Introduction
Sepsis is a prevalent, severe disease characterized
by a systemic inflammatory response to infection (1). It is most apparent in the pulmonary
circulation as lungs experience continuous exposure to circulating
pathogen-associated molecular patterns, such as endotoxin
lipopolysaccharide (LPS), which may initiate an innate immune
response (2). Acute lung injury
(ALI), characterized by neutrophilic inflammation and pulmonary
vascular hyperpermeability, develops in >40% of individuals with
sepsis (3). The onset of ALI
results in a significant decline in patient prognosis and an
increase in intensive care unit mortality from 11 to 38% in septic
shock patients (4). Patients that
do not succumb to ALI often suffer from long-term morbidity with
high healthcare expenditures (5).
However, at present, there are no sepsis-specific therapies to
prevent the onset of inflammatory lung injury and the underlying
mechanisms of septic ALI pathogenesis remain to be fully
elucidated.
Tumor necrosis factor-α (TNF-α)-induced protein-8
(TNFAIP8) has important regulatory roles in cell apoptosis, signal
transduction, tumor occurrence and development as well as the cell
invasion process (6). Numerous
studies have focused on the major member of the TNFAIP8 family;
TNFAIP8-like 2 (TIPE2) was reported to be necessary for the
maintenance of immune homeostasis and was highly expressed in
inflammatory tissues, exhibiting negative regulatory effects on the
natural immune response (7,8).
Previous studies have demonstrated that in the resting state,
nuclear factor (NF)-κB within cells exists as a trimer complex
composed of two subunits, P65 and P50, and inhibitor of κB kinase
(IκB) (9,10). In the absence or mutation of TIPE2,
IκB was degraded from the trimer complex by protein kinase C,
therefore releasing NF-κB from the cytoplasm and enabling it to
translocate to the nucleus. Furthermore, NF-κB, combined with the
binding site, initiated the transcription and translation processes
of a variety of cytokine genes, including TNF-α, interleukin (IL)-1
and IL-6, therefore inducing the activation of inflammatory cells
(11).
Traditional Chinese Medicine and botanical folk
medicines, particularly Chinese medicines, which have beneficial
effects on fevers and toxicity, were verified to have
anti-inflammatory and antioxidant effects by modern pharmacological
experiments (12,13). The investigation and application of
botanical anti-inflammatory folk medicines which avoid the adverse
effects of western medicines is currently a controversial topic,
which has been a focus of modern medical studies (14). Melilotus suaveolens Ledeb, a
type of annual or biennial herbage belonging to the
Melilotus family of Leguminosae, functions to reduce
fever, remove toxicity and exert anti-inflammatory effects and
detumescence (15). In addition,
members of the Melilotus family were reported to be
applicable to a variety of diseases, including spleen disease,
twisted intestinal fever, diphtheria and tonsillitis (16). Several studies have shown that
Melilotus extract, containing active components including
coumarin, flavonoids and tannic acid, functions to inhibit the
synthesis and release of inflammatory factors, reduce capillary
permeability, improve microcirculation and promote the absorption
of edema fluid (17,18,19).
Melilotus extract tablets have been widely used for clinical
purposes; however, they have not been studied in the literature
regarding their protection against sepsis-induced lung injury.
The aim of the present study was to determine
whether Melilotus extract decreased Toll-like receptor
(TLR)4 and NF-κB expression via the promotion of TIPE2 expression,
which would therefore indicate its protective role against lung
injury. Mice with cecal ligation-perforation (CLP)-induced sepsis
were used as a model system.
Materials and methods
Animals
C57BL/6J (B6) mice were purchased from Kunming
Medical University Laboratory Animal Center (Kunming, China). All
mice were housed in the Kunming Medical University animal care
facility and were maintained in a pathogen-free environment. The
mice were aged 8–9 weeks and weighed 20–30 g at the initiation of
the experiment, were housed in a vivarium maintained at 23°C with a
12:12 h light/dark cycle (lights off at 7.00pm) and a standard
laboratory diet and water were provided ad libitum. All
experiments were approved by the Ethics Committee of Kunming
Medical University (Yunnan, China) and performed according to the
guidelines of the Animal Care Committee of Kunming Medical
University.
Reagents
The reverse transcription (RT) reaction kit was
obtained from Takara Biotechnology Co. Ltd., (Dalian, China). The
polymerase chain reaction (PCR) amplification reagent kit and DNA
ladder marker were obtained from Sangon Biological Engineering Co.
Ltd (Shanghai, China). β-actin was obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). TNF-α, IL-6, IL-10 and IL-12
ELISA kits were obtained from Pierce Biotechnology Inc. (Rockford,
IL, USA). Melilotus extracts were obtained from Seiko Eiyo
Yakuhin Co. Ltd (Osaka, Japan).
Generation of the animal model
C57BL/6J (B6) mice weighing 25–30 g were
acclimatized for 1 week following purchase. In order to induce
sepsis, mice were anesthetized with isofluorane (4% induction, 2%
maintenance; Guangzhou Jin Kang Medical Technology Co., Ltd,
Guangzhou, China) and placed on a warming pad (Jinan Ron Trade LLC,
Jinan, China). Following laparotomy, the cecum was exteriorized and
the membrane between the cecum and mesentery was carefully
dissected to release the cecum. The cecum was ligated 4 cm from the
tip. Four punctures were made using an 18-gauge needle and 1 mm of
faecal material was expressed from the punctures. The incision was
sutured in two layers with 4–0 silk. In the sham group, the cecum
was located but was not ligated or punctured. Following the
procedure, 5 ml warm saline was administered intraperitoneally, the
animals were placed on a warming pad and then allowed to recover in
individual cages with free access to food and water.
Generation of TIPE2-deficient mice
TIPE2 genomic fragments of 2.2 and 5.0 kb were
amplified using a PCR amplification reagent kit (Sangon Biological
Engineering Co., Ltd, Shanghai, China) (20) and cloned, respectively, into the
XhoI/NheI and NotI/SalI sites of the pOSDUPDEL vector (a gift from
Dr Xiao-Ping Zhong, Department of Pediatrics, Duke University;
Durham, NC, USA). TL1 embryonic stem (ES) cells obtained from
129S6/SvEvTac mice were transfected with the targeting vector and
subjected to positive and negative selection using G418 (Guangzhou
Huowei Chemical Co., Ltd, Guangzhou, China) and ganciclovir (Jena
Biosciences, Jena, Germany), respectively. The 129S6/SvEvTac mice,
weighing 13–20 g, were purchased from Shanghai Laboratory Animal
Center of the Chinese Academy of Science (Shanghai, China), were
housed in a temperature-controlled and closed aseptic environment
(at a constant temperature of 18–22°C and humidity of 50–80%) under
a 12 h light/dark cycle, and provided with free access to sterile
water and food. Two ES cell clones were identified using a Southern
blot, in which a copy of the TIPE2 gene (including exons 1 and 2)
was replaced by the neomycin resistance gene cassette. Mutant ES
cells were injected into four-day-old C57BL/6J mouse blastocysts.
The resultant chimeric male offspring were crossed with
129S6/SvEvTac females for germline transmission. Unless indicated
otherwise, all mice used in the present study were of the
129S6/SvEvTac genetic background. Age- and gender-matched
littermates were used as controls.
Groupings and treatment
Using a random number table, 80 mice were divided
into the following four groups: Normal control group, sham-operated
group (sham group), sepsis model group (model group) and
Melilotus treatment group (treatment group), with 20 mice in
each group. The model and treatment groups were induced by cecal
ligation-perforations (CLP). Animals in the treatment group were
administered 25 mg/kg Melilotus extract two hours prior to
surgery and subsequently every 8 h. The normal control, sham and
control groups were administered an identical volume of normal
saline. Animals in each group were anesthetized with ether (Wei
Sheng Chemical Co., Ltd, Nanjing, China) and sacrificed 24 h
following surgery, and the right internal carotid artery was
isolated. Blood was extracted (1.5 ml) and centrifuged (10,000 × g
for 5 min) to collect the supernatant. The blood was then dispensed
into two sterile tubes, which were sealed and stored at −20°C until
further use. Furthermore, 2 ml peripheral venous blood was
extracted and added to the EDTA anticoagulant, and peripheral blood
mononuclear cells (PBMCs) were isolated using the Ficoll density
gradient centrifugation method (15,000 × g for 5 min) (21) to detect TIPE2.
RT quantitative PCR (RT-qPCR)
analysis
Total RNA was extracted using Gibco®
TRIzol (Thermo Fisher Scientific, Waltham, MA, USA) according to
the manufacturer’s instructions. RNA samples were electrophoresed
in agarose gels (Shanghai Yuanye Biochemicals Ltd. Shanghai, China)
and visualized the gel image using Kodak 1D software (Life
Technologies, Grand Island, NY, USA), with ethidium bromide
(Beijing Xin Hua Luyuan Science and Technology Co., Ltd, Beijing,
China) as a quality control. RNA (3 μg) was incubated with reverse
transcriptase for 1 h at 37°C to allow for complementary (c)DNA
synthesis. Quantitative changes in messenger (m)RNA expression were
assessed using the CFX 96 Real-Time PCR Detection System (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and SYBR Green PCR Master
Mix (Shanghai Star-Biological Technology Co., Ltd, Shanghai,
China). The PCR master mix consisted of 0.5 units of Taq
polymerase, 2 μl of each primer and 3 μl of each cDNA sample in a
final volume of 20 μl. All amplifications were repeated three
times. Primer sequences used for RT-qPCR are shown in Table I. β-actin was used as an endogenous
control and each sample was normalized on the basis of its β-actin
content. Relative quantification was calculated using the
comparative CT method (2−ΔΔCt method:
ΔΔCt=ΔCtsample - ΔCtreference).
Low ΔCT and ΔΔCT values reflect a relatively high volume of gene
transcript. Statistical analyses were then performed for 6–15
replicate experimental samples in each set.
 | Table IReverse transcription-quantitative
polymerase chain reaction primer sequences for genes used to
validate the microarray analysis. |
Table I
Reverse transcription-quantitative
polymerase chain reaction primer sequences for genes used to
validate the microarray analysis.
| Gene name | Primer
sequence | Size (bp) |
|---|
| TIPE2 mRNA | F,
5′-GGGAACATCCAAGGCAAG-3′
R, 5′-AGCTCATCTAGCACCTCACT-3′ | 195 |
| TLR4 mRNA | F,
5′-CGCTTTCACCTCTGCCTTCACTACAG-3′
R, 5′-ACACTACCACAATAACCTTCGGCTC-3′ | 270 |
| NF-κB mRNA | F,
5′-GCACGGATGACAGAGGCGTGTATAAGG-3′
R, 5′-GGCGGATGATCTCCTTCTCTCTGTCTG-3′ | 420 |
| IκB mRNA | F,
5′-TGCTGAGGCACTTCTGAG-3′
R, 5′-CTGTATCCGGGTGCTTGG-3′ | 42l |
| β-actin | F,
5′-GATTACTGCTCTGGCTCCTGC-3′
R, 5′-GACTCATCGTACTCCTGCTTGC-3′ | 190 |
Western blot analysis
Lung tissues and isolated PBMCs were snap-frozen in
liquid nitrogen, pulverized and resuspended in ice-cold lysis
buffer (Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China). Protein concentrations were determined using the
Bradford method (22). Lysates
were solubilized on ice for 30 min and the particulate mass was
removed using centrifugation (15,000 xg) for 15 min at 4°C.
Supernatants were analyzed using 10% SDS-PAGE (Beijing Saichi
Biological Technology Co., Ltd, Beijing, China). The primary
antibodies used included rabbit anti-TIPE2 monoclonal antibody
(1:400), rabbit anti-HO-1 monoclonal antibody (1:400), rabbit
anti-NF-κB monoclonal antibody (1:400), mouse anti-IκB monoclonal
antibody (1:400) and were purchased from Santa Cruz Biotechnology,
Inc. The secondary antibodies used were horseradish peroxidase
(HRP)-linked goat anti-rabbit immunoglobulin G (IgG) (1:4,000
dilution; Amersham Pharmacia Biotech, Piscataway, NJ, USA) and
sheep anti-mouse IgG-HRP (1:8,000 dilution; Amersham Pharmacia
Biotech). The blots were visualized by enhanced chemiluminescence
(ECL) using a Pierce ECL western blotting substrate (Pierce
Biotechnology, Inc.) and a Johnson enhanced chemiluminescence
immunoassay analyzer (Shanghai Qian Jin Industrial Co., Ltd,
Shanghai, China).
Myeloperoxidase (MPO) activity
determination
MPO activity was determined using an MPO kit
purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing,
China) and performed according to the manufacturer’s instructions.
In brief, frozen lung samples were thawed and homogenized in
ice-cold buffer. Homogenates were then centrifuged at 5,000 × g for
10 min and the pellets were suspended in 0.5% hexadecyl trimethyl
ammonium bromide (Shanghai Jinshan Jingwei Chemical Co., Ltd.,
Shanghai, China) in 50 mM phosphate-buffered saline (PBS; pH 6.0;
Wuhan Institute of Biological Products Co., Ltd, Wuhan, China) and
incubated at 60°C for 2 h. Following additional centrifugation
(5,000 × g for 5 min), the supernatants were collected. The protein
concentrations were measured using a protein assay kit (A045;
Nanjing Jiancheng Bioengineering Institute). In a 96-well plate, 15
μg protein was incubated with 100 μl
3,3R,5,5R-tetramethylbenzidine [Yuan (Suqian)
Biological Technology Co., Ltd, Suqian, China] for 3 min.
Subsequently, 100 μl sulfuric acid (1 N) was added and the
absorbance was determined using a UV visible spectrophotometer (
UV-9200; Beijing Rayleigh Analytical Instruments Ltd, Beijing,
China) at a wavelength of 450 nm. The original MPO value was
normalized against the protein content.
Superoxide dismutase assay (SOD)
SOD activity was estimated as previously described
by Kakkar et al (23). The
reaction mixture contained 0.1 ml phenazine methosulphate (186
μmol; (Shanghai Yuanye Biotechnology Ltd, Shanghai, China)) and 1.2
ml sodium pyrophosphate buffer (0.052 mmol; pH 7.0; Zhengzhou Lanyu
Chemical Co., Ltd, Zhengzhou, China). Following centrifugation
(1,500 xg for 10 min followed by 10,000 xg for 15 min) of the
homogenate, 0.3 ml supernatant was added to the reaction mixture.
The enzyme reaction was initiated by the addition of 0.2 ml NADH
(780 μmol; Biotium, Hayward, CA, USA) and terminated after 1 min by
the addition of 1 ml glacial acetic acid (Baoding City Bai Yun
Chemical Co., Ltd, Shanxi, China). The amount of chromogen formed
was measured by recording the color intensity at 560 nm. Results
were expressed as U/mg protein.
Quantification of malondialdehyde (MDA)
content
MDA quantification was used to determine lipid
peroxidation levels, MDA was quantified as thiobarbituric acid
reactive substances (TBARS) kit (Xiao Ke Yuan Biological Technology
Co., Ltd, Beijing, China) as previously described (24). In brief, weighed samples were
homogenized in 1 ml 5% trichloroacetic acid. The samples were
centrifuged (1,500 × g for 10 min), and 250 ml supernatant
incubated with the same volume of 20 mM thiobarbituric acid for 35
min at 95°C, followed by 10 min at 4°C. The sample fluorescence was
read using a spectrophotometric plate reader with an excitation
wavelength of 515 nm and emission wavelength of 553 nm.
Inflammatory cell quantification in
bronchoalveolar lavage fluid (BAL)
As previously described (25), BAL analysis was performed by
instilling 0.9% NaCl with 0.6 mmol/l ethylenediaminetetraacetic
acid (Qingdao Xinben Chemical Co., Ltd, Qingdao, China) into two
separate 0.5-ml aliquots. The fluid was recovered by gentle suction
and placed on ice for immediate processing. An aliquot of the BAL
was processed for total and differential cell counts; the remainder
of the lavage fluid was centrifuged (1,500 × g for 10 min) and the
supernatant was removed aseptically and stored in individual
aliquots at −70°C. Total cell counts in the BAL were determined
using a hemocytometer. The number of different inflammatory cells
was calculated as the percentage of certain inflammatory cells
multiplied by the total number of cells in the BAL sample. All
analyses were performed in a blind manner.
Cytokine analysis
TNF-α, IL-6, IL-1β, IL-10 and IL-12 levels in BAL
were determined using commercially available Mouse
cytokine-specific Quantikine ELISA kits (Pierce Biotechnology
Inc.), according to the manufacturer’s instructions.
Vascular permeability assessment
The Evans Blue-conjugated albumin (EBA)
extravasation assay was performed as previously described (26). Retroorbital injection of 20 mg/kg
EBA (HuanYu Biology Technology Co., Ltd, Suzhou, China) was
administered to mice 30 min prior to tissue collection. Lungs were
perfused free of blood using PBS, blotted dry and then weighed.
Lung tissue was homogenized in 1 ml PBS and incubated with 2X
formamide (Suqian Xinya Technology Co., Ltd, Suqian, China) at 60°C
for 18 h. The homogenate was then centrifuged at 5,000 xg for 30
min. The optical density of the supernatant was measured at 620 nm
and 740 nm. The extravasated EBA in the lung homogenate was
expressed as mg Evans Blue dye per g lung tissue.
Albumin concentration of BAL
The albumin content of the BAL supernatants was
assessed using an albumin ELISA kit (E91028Mu; Uscn Life Science,
Inc., Hubei, China). Absorbance was measured at 450/540 nm using a
microplate reader (Infinite 200; Tecan Group, Ltd, Maennedorf,
Switzerland).
Lung wet/dry (W/D) weight ratio
Following sacrificing the mice, the lungs were
surgically dissected away from the heart, trachea and primary
bronchi. Each lung was blotted dry, weighed and dried to a constant
weight by placing the lung specimen in an oven at 70°C for 48 h.
The ratio of the wet lung to dry lung was calculated in order to
determine the level of lung edema.
Histology
A section of the right lung was fixed in formalin,
embedded in paraffin wax and stained with Mayer’s hematoxylin and
eosin (Merck Millipore, Darmstadt, Germany) for histological
examination using a Nikon Eclipse E800 microscope (Nikon Corp.,
Tokyo, Japan) (27).
Histology scoring system
Lung sections were evaluated and scored
independently by two members of the lab trained in histological
assessment, with the use of the scoring system described below. For
each mouse, three different lobes were examined for the following
features: Interstitial edema, hemorrhage and neutrophil
infiltration. Each feature was scored as follows: 0, no injury; 1,
minimal injury; 2, moderate injury; and 3, severe injury. The sum
of these three scores indicated the total for each lobe and the
three lobes were averaged to generate an overall ALI pathological
score for each mouse, resulting in a minimum score of 0 and a
maximum score of 9.
Statistical analysis
Values are expressed as the mean ± standard
deviation. Statistical calculations were performed using GraphPad
Prism 5 (GraphPad Software, Inc, San Diego, CA, USA). For
comparisons among multiple groups, a one-way or two-way analysis of
variance followed by a Bonferroni post-hoc test were performed.
Analysis of linear correlation was used to evaluate the correlation
between two variances. P<0.05 was considered to indicate a
statistically significant difference between values.
Results
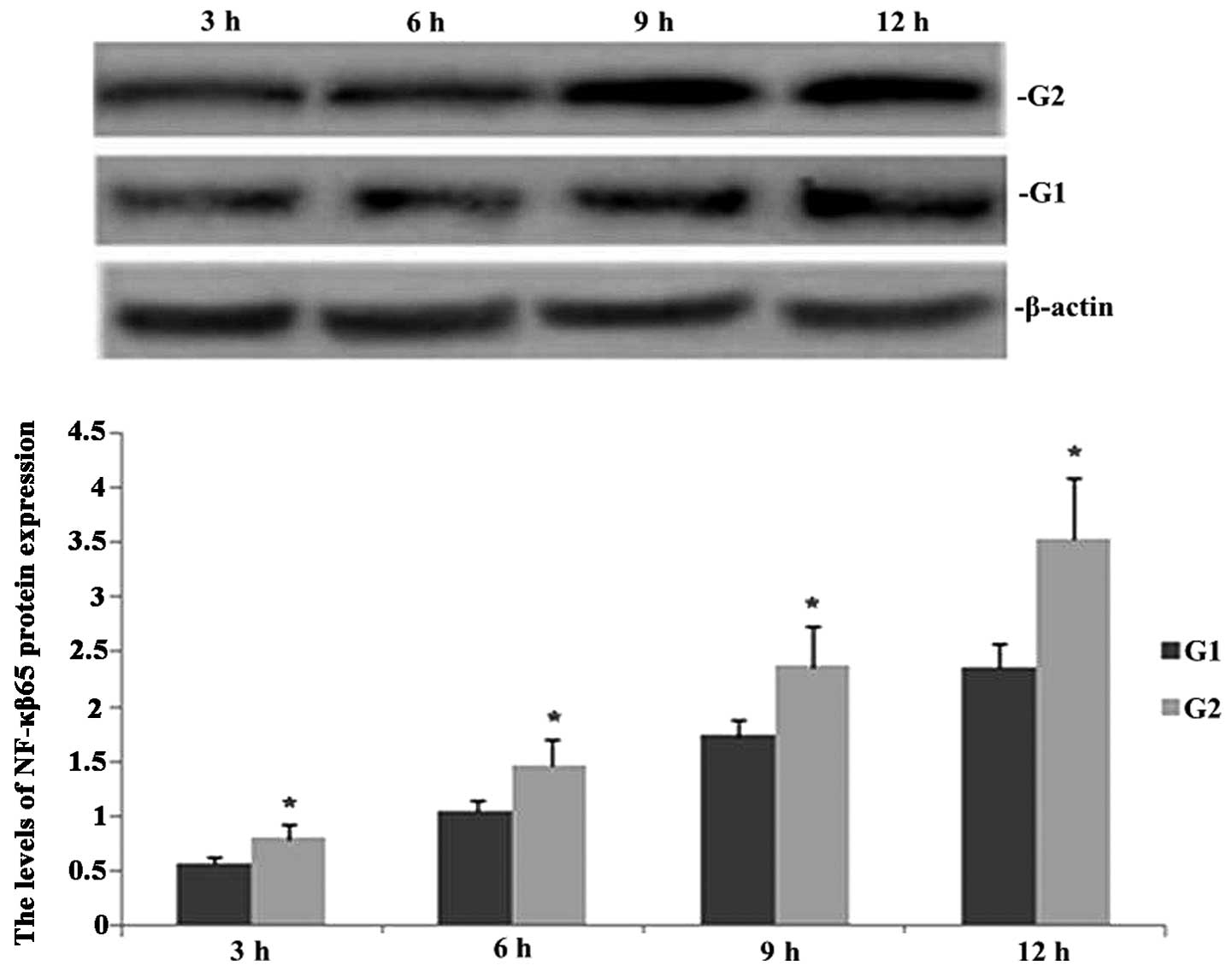
TIPE2 deficiency increases NF-κB65
expression in septic mice
Western blot analysis was performed in order to
observe the effect of TIPE2 on NF-κB65 protein in septic mice. As
shown in Fig. 1, following CLP
surgery, the expression of NF-κB65 was enhanced in TIPE2-/- and
wild-type (WT) mice in a time-dependent manner. However, the
protein expression of NF-κB65 was significantly increased at each
time-point in the TIPE2 deficient mice compared with that of the WT
mice.
Melilotus extracts upregulate TIPE2 and
IκB expression and inhibit TLR4 and NF-κB expression
RT-qPCR and western blot analyses were performed in
order to observe the effect of Melilotus extract on TIPE2,
TLR4, NF-κB and IκB protein and gene expression in septic mice. As
shown in Figs. 2 and 3, following CLP surgery, the mRNA and
protein expression levels of TIPE2, TLR4 and NF-κB in the untreated
control group were significantly upregulated and IκB expression was
downregulated. However, in mice treated with Melilotus
extract tablets, TIPE2 and IκB mRNA and protein expression levels
were significantly upregulated, whereas TLR4 and NF-κB expression
was significantly downregulated.
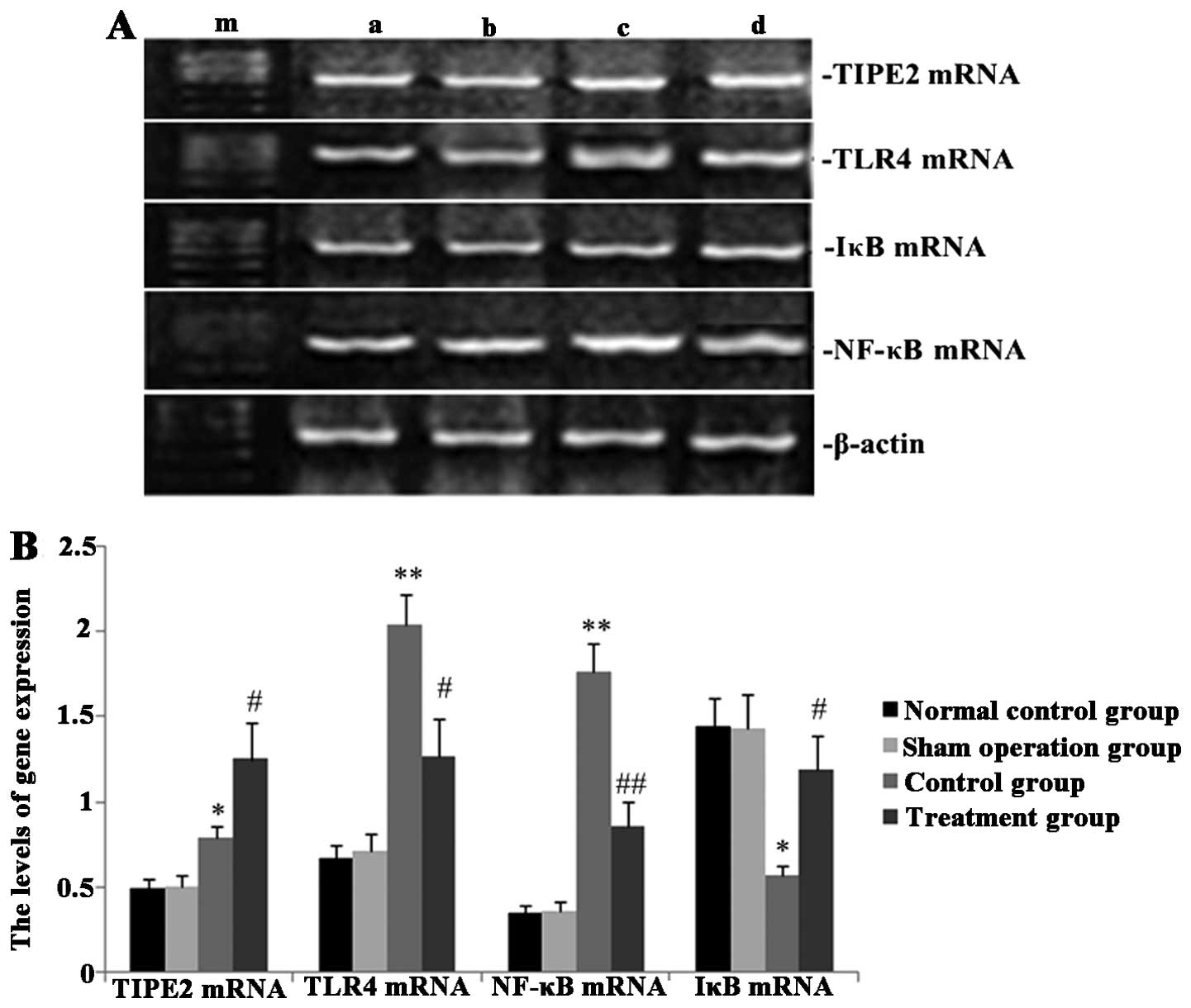 | Figure 2Effect of Melilotus extract
treatment on TIPE2, TLR4, NF-κB and IκB mRNA expression in septic
mice. Reverse transcription-quantitative polymerase chain reaction
was used to determine the mRNA expression levels of TIPE2, TLR4,
NF-κB and IκB at 24 h post-administration of Melilotus
extract and exposure to cecal ligation-perforation. (A)
Representative image of mRNA expression. Lanes: a, normal control
group; b, sham-operated group; c, control group; d, treatment
group; m, marker. (B) Quantitative analysis of mRNA expression
levels. Values are presented as the mean ± standard deviation.
*P<0.05 or **P<0.01 vs. sham-operated
and normal control groups, #P<0.05 or
##P<0.01 vs. control group. TIPE2, tumour necrosis
factor-α-induced protein-8-like 2; NF-κB65, nuclear factor κB65;
TLR4, toll-like receptor 4; IκB, inhibitor of κB kinase. |
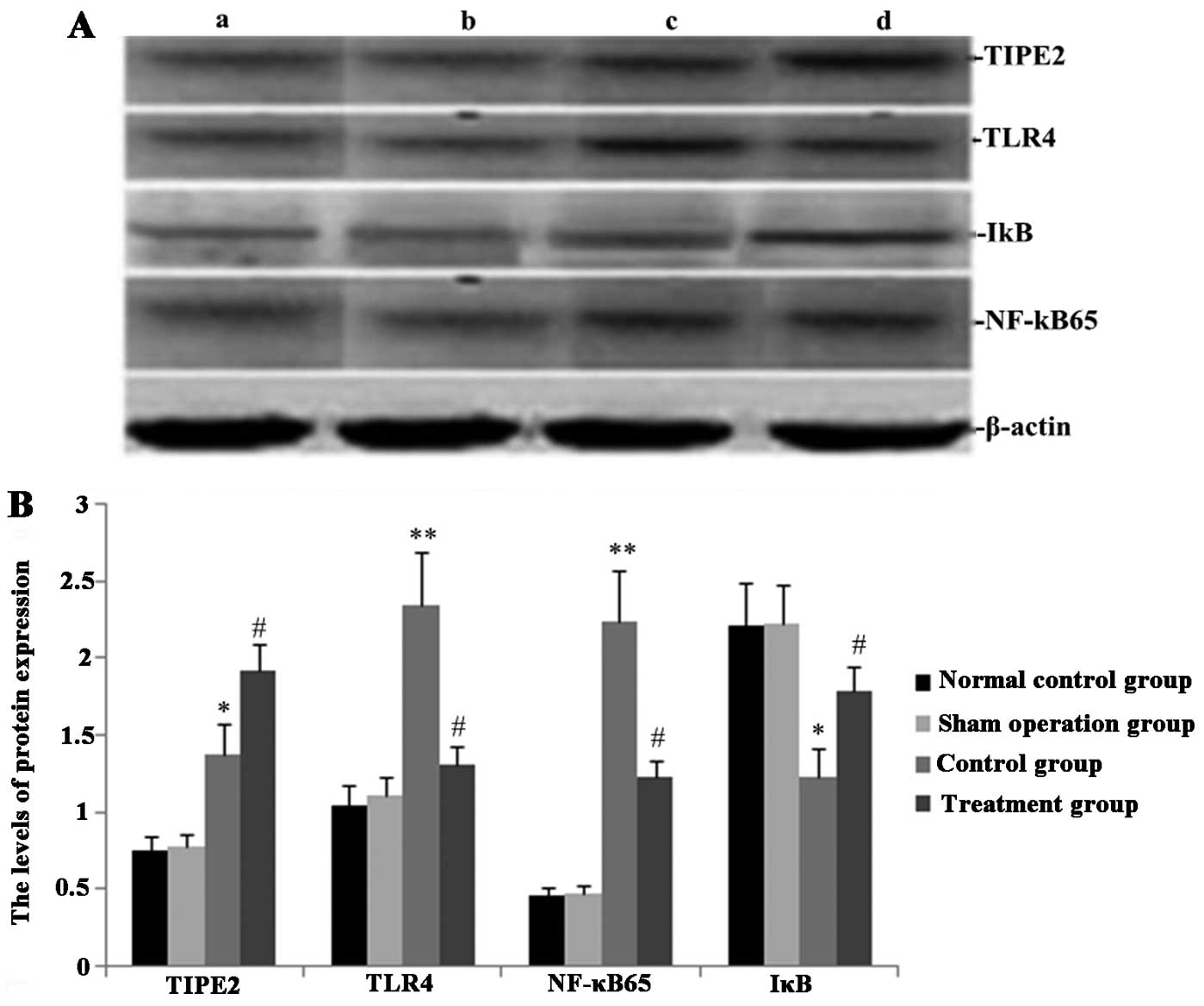 | Figure 3Effect of Melilotus extract
treatment on TIPE2, TLR4, NF-κB and IκB protein expression in
septic mice. Western blot analysis was used to determine the
protein expression levels of TIPE2, TLR4, NF-κB and IκB at 24 h
post-administration of Melilotus extract and exposure to
cecal ligation-perforation. (A) Representative western blots of
protein expression. Lanes: a, normal control group; b,
sham-operated group; c, control group; d, treatment group. (B)
Quantitative analysis of protein expression levels. Values are
presented as the mean ± standard deviation. *P<0.05
or **P<0.01 vs. sham-operated and normal control
groups, #P<0.05 or ##P<0.01 vs. control
group. TIPE2, tumour necrosis factor-α-induced protein-8-like 2;
NF-κB65, nuclear factor κB65; TLR4, toll-like receptor 4; IκB,
inhibitor of κB kinase. |
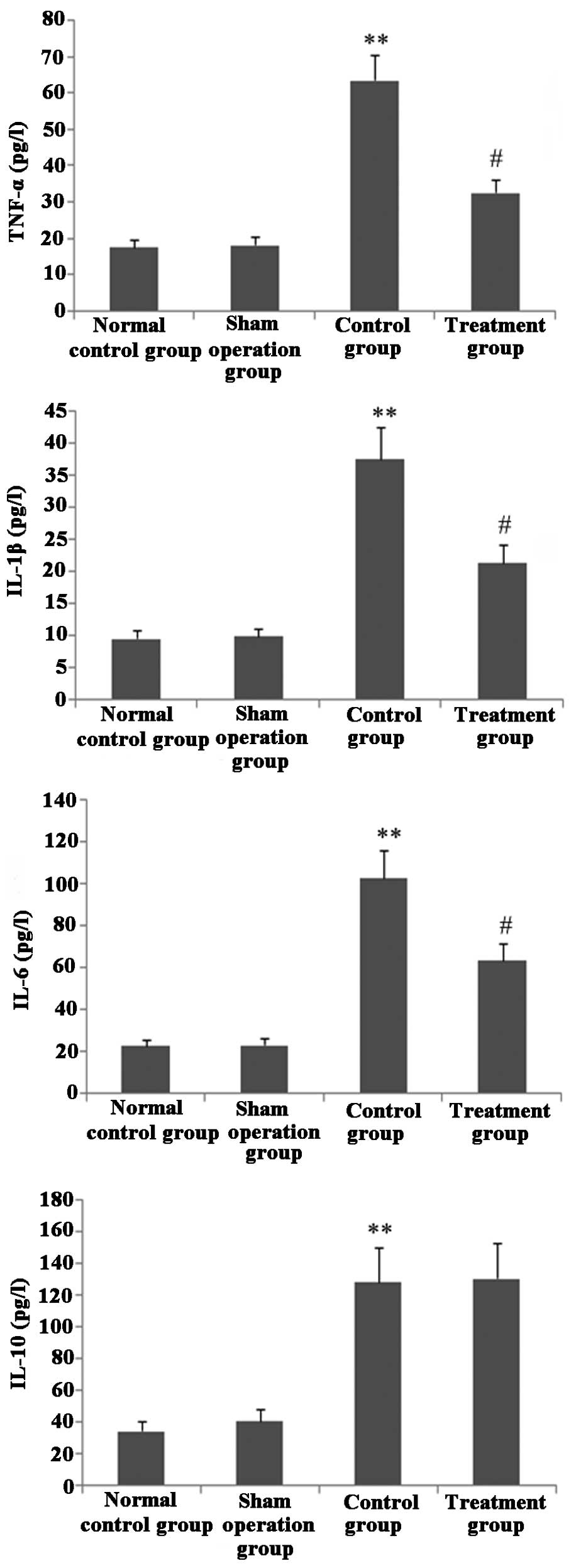
Melilotus extract decreases
proinflammatory cytokine production
As shown in Fig. 4,
following treatment with Melilotus extract, CLP-induced mice
exhibited significantly decreased levels of the proinflammatory
cytokines TNF-α, IL-1β and IL-6 compared with those in the control
group, as determined by BAL. However, the BAL levels of the
anti-inflammatory cytokine IL-10 did not significantly change.
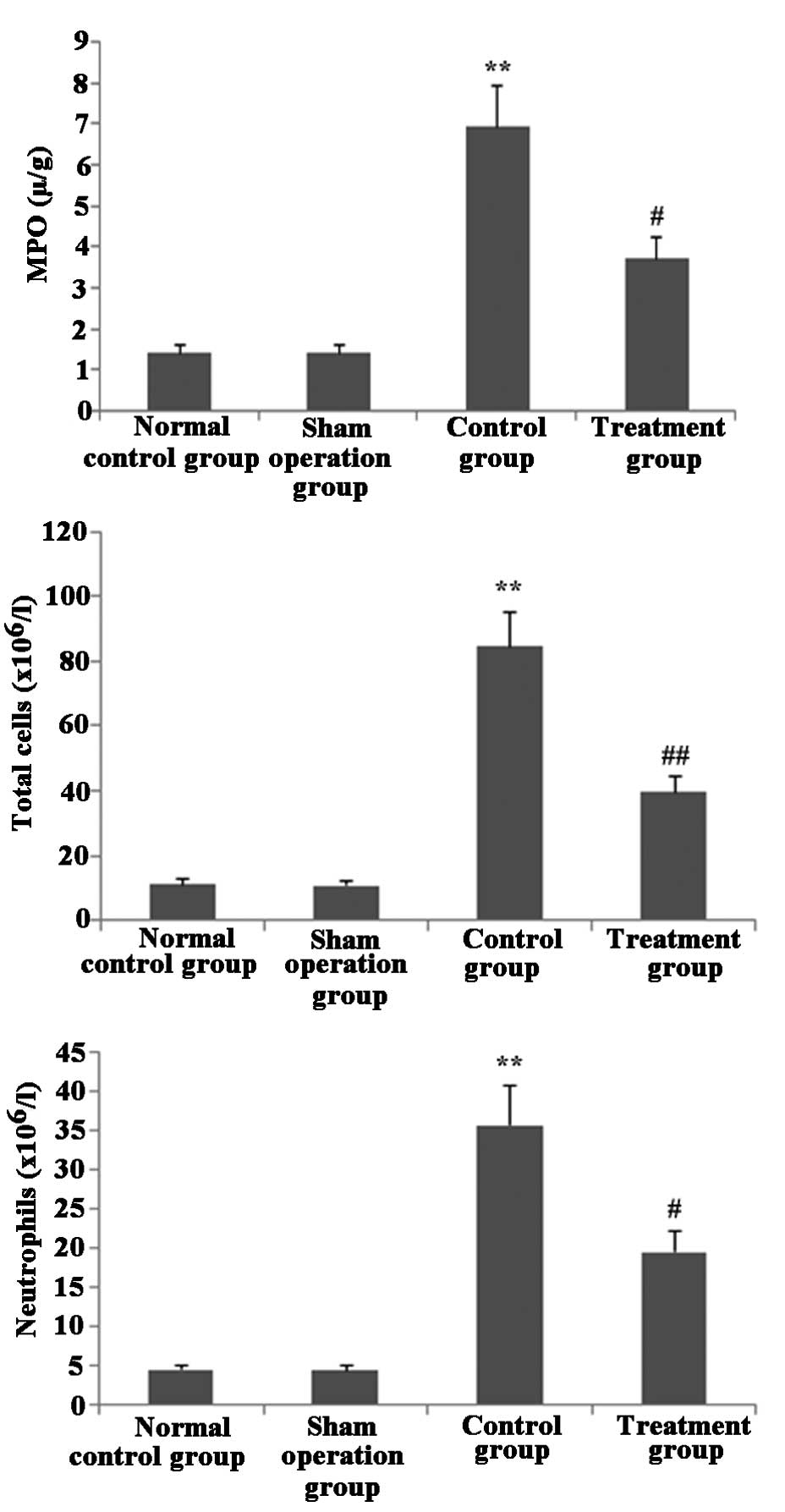
Melilotus extract decreases MPO activity
and blocks inflammatory cell infiltration in lung tissue
As shown in Fig. 5,
following treatment with Melilotus extract, the total
numbers of inflammatory cells and neutrophils in BAL and MPO
activity in lung tissue were significantly decreased compared with
those in the control group.
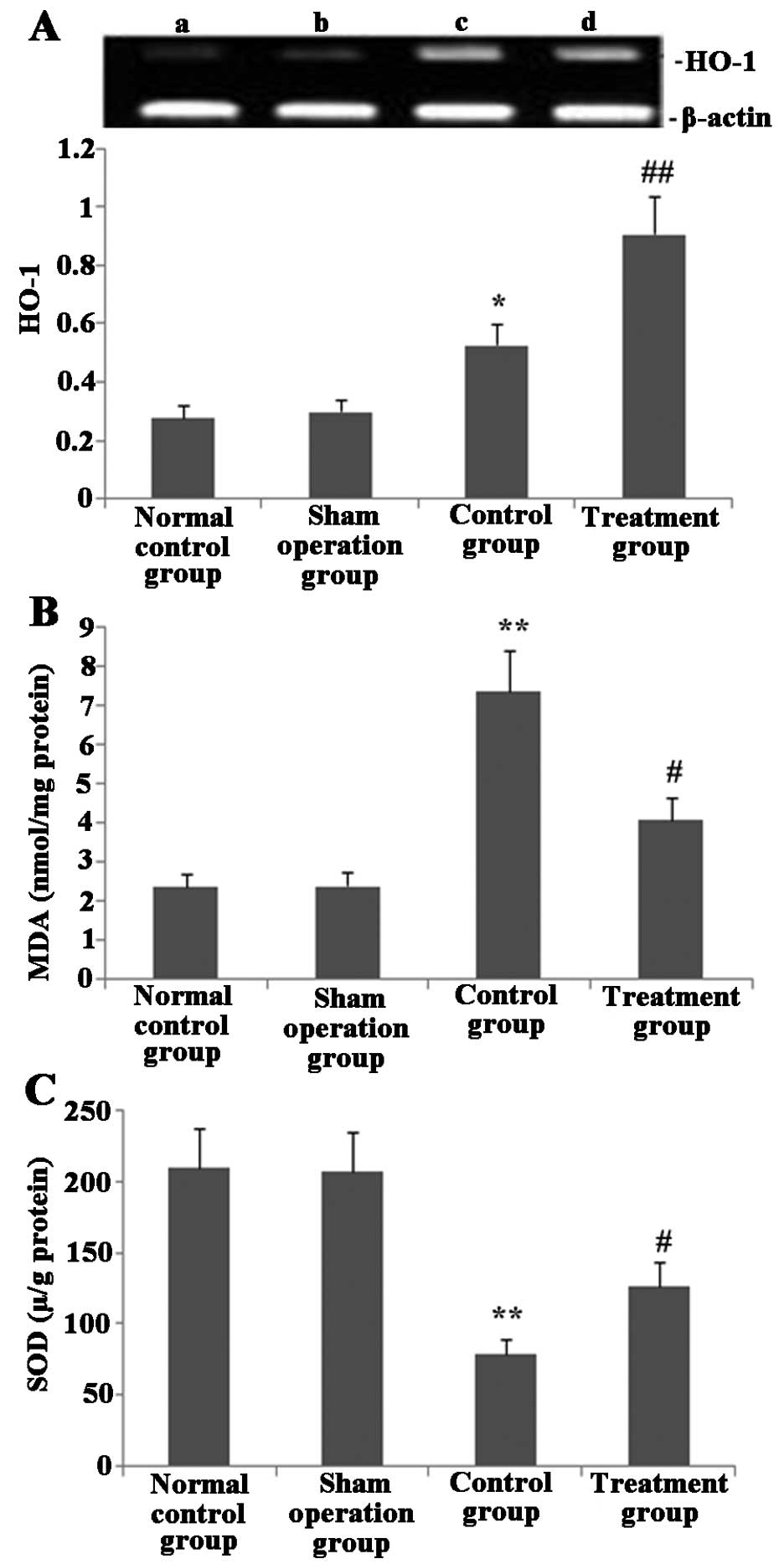
Melilotus extract upregulates HO-1
expression, increases SOD activity and prevents MDA activity in
lung tissue
As shown in Fig. 6,
24 h post-administration of Melilotus extract and exposure
to CLP operation, HO-1 expression and SOD activity in lung tissue
were significantly enhanced compared with those in the untreated
control group. In addition, the MDA activity in the treatment group
was significantly decreased compared with that of the normal
control and sham-operated groups.
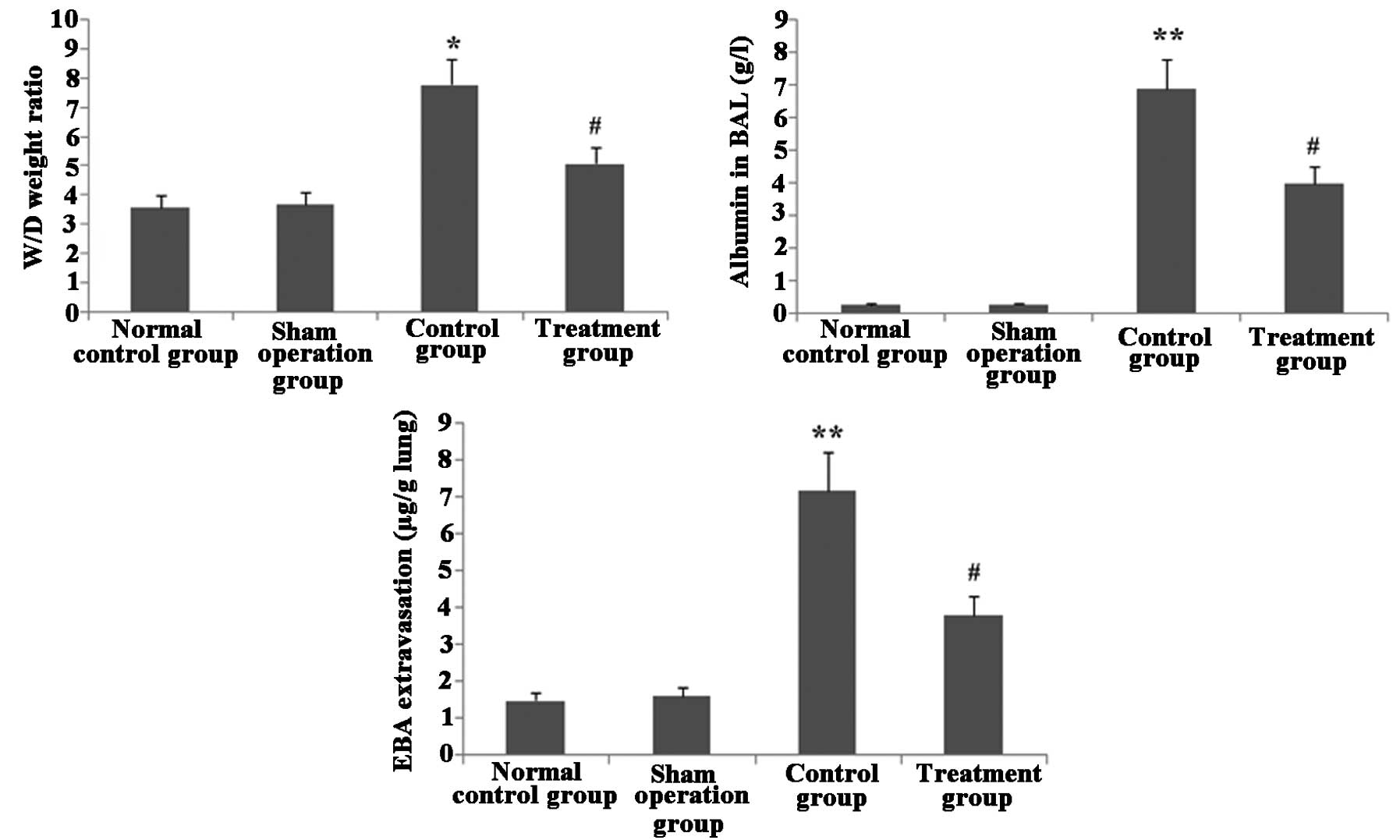
Melilotus extract ameliorates lung
vascular integrity in septic mice
As shown in Fig. 7,
following CLP surgery, the W/D weight ratio and EBA extravasation
in lung tissue as well as albumin in BAL were significantly
increased in the untreated control group compared with those in the
normal control and sham-operated groups. However, following
administration of Melilotus extract, there was a significant
decrease in the W/D weight ratio, EBA extravasation in lung tissue
and albumin in BAL compared to those in the untreated control
group.
Melilotus extract reduces pathological
lung injury in CLP-induced mice
As shown in Fig. 8,
histological analyses of lungs following CLP exposure revealed
alveolar septal thickening, accumulation of inflammatory cells in
the interstitium and alveoli and an influx of protein-rich fluid
into the alveolar space; in addition, ALI pathological score of
this untreated CLP surgery group was significantly increased
compared with that of the sham-surgery group. However, mice treated
with Melilotus extracts demonstrated reduced structural
changes following CLP exposure and a significantly decreased ALI
pathology score compared with that of the untreated group.
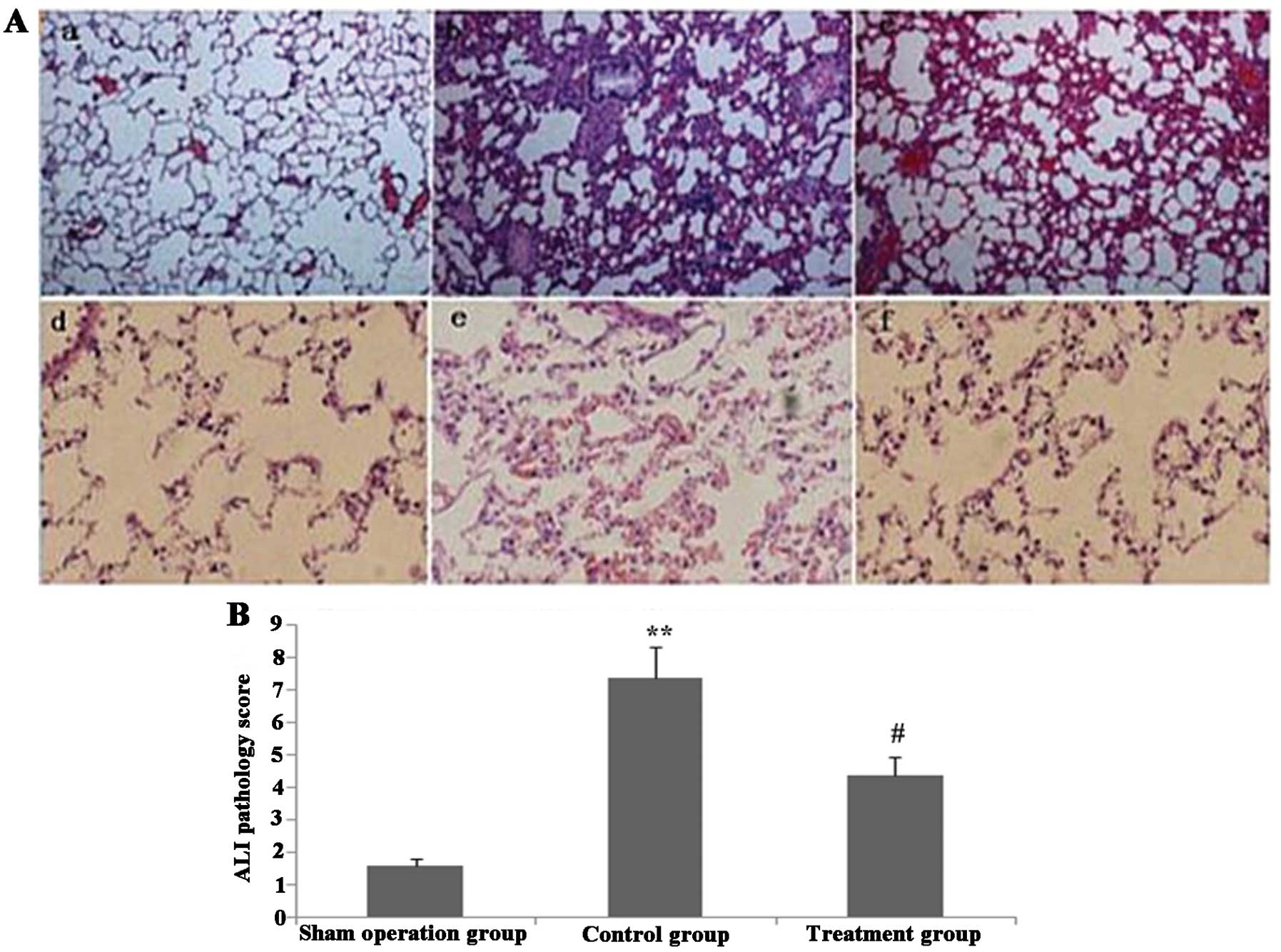 | Figure 8Melilotus extract ameliorates
the histopathological changes of lung tissue in septic mice. (A)
Histopathological changes were determined using hematoxylin and
eosin staining in the lung tissue of mice in each of the following
groups: a, sham-operated group; b, control group; c, treatment
group; d, sham-operated group; e, control group; f, treatment group
(magnification: a-c, ×100; d-f, ×400). (B) Quantitative analysis of
ALI pathology scores in mice from each group. Values are presented
as the mean ± standard deviation (n=3). **P<0.01 vs.
sham-operated group, #P<0.05 vs. control group. ALI,
acute lung injury. |
Discussion
In the animal models in the present study, cecal
ligation and perforation were used to induce diffused peritonitis
in the abdominal cavity by contamination of bacteria present in the
intestinal content (28,29), therefore resulting in a wide-range
systemic inflammatory response. Mice initially demonstrated
hyper-dynamic circulation and hypermetabolism and lower dynamic
circulation during a later period, which was consistent with
clinical symptoms observed in humans (30).
Medicinal chemistry studies have demonstrated that
Melilotus plants contain a number of substances which exert
anti-inflammatory, antibacterial and antioxidant activities; these
substances include coumarin, flavonoids, phenolic acids and
saponins. Coumarin constitutes the primary active anti-inflammatory
component in Melilotus plants (31). Using the erythrocyte sedimentation
rate adoption method, Trouillas et al (32) investigated the antioxidant
properties of water-soluble sites in 16 plant types, including
Melilotus officinalis. Their results indicated that the
antioxidant activities of these plants were positively correlated
with the total quantity of phenolic acids. In addition, Parejo
et al (33) compared the
free radical-scavenging activities and antioxidant capacities of 36
different extracts of six plant types, including Melilotus,
and tested the total phenolic acids using Folin-Ciocalteu
colorimetry. The results showed that the extracts of ethyl acetate
and dichloromethane contained numerous phenolics, which functioned
to scavenge free radicals. A previous study by Pabst et al
(34) suggested that
Melilotus extracts containing 0.9% coumarin, 0.2% hydroxyl
coumarin and certain flavonoids may improve blood circulation
across tissues. Furthermore, Kang et al (35) demonstrated that the degree of
leukocyte inhibition was an indicator for the evaluation of
antiinflammatory activity and revealed that 6 mg azukisaponin V
isolated from Melilotus extract administered to rats
resulted in the suppression of leukocyte production. Zhang et
al (36) performed an
inflammatory swelling and granuloma experiment, which verified that
different extracts and coumarin inhibited LPS stimulation in
RAW264.7 cells in order to generate pro-inflammatory factors,
including IL-6, TNF-α, IL-1β and nitric oxide, as well as promoted
the production of the anti-inflammatory factor IL-10. The results
of the present study showed that Melilotus officinalis
enhanced TIPE2 expression, suppressed TLR4 and NF-κB expression,
reduced the inflammatory response, increased HO-1 expression,
prevented oxidative stress as well as significantly alleviated lung
injury; this therefore indicated that Melilotus officinalis
exhibited effective lung protective abilities.
TLR4, a specific LPS transmembrane receptor,
initiates the production of proinflammatory cytokines and
corresponding immune response following LPS activation (37). LPS combines with LPS-binding
protein (LBP) to form the LPS-LBP complex, which then binds with
CD14 (mCD14) on the surface of the cell membrane to form a complex.
Following depolarization, LPS in the complex interacts with TLR4 to
activate LPS signal transduction pathways, which reinforce NF-κB
activity (38). NF-κB, a
transcription factor which regulates the expression of
proinflammatory cytokines and proteins, is activated in response to
several extracellular stimuli and oxidative stress. Activated NF-κB
enhances the transcription of numerous cytokines, including TNF-α
and IL-6 (38), therefore
decreasing the time and increasing the quantity of inflammatory
factor synthesis in the inflammatory cells (39,40).
In the resting state, NF-κB combines with the inhibitory protein
IκB to form an inactive complex within the cytoplasm. When this
occurs, cells are stimulated by endotoxins, tumor necrosis factors
and other extracellular signals, while IκB kinase phosphorylates
and decomposes IκB; subsequently, NF-κB rapidly translocates to the
nucleus prior to combining with specific IκB sequences, which
induces the transcription of TNF-α, IL-6 and other inflammatory
factors as well as adhesion molecules, colony stimulating factors,
cyclooxygenase 2 and inducible nitric oxide synthase, thus
triggering a systematic inflammatory response (39). Furthermore, inhibition of TLR
expression through increasing TIPE2 expression may suppress the
activation of NF-κB or promote IκB expression, which in turn
contributes to the suppression of inflammation mediator production,
thereby inhibiting the occurrence and development of ALI in septic
mice.
TIPE2 is a recently identified member of the TNFAIP8
family of immune regulators (41).
Certain immune-negative regulatory molecules have important effects
acute injury or sepsis. TIPE2, which is thought to be necessary to
maintain immune homeostasis, was found to be highly expressed in
inflammatory tissues (42).
Several studies have reported that LPS, via the stimulation of
macrophage TIPE2, downregulated multiple signal transduction
pathways (11,43,44).
TIPE2 cannot directly act on extracellular signal regulating kinase
pathways; however, it was shown to inhibit the activation of
terminal kinase of c-jun amino- and p38 mitogen-activated protein
kinase, therefore weakening the activity of the transcription
factor activator proteins (AP) (45) and depleting TIPE2 expression
levels. This may result in the enhancement of the NF-κB sequence
and phosphorylation of IκB; in concurrence with this, it was
demonstrated that TIPE2 suppressed the activation of AP-1 and
NF-κB. Furthermore, TIPE2-deficient cells were found to be highly
responsive to the activation of TLR and T-cell receptor signals
(46); in addition, in the
low-dose LPS-induced sepsis model, TIPE2-knockout mice demonstrated
clear septic shock responses compared with those of normal WT mice
(47). According to serum
analysis, decreased TIPE2 expression resulted in continual
lymphocyte activation, which promoted Fas expression and lymphocyte
apoptosis (48). However, TIPE2
gene defects may result in the increased production of cell
factors, including IL-4, IL-6, IL-12 and IFN-γ (47). The present study demonstrated that
the promotion of TIPE2 expression by Melilotus extract
inhibited the expression of NF-κB and TLR4, thereby inhibiting the
production of proinflammatory mediators, including TNF-α and
IL-6.
Oxidative stress is an indicator of inflammatory
processes. Previous studies have demonstrated that oxidative stress
and damage were associated with the pathogenesis and severity of
ALI (49–51). The production and release of
reactive oxygen species (ROS) is a fundamental anti-microbicidal
mechanism, by which ROS upregulation induces tissue damage in
sepsis and ALI. MDA, as the primary product of lipid peroxidation,
is commonly used as an indicator of the degree of oxidative damage
in the body (52). SOD is an
important enzyme involved in the dismutation of superoxide
radicals, which results from cellular oxidative metabolism into
hydrogen peroxide and inhibits LPS-induced penetration. HO-1, also
named heat shock protein 32, is a microsomal and rate-limiting
enzyme, which catalyzes the degradation of heme into biliverdin,
iron atoms and carbon monoxide (53). HO-1 and its breakdown products have
vital physiological roles in anti-inflammation, anti-oxidation and
the regulation of apoptosis (54,55).
The results of the present study demonstrated that upregulating
TIPE2 expression using Melilotus extracts significantly
enhanced HO-1 expression, reduced MDA levels and upregulated SOD
activity in damaged lung tissue, indicating that the redox
environment of the lungs was improved.
ALI and the more severe stage of acute respiratory
distress syndrome (ARDS) are induced by a variety of factors within
and outside the lung. ALI/ARDS is characterized by progressive
dyspnea and refractory hypoxemia; these are acute syndromes induced
through excessive inflammatory responses in the body. Endothelial
cell damage and dysfunction are important pathological features of
ALI/ARDS (56), which manifest as
extensive damage of pulmonary vascular endothelial and alveolar
epithelial cells as well as increased pulmonary vascular
permeability (57). In the present
study, CLP-induced sepsis resulted in increased levels of albumin
in BAL, W/D ratio and extravasated EBA in lung tissue as well as
revealed an increase in pulmonary vascular permeability. However,
these effects were all attenuated by treatment with
Melilotus extracts, indicating that Melilotus
extracts reduced pulmonary vascular permeability.
ALI is characterized by an intense inflammatory
response. This activates a cascade of proinflammatory events that
result in leukocyte infiltration into the lung (56). Therefore, in the present study, MPO
activity was measured in lung tissue and the number of inflammatory
cells in BAL was quantified. As expected, the increased MPO
activity in lung tissues and inflammatory cells count in BAL in the
untreated control group was significantly inhibited in mice treated
with Melilotus extract; in addition, the pathological
observations revealed that paraquat-induced lung inflammatory
changes were extenuated by Melilotus extract treatment.
In conclusion, inflammation immune dysfunction has
an important role in the pathogenesis of sepsis. The results of the
present study showed that the Traditional Chinese Medicine
Melilotus officinalis inhibited TLR4 and NF-κB expression,
enhanced IκB and HO-1 expression and decreased the inflammatory
response and oxidative stress via upregulation of TIPE2 expression
in CLP-induced lung injury. Furthermore, early Melilotus
officinalis treatment following CLP was found to have
protective effects, which indicated its potential role in the
prevention of LPS-induced ALI.
Acknowledgements
The authors would like to thank Professor Qin-qin
Huang and Professor Lin-jun Wang for their excellent technical
assistance.
Abbreviations:
|
TIPE2
|
tumor necrosis factor-α-induced
protein-8-like 2
|
|
NF-κB
|
nuclear factor κB
|
|
IL-6
|
interleukin-6
|
|
TNF-α
|
tumor necrosis factor-α
|
|
LPS
|
lipopolysaccharide
|
|
CLP
|
cecal ligation and puncture
|
|
RT-PCR
|
reverse transcription polymerase chain
reaction
|
|
NE
|
neutrophil elastase
|
|
ALI
|
acute lung injury
|
|
BAL
|
bronchoalveolar lavage fluid
|
|
TNFAIP8
|
tumor necrosis factor-α induced
protein-8
|
|
TLR4
|
toll-like receptor 4
|
|
HO-1
|
heme oxygenase-1
|
|
IκB
|
inhibitor of κB kinase
|
|
MPO
|
myeloperoxidase
|
|
MDA
|
malondialdehyde
|
|
SOD
|
superoxide dismutase
|
References
|
1
|
Seoane L, Winterbottom F, Nash T,
Behrhorst J, Chacko E, Shum L, Pavlov A, Briski D, Thibeau S,
Bergeron D, et al: Using quality improvement principles to improve
the care of patients with severe sepsis and septic shock. Ochsner
J. 13:359–366. 2013.PubMed/NCBI
|
|
2
|
Lee JW, Kwon JH, Lim MS, Lee HJ, Kim SS,
Lim SY and Chun W: 3,4,5-Trihydroxycinnamic acid increases
heme-oxygenase-1 (HO-1) and decreases macrophage infiltration in
LPS-induced septic kidney. Mol Cell Biochem. 397:109–116. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koch A, Meesters MI, Scheller B, Boer C
and Zacharowski K: Systemic endotoxin activity correlates with clot
formation: an observational study in patients with early systemic
inflammation and sepsis. Crit Care. 17:R1982013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brichon PY, Poquet C, Arvieux C and Pison
C: Successful treatment of a life-threatening air leakage,
complicating severe abdominal sepsis, with a one-way endobronchial
valve. Interact Cardiovasc Thorac Surg. 15:779–780. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rivers EP, Katranji M, Jaehne KA, Brown S,
Abou Dagher G, Cannon C and Coba V: Early interventions in
severesepsis and septic shock: a review of the evidence one decade
later. Minerva Anestesiol. 78:712–724. 2012.PubMed/NCBI
|
|
6
|
Lou Y and Liu S: The TIPE (TNFAIP8) family
in inflammation, immunity, and cancer. Mol Immunol. 49:4–7. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Luan YY, Yao YM, Zhang L, Dong N, Zhang
QH, Yu Y and Sheng ZY: Expression of tumor necrosis factor-α
induced protein 8 like-2 contributes to the immunosuppressive
property of CD4(+) CD25(+) regulatory T cells in mice. Mol Immunol.
49:219–226. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lou Y, Sun H, Morrissey S, Porturas T, Liu
S, Hua X and Chen YH: Critical roles of TIPE2 protein in murine
experimental colitis. J Immunol. 193:1064–1070. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Q, Huber N, Noel G, Haar L, Shan Y,
Pritts TA and Ogle CK: NF-κB inhibition is ineffective in blocking
cytokine-induced IL-8 production but P38 and STAT1 inhibitors are
effective. Inflamm Res. 61:977–985. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li X, Wu G, Wu M, Chen W and Liu X: In
vitro study of inhibitory millimeter wave treatment effects on the
TNF-α-induced NF-κB signal transduction pathway. Int J Mol Med.
27:71–78. 2011.
|
|
11
|
Sun X, Lv Z, Peng H, Fung M, Yang L, Yang
J, Zheng H, Liang J and Wu Z: Effects of a recombinant
schistosomal-derived anti-inflammatory molecular (rSj16) on the
lipopolysaccharide (LPS)-induced activated RAW264.7. Parasitol Res.
110:2429–2437. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu F, Zeng W, Mao X and Fan GK: The
efficacy of Melilotus extract in the management of postoperative
ecchymosis and edema after simultaneous rhinoplasty and
blepharoplasty. Aesthetic Plast Surg. 32:599–603. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pleşca-Manea L, Pârvu AE, Pârvu M, Taămaş
M, Buia R and Puia M: Effects of Melilotus officinalis on acute
inflammation. Phytother Res. 16:316–319. 2002. View Article : Google Scholar
|
|
14
|
Namsa ND, Tag H, Mandal M, Kalita P and
Das AK: An ethnobotanical study of traditional anti-inflammatory
plants used by the Lohit community of Arunachal Pradesh. India J
Ethnopharmacol. 125:234–245. 2009. View Article : Google Scholar
|
|
15
|
Pang R, Zhang SL, Zhao L, Liu SL, Dong JH
and Tao JY: Effect of petroleum ether extract from Melilotus
suaveolens Ledeb on the expression of NF-kappaB and Heme oxygenase
1. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 24:861–863. 2008.(In
Chinese). PubMed/NCBI
|
|
16
|
Asres K, Gibbons S, Hana E and Bucar F:
Anti-inflammatory activity of extracts and a saponin isolated from
Melilotus elegans. Pharmazie. 60:310–312. 2005.PubMed/NCBI
|
|
17
|
Zhang XY, Tao JY, Zhao L, Huang ZJ, Xiong
FL, Zhang SL, Li CM and Xiao F: In vitro anti-inflammatory effects
of different solution fractions of ethanol extract from Melilotus
suaveolens Ledeb. Chin Med J (Engl). 120:1992–1998. 2007.
|
|
18
|
Cataldi A, Gasbarro V, Viaggi R, Soverini
R, Gresta E and Mascoli F: Effectiveness of the combination of
alpha tocopherol, rutin, melilotus, and centella asiatica in the
treatment of patients with chronic venous insufficiency. Minerva
Cardioangiol. 49:159–163. 2001.PubMed/NCBI
|
|
19
|
Forte R, Cennamo G, Finelli ML,
Bonavolontà P, de Crecchio G and Greco GM: Combination of
flavonoids with Centella asiatica and Melilotus for diabetic
cystoid macular edema without macular thickening. J Ocul Pharmacol
Ther. 27:109–113. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu Z, Zhou X, Yu S, Xie H and Zheng S:
IL-15 is decreased upon CsA and FK506 treatment of acute rejection
following heart transplantation in mice. Mol Med Rep. 11:37–42.
2015.
|
|
21
|
Wang LY, Fan YC, Zhao J, Gao S, Sun FK,
Han J, Yang Y and Wang K: Elevated expression of tumour necrosis
factor-α-induced protein 8 (TNFAIP8)-like 2 mRNA in peripheral
blood mononuclear cells is associated with disease progression of
acute-on-chronic hepatitis B liver failure. J Viral Hepat.
21:64–73. 2014. View Article : Google Scholar
|
|
22
|
Bradford MM: Rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kakkar P, Das B and Viswanathan PN: A
modified spectrophotometric assay of superoxide dismutase. Indian J
Biochem Biophys. 21:130–132. 1984.PubMed/NCBI
|
|
24
|
Farooq MA, Ali S, Hameed A, Ishaque W,
Mahmood K and Iqbal Z: Alleviation of cadmium toxicity by silicon
is related to elevated photosynthesis, antioxidant enzymes;
suppressed cadmium uptake and oxidative stress in cotton.
Ecotoxicol Environ Saf. 96:242–249. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dada L, Gonzalez AR, Urich D, Soberanes S,
Manghi TS, Chiarella SE, Chandel NS, Budinger GR and Mutlu GM:
Alcohol worsens acute lung injury by inhibiting alveolar sodium
transport through the adenosine A1 receptor. PLoS One.
7:e304482012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Z and Jin ZQ: Ischemic preconditioning
enhances integrity of coronary endothelial tight junctions. Biochem
Biophys Res Commun. 425:630–635. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luna LG: Routine staining procedures
Hematoxylin and eosin stains. Manual of Histologic Staining Methods
of the Armed Forces Institute of Pathology. 3rd edition.
McGraw-Hill; New York, NY: pp. 32–39. 1968
|
|
28
|
Seely KA, Holthoff JH, Burns ST, Wang Z,
Thakali KM, Gokden N, Rhee SW and Mayeux PR: Hemodynamic changes in
the kidney in a pediatric rat model of sepsis-induced acute kidney
injury. Am J Physiol Renal Physiol. 301:F209–F217. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Iba T, Okamoto K, Kawasaki S, Nakarai E
and Miyasho T: Effect of hemoperfusion using polymyxin
B-immobilized fibers on non-shock rat sepsis model. J Surg Res.
171:755–761. 2011. View Article : Google Scholar
|
|
30
|
Chihara R, Nakamoto H, Arima H, Moriwaki
K, Kanno Y, Sugahara S, Okada H and Suzuki H: Systemic capillary
leak syndrome. Intern Med. 41:953–956. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Miliauskas G, Venskutonis PR and Beek TA:
Screening of radical scavenging activity of some medicinal and
aromatic plant extracts. Food Chem. 85:231–237. 2004. View Article : Google Scholar
|
|
32
|
Trouillas P, Calliste CA, Allais DP, Simon
A, Marfak A, Delage C and Duroux JL: An tioxidant,
anti-inflammatory and an tip roliferative properties of sixteen
water plant extracts used in the Limousin countryside as herbal
teas. Food Chem. 80:399–407. 2003. View Article : Google Scholar
|
|
33
|
Parejo I, Viladomat F, Bastida J,
Schmeda-Hirschmann G, Burillo J and Codina C: Bioguided isolation
and identification of the nonvolatile antioxidant compounds from
fennel(Foeniculum vulgare Mill) waste. Agric Food Chem.
52:1890–1897. 2004. View Article : Google Scholar
|
|
34
|
Pabst HW and Klemm H: Effect of extracts
of Melilotus officinalis. Med Monatsschr. 4:589–591. 1960.(In
German).
|
|
35
|
Kang SS, Lee YS and Lee EB: Isolation of
azukisaponin V possessing leucocyte migration inhibitory activity
from Melilotus officinalis. Natural Products. 18:89–93. 1987.
|
|
36
|
Zhang XY, Tao JY, Zhao L, Huang ZJ, Xiong
FL, Zhang SL, Li CM and Xiao F: In vitro anti-inflammatory effects
of different solution fractions of ethanol extract from Melilotus
suaveolens Ledeb. Chin Med J (Engl). 120:1992–1998. 2007.
|
|
37
|
Wang TY, Li J, Jin Z, Wu F and Zhou Q:
Inhibitory effect of TGF-β1 on no production in peritoneal
macrophages from collagen-induced arthritis rats involving the
LPS-TLR4 pathway. Mol Med Rep. 8:1143–1148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Black KE, Collins SL, Hagan RS, Hamblin
MJ, Chan-Li Y, Hallowell RW, Powell JD and Horton MR: Hyaluronan
fragments induce IFNbeta via a novel TLR4-TRIF-TBK1-IRF3-dependent
pathway. Inflamm (Lond). 10:23–29. 2013. View Article : Google Scholar
|
|
39
|
Ren W, Hu L, Hua F, Jin J, Wang Y and Zhu
L: Myeloid differentiation protein 2 silencing decreases
LPS-induced cytokine production and TLR4/MyD88 pathway activity in
alveolar macrophages. Immunol Lett. 141:94–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kannan Y, Sundaram K, Aluganti Narasimhulu
C, Parthasarathy S and Wewers MD: Oxidatively modified low density
lipoprotein (LDL) inhibits TLR2 and TLR4 cytokine responses in
human monocytes but not in macrophages. J Biol Chem.
287:23479–23488. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang Y, Wei X, Liu L, Liu S, Wang Z,
Zhang B, Fan B, Yang F, Huang S, Jiang F, Chen YH and Yi F: TIPE2,
a novel regulator of immunity, protects against experimental
stroke. J Biol Chem. 287:32546–32555. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li D, Song L, Fan Y, Li X, Li Y, Chen J,
Zhu F, Guo C, Shi Y and Zhang L: Down-regulation of TIPE2mRNA
expression in peripheral blood mononuclear cells from patients with
systemic lupus erythematosus. Clin Immunol. 133:422–427. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang X, Wang J, Fan C, Li H, Sun H, Gong
S, Chen YH and Shi Y: Crystal structure of TIPE2 provides insights
into immune homeostasis. Nat Struct Mol Biol. 16:89–90. 2009.
View Article : Google Scholar
|
|
44
|
Liu MW, Su MX, Zhang W, Wang L and Qian
CY: Atorvastatin increases lipopolysaccharide-induced expression of
tumour necrosis factor-α-induced protein 8-like 2 in RAW264.7
cells. Exp Ther Med. 8:219–228. 2014.PubMed/NCBI
|
|
45
|
Gus-Brautbar Y, Johnson D, Zhang L, Sun H,
Wang P, Zhang S, Zhang L and Chen YH: The anti-inflammatory TIPE2
is an inhibitor of the oncogenic Ras. Mol Cell. 45:610–618. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sun H, Gong S, Carmody RJ, Hilliard A, Li
L, Sun J, Kong L, Xu L, Hilliard B, Hu S, Shen H, Yang X and Chen
YH: TIPE2, a negative regulator of innate and adaptive immunity
that maintain immune Homeostasis. Cell. 133:415–426. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xi W, Hu Y, Liu Y, Zhang J, Wang L, Lou Y,
Qu Z, Cui J, Zhang G, et al: Roles of TIPE2 in hepatitis B
virus-induced hepatic inflammation in humans and mice. Mol Immunol.
48:1203–1208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Freundt EC, Bidere N and Lenardo MJ: A
different TIPE2 of immune homeostasis. Cell. 133:401–402. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang HT, Liu CF, Tsai TH, Chen YL, Chang
HW, Tsai CY, Leu S, Zhen YY, Chai HT, et al: Effect of obesity
reduction on preservation of heart function and attenuation of left
ventricular remodeling, oxidative stress and inflammation in obese
mice. J Transl Med. 10:145–148. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lv R, Zheng J, Ye Z, Sun X, Tao H, Liu K,
Li R, Xu W, Liu W and Zhang R: Advances in the therapy of
hyperoxia-induced lung injury: findings from animal models.
Undersea Hyperb Med. 41:183–202. 2014.PubMed/NCBI
|
|
51
|
Su ZQ, Mo ZZ, Liao JB, Feng XX, Liang YZ,
Zhang X, Liu YH, Chen XY, Chen ZW, Su ZR and Lai XP: Usnic acid
protects LPS-induced acute lung injury in mice through attenuating
inflammatory responses and oxidative stress. Int Immunopharmacol.
22:371–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wu KL, Chan SH and Chan JY:
Neuroinflammation and oxidative stress in rostral ventrolateral
medulla contribute to neurogenic hypertension induced by systemic
inflammation. J Neuroinflammation. 9:212–218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lino-dos-Santos-Franco A, Correa-Costa M,
Durão AC, de Oliveira AP, Breithaupt-Faloppa AC, de Bertoni JA,
Oliveira-Filho RM, Câmara NO, Marcourakis T and Tavares-de-Lima W:
Formaldehyde induces lung inflammation by an oxidant and
antioxidant enzymes mediated mechanism in the lung tissue. Toxicol
Lett. 207:278–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
de Sousa Oliveira Vanderlei E, de Araújo
IW, Quinderé AL, Fontes BP, Eloy YR, Rodrigues JA, e Silva AA,
Chaves HV, Jorge RJ, et al: The involvement of the HO-1 pathway in
the anti-inflammatory action of a sulfated polysaccharide isolated
from the red seaweed Gracilaria birdiae. Inflamm Res. 60:1121–1130.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yeligar SM, Machida K and Kalra VK:
Ethanol-induced HO-1 and NQO1 are differentially regulated by
HIF-1alpha and Nrf2 to attenuate inflammatory cytokine expression.
J Biol Chem. 285:35359–35373. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hu ZF and Sun HC: The mechanism of
inflammatory mediators of vascular endothelial cells in acute lung
injury. Traum Surg. 11:281–290. 2009.
|
|
57
|
Campos R, Shimizu MH, Volpini RA, de
Bragança AC, Andrade L, Lopes FD, Olivo C, Canale D and Seguro AC:
N-acetylcysteine prevents pulmonary edema and acute kidney injury
in rats with sepsis submitted to mechanical ventilation. Am J
Physiol Lung Cell Mol Physiol. 302:L640–L650. 2012. View Article : Google Scholar : PubMed/NCBI
|