce, Rockford, IL, USA).
Statistical analysis
All the data are shown as the means ± SEM of
independent experiments. One-way analysis of variance was used to
determine differences among groups. P≤0.05 was considered to
indicate a statistically significantly difference.
Results
Capsinoids decrease fat growth
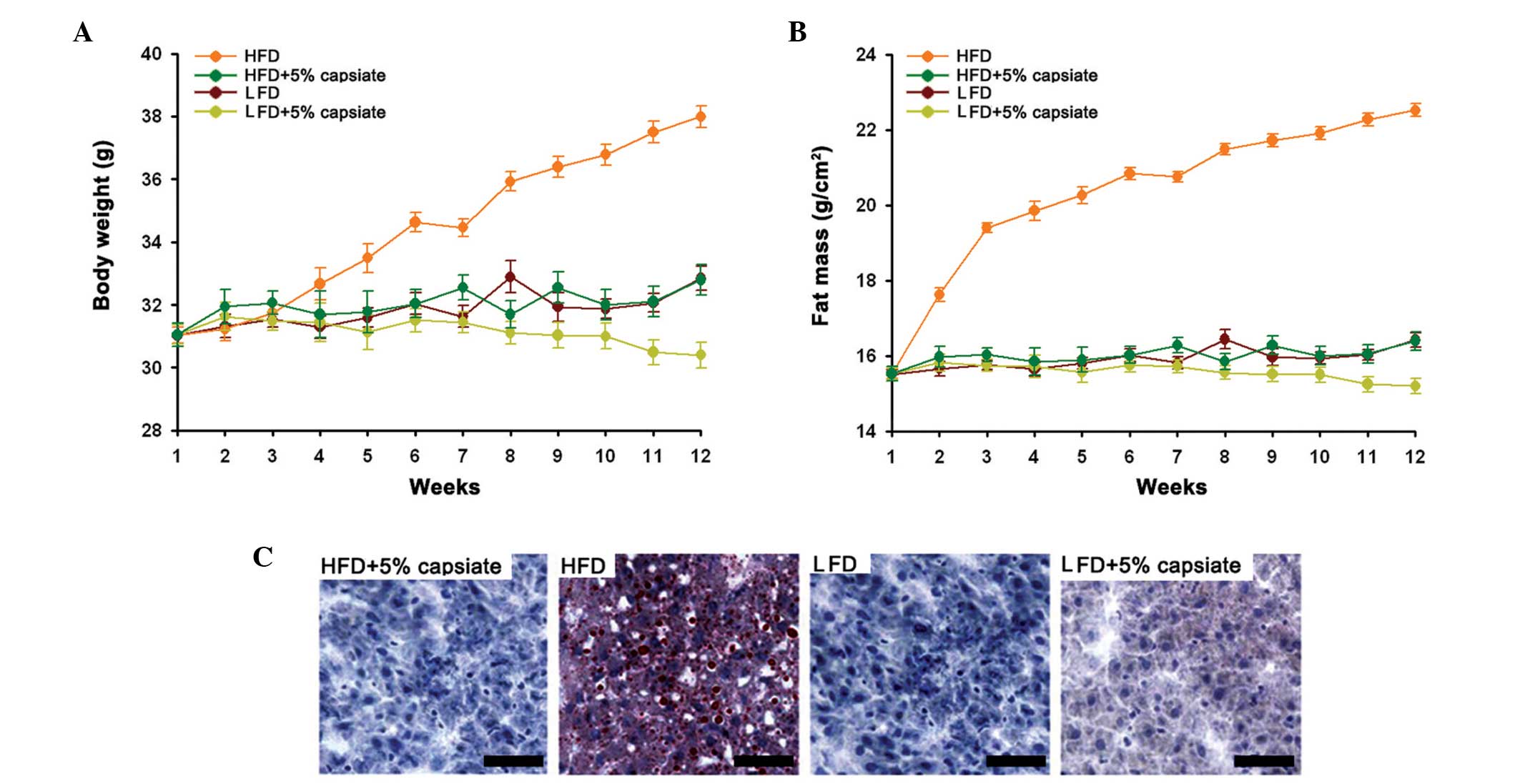
We first induced mouse models of obesity and assayed
the body growth and fat growth. The results demonstrated that after
feeding with the HFD, the body weight increased significantly
compared with the other groups (Fig.
1A). Mice in the LFD+5% capsinoids group had the lowest body
weight among all the tested groups. Similarly, the fat mass was the
higher in the HFD group than in the other tested groups (Fig. 1B). In addition, the tissue sections
also confirmed that the capsinoids decreased fat growth in liver
tissue as they demonstrated significant oil red O signals while
other groups had almost no fat accumulation in the tissues
(Fig. 1C).
Capsinoids induce lipid metabolism in
liver
Subsequently, the changes in the key genes which
participate in lipid metabolism, including HMG-CoA reductase,
CPT-1, FAT/CD36 and GLUT4, were assayed in the liver of obese mouse
models. The results revealed that, for all the studied genes, the
HFD group had the lowest mRNA expression (Fig. 2A–D) as well as protein expression
(Fig. 2E). The HFD+5% capsinoids
group demonstrated an increase in HMG-CoA reductase, CPT-1,
FAT/CD36 and GLUT4 expression levels, which suggested that 5%
capsinoid diets may promote lipid metabolism in liver.
Capsinoids induce lipid metabolism in
adipose tissue
We also analyzed the lipid metabolism in adipose
tissue. The results revealed that all the tested genes had the
lowest mRNA expression as well as protein expression in the HFD
group (Fig. 3A), which indicated
that the 5% capsinoid diets increased lipid metabolism. The in
vitro study also supported this finding. In induced adipocytes,
by adding capsinoids, the expression of HMG-CoA reductase, CPT-1,
FAT/CD36 and GLUT4 increased significantly compared with the
control group (Fig. 3B). In
addition, oil red O staining also indicated that 10−10 M
capsinoids decreased fat accumulation significantly in the
adipocytes (Fig. 3C).
Discussion
In the present study, we demonstrated that
capsinoids have an inhibitory effect on fat accumulation which is
due to the increasing effect of capsinoids on the lipid metabolism
in liver and adipose tissues. Capsinoids, as an analog of
capsaicin, also exhibited similar effects to capsaicin (20). However, capsinoids have a great
advantage over capsaicin as they are non-pungent (21). It has been reported that capsinoids
induce immune responses, having anti-inflammatory and
antiproliferative effects on T cells (22). In addition, capsinoids are able to
stimulate an antioxidant effect. It has also been reported by
Haramizu et al (23) that
after 2 weeks of treatment with capsinoids, human body fat
accumulation was suppressed. In the present study, we also
indicated that following intake of capsinoids, the body weight and
fat mass index were suppressed even when feeding with a HFD. Thus,
the mechanism of the suppression effect was also illustrated in the
present study.
We then elucidated the expression changes of the
lipid metabolism in liver and adipose tissues following treatment
with capsinoids for various genes including HMG-CoA reductase,
CPT-1, FAT/CD36 and GLUT4. All of these genes were significantly
increased in liver and adipose tissues. These results suggested
that the lipid metabolism was also upregulated by capsinoid
treatment. HMG-CoA reductase is a rate-controlling enzyme which
participates in the mevalonate pathway and is responsible for
producing cholesterol and other isoprenoids. In normal cells of
animals, HMG-CoA reductase is suppressed and degraded by
low-density lipoprotein (24). In
addition, HMG-CoA reductase has been considered to play a role in
cholesterol synthesis and demonstrated activity in lipid metabolism
(25). CPT1 is an essential enzyme
in the beta-oxidation of long-chain fatty acids which participates
in fatty acid activation and oxidization within the mitochondrial
matrix (26. FAT/CD36 has functions in long-chain fatty acid uptake
and could be irreversibly inhibited by sulfo-N-succinimidyl oleate,
which is associated with myocardial fatty acid uptake (27). GLUT4 is a insulin-regulated glucose
transporter which was identified in adipose tissues and striated
muscle and has demonstrated a facilitated diffusion of circulating
glucose in fat cells (28). Thus,
these genes are significant components in lipid metabolism. In
liver, the upregulated genes provide an indication that, following
intake of capsinoids, the lipid metabolism pathway is significantly
stimulated. The same effect was also observed in adipose tissues.
In mice, it has been reported that capsinoids upregulate uncoupling
protein in skeletal muscle and brown adipose tissue, which
indicates that capsinoids play a notable role in energy
expenditure, body weight and thermoregulation (29). A previous study has revealed that
capsinoids suppressed body fat accumulation and raised oxygen
consumption in the same way as exercise (29). Our findings correspond with these
studies. However, the present study provides new evidence of the
role of capsinoids in lipid metabolism.
It has been reported that capsaicin stimulated UCP1
expression in brown adipose tissue as well as in the concentration
of serum adrenaline, which induced a depression of perirenal
adipose tissue (30). Capsinoids
are non-pungent, but have a similar function to capsaicin in
stimulating UCP1 expression, resulting in changes in energy
metabolism, adrenaline release and body fat accumulation. Thus,
studies of capsinoids may provide information to support their
potential application in decreasing fat. Capsaicin also
demonstrated physiological effects on adrenaline release and
increase in body temperature. These results also confirm that the
metabolic effects of capsinoids are the same as those of capsaicin.
Obesity occurs due to the imbalance between energy intake and
consumption, which leads to weight gain and abdominal adipose
tissue accumulation. Faraut et al demonstrated that
capsinoid-induced UCP3 and UCP3 expression is a causative factor of
weight loss (31). We have
demonstrated in the present study that capsinoid intake reduced
abdominal fat mass. Thus, it was also confirmed that capsinoids
stimulate the lipid metabolism.
In conclusion, the results of the present study
indicated that capsinoid intake stimulates fat metabolism, which
may lead to a reduction in fat accumulation. The present findings
suggest that compound capsinoids may be worth investigating as a
cure for obesity.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81302421/H2603) and the
Natural Science Foundation of Hunan Province, China (grant no.
12JJ6098).
References
|
1
|
Akpinar EK, Bicer Y and Yildiz C: Thin
layer drying of red pepper. J Food Eng. 59:99–104. 2003. View Article : Google Scholar
|
|
2
|
Kang JH, Goto T, Han IS, Kawada T, Kim YM
and Yu R: Dietary capsaicin reduces obesity-induced insulin
resistance and hepatic steatosis in obese mice fed a high-fat diet.
Obesity (Silver Spring). 18:780–787. 2010. View Article : Google Scholar
|
|
3
|
Szallasi A, Cortright DN, Blum CA and Eid
SR: The vanilloid receptor TRPV1: 10 years from channel cloning to
antagonist proof-of-concept. Nat Rev Drug Discov. 6:357–372. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dinis P, Charrua A, Avelino A, et al: The
distribution of sensory fibers immunoreactive for the TRPV1
(capsaicin) receptor in the human prostate. Eur Urol. 48:162–167.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chan CL, Facer P, Davis J, et al: Sensory
fibres expressing capsaicin receptor TRPV1 in patients with rectal
hypersensitivity and faecal urgency. Lancet. 361:385–391. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matias I, Gonthier MP, Petrosino S, et al:
Role and regulation of acylethanolamides in energy balance: focus
on adipocytes and β-cells. Br J Pharmacol. 152:676–690. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Diepvens K, Westerterp KR and
Westerterp-Plantenga MS: Obesity and thermogenesis related to the
consumption of caffeine, ephedrine, capsaicin, and green tea. Am J
Physiol Regul Integr Comp Physiol. 292:R77–R85. 2007. View Article : Google Scholar
|
|
8
|
Uno K, Katagiri H, Yamada T, et al:
Neuronal pathway from the liver modulates energy expenditure and
systemic insulin sensitivity. Science. 312:1656–1659. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kang JH, Kim CS, Han IS, Kawada T and Yu
R: Capsaicin, a spicy component of hot peppers, modulates adipokine
gene expression and protein release from obese-mouse adipose
tissues and isolated adipocytes, and suppresses the inflammatory
responses of adipose tissue macrophages. FEBS Lett. 581:4389–4396.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kang JH, Tsuyoshi G, Le Ngoc H, et al:
Dietary capsaicin attenuates metabolic dysregulation in genetically
obese diabetic mice. J Med Food. 14:310–315. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Capsaicin Study Group. Effect of treatment
with capsaicin on daily activities of patients with painful
diabetic neuropathy. Diabetes Care. 15:159–165. 1992. View Article : Google Scholar
|
|
12
|
Yuen KC, Baker NR and Rayman G: Treatment
of chronic painful diabetic neuropathy with isosorbide dinitrate
spray a double-blind placebo-controlled cross-over study. Diabetes
Care. 25:1699–1703. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Surh YJ and Lee SS: Capsaicin in hot chili
pepper: carcinogen, co-carcinogen or anticarcinogen? Food Chem
Toxicol. 34:313–316. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Iida T, Moriyama T, Kobata K, et al: TRPV1
activation and induction of nociceptive response by a non-pungent
capsaicin-like compound, capsiate. Neuropharmacology. 44:958–967.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tilg H and Moschen AR: Adipocytokines:
mediators linking adipose tissue, inflammation and immunity. Nat
Rev Immunol. 6:772–783. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsuzawa Y, Funahashi T and Nakamura T:
Molecular mechanism of metabolic syndrome X: contribution of
adipocytokines adipocyte-derived bioactive substances. Ann N Y Acad
Sci. 892:146–154. 1999. View Article : Google Scholar
|
|
17
|
Yamauchi T, Kamon J, Minokoshi Y, et al:
Adiponectin stimulates glucose utilization and fatty-acid oxidation
by activating AMP-activated protein kinase. Nat Med. 8:1288–1295.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Posey KA, Clegg DJ, Printz RL, et al:
Hypothalamic proinflammatory lipid accumulation, inflammation, and
insulin resistance in rats fed a high-fat diet. Am J Physiol
Endocrinol Metab. 296:E1003–E1012. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chawla A, Schwarz EJ, Dimaculangan DD and
Lazar MA: Peroxisome proliferator-activated receptor (PPAR) gamma:
adipose-predominant expression and induction early in adipocyte
differentiation. Endocrinology. 135:798–800. 1994.PubMed/NCBI
|
|
20
|
Iwai K, Yazawa A and Watanabe T: Roles as
metabolic regulators of the non-nutrients, capsaicin and capsiate,
supplemented to diets. Proc Jpn Acad Ser B Phys Biol Sci.
79B:207–212. 2003. View Article : Google Scholar
|
|
21
|
Ludy MJ, Moore GE and Mattes RD: The
effects of capsaicin and capsiate on energy balance: critical
review and meta-analyses of studies in humans. Chem Senses.
37:103–121. 2012. View Article : Google Scholar :
|
|
22
|
Lee EJ, Jeon MS, Kim BD, et al: Capsiate
inhibits ultraviolet B-induced skin inflammation by inhibiting Src
family kinases and epidermal growth factor receptor signaling. Free
Radic Biol Med. 48:1133–1143. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Haramizu S, Mizunoya W, Masuda Y, et al:
Capsiate, a nonpungent capsaicin analog, increases endurance
swimming capacity of mice by stimulation of vanilloid receptors.
Biosci Biotechnol Biochem. 70:774–781. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nawrocki JW, Weiss SR, Davidson MH, et al:
Reduction of LDL cholesterol by 25 to 60% in patients with primary
hypercholesterolemia by atorvastatin, a new HMG-CoA reductase
inhibitor. Arterioscler Thromb Vasc Biol. 15:678–682. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wanders R, Ferdinandusse S, Jansen G, et
al: Peroxisomal fatty acid alpha-and beta-oxidation in humans:
enzymology, peroxisomal metabolite transporters and peroxisomal
diseases. Biochem Soc Trans. 29:250–267. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brown MS and Goldstein JL: Multivalent
feedback regulation of HMG CoA reductase, a control mechanism
coordinating isoprenoid synthesis and cell growth. J Lipid Res.
21:505–517. 1980.PubMed/NCBI
|
|
27
|
Coort SL, Willems J, Coumans WA, et al:
Sulfo-N-succinimidyl esters of long chain fatty acids specifically
inhibit fatty acid translocase (FAT/CD36)-mediated cellular fatty
acid uptake. Mol Cell Biochem. 239:213–219. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shepherd PR, Gnudi L, Tozzo E, Yang H,
Leach F and Kahn BB: Adipose cell hyperplasia and enhanced glucose
disposal in transgenic mice overexpressing GLUT4 selectively in
adipose tissue. J Biol Chem. 268:22243–22246. 1993.PubMed/NCBI
|
|
29
|
Masuda Y, Haramizu S, Oki K, et al:
Upregulation of uncoupling proteins by oral administration of
capsiate, a nonpungent capsaicin analog. J Appl Physiol.
95:2408–2415. 2003.PubMed/NCBI
|
|
30
|
Cannon B and Nedergaard J: Brown adipose
tissue: function and physiological significance. Physiol Rev.
84:277–359. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Faraut B, Giannesini B, Matarazzo V, et
al: Downregulation of uncoupling protein-3 in vivo is linked to
changes in muscle mitochondrial energy metabolism as a result of
capsiate administration. Am J Physiol Endocrinol Metab.
292:E1474–E1482. 2007. View Article : Google Scholar : PubMed/NCBI
|