Introduction
Epilepsy is the most prevalent chronic neurological
disorder. Although the exact pathogenic mechanisms remain unclear,
mossy fiber sprouting (MFS) is a pathological phenomenon observed
in both animal models of temporal lobe epilepsy (TLE) and brain
sections of epileptic patients (1–3). The
majority of studies support the hypothesis that MSF contributes to
increased seizure susceptibility by forming recurrent excitatory
circuits. However, the mechanisms underlying these structural
changes are not fully understood. The hippocampal mossy fibers are
normally guided to the CA3 and form synapses with the pyramidal
cells. However, in the epileptic hippocampus, the mossy fibers
abnormally innervate into the inner molecular layer and/or stratum
oriens of the CA3, establishing hyperexcitable recurrent circuits,
which is called MFS (4). Based on
recent findings (5–7), MFS may be regarded as a result of the
disruption of molecular mechanisms underlying axonal growth and
axonal guidance.
The repulsive guidance molecule (RGM) was originally
known as a glycosylphosphatidylinositol (GPI)-anchored glycoprotein
involved in guiding axons in the developing chick retina (8). Repulsive guidance molecule A (RGMa)
is one of the three homologs of RGM, and is principally expressed
in the central nervous system (9).
In addition to regulating axonal guidance, RGMa also functions as a
myelin-derived neurite outgrowth inhibitor in vitro and
in vivo (10–11). These studies indicate the essential
role of RGMa in the neural circuit formation.
The Ras superfamily GTPase proteins play essential
roles in mediating neurite outgrowth and maintaining growth cone
morphology by regulating cytoskeletal reorganization. Ras, one of
the GTPase proteins that is abundantly distributed in neuronal
axons and growth cones, promotes axonal extension during
development (12–13). A previous study has shown that RGMa
may exert its biological effects by dephosphorylating focal
adhesion kinase (FAK) at Tyr397, then regulating the activation of
Ras (14). However, the role of
RGMa in epileptogenesis and MFS remains unclear, and the potential
signaling pathway remains unexplored.
Considering the possible functions of RGMa in the
adult brain, we hypothesized that RGMa may also participate in the
plastic changes that occur during TLE development through the
RGMa-FAK-Ras pathway. In this study, we investigated this
hypothesis using the pentylenetetrazole (PTZ) kindling model, which
has been widely adopted as a model of synaptic rearrangement and
neuronal plasticity in the epileptic brain (15,16).
Materials and methods
Animals and the PTZ model
A total of 120 adult male Sprague-Dawley rats
(Animal Experimental Centre, Central South University, China)
weighing 180–220 g were equally divided into a control and a PTZ
group, each containing five subgroups of 12 rats. The PTZ group
received a dose of 30 mg/kg PTZ (Sigma, St. Louis, MO, USA)
intraperitoneally once per day until the rats were kindled or
sacrificed (rats in the PTZ group were not kindled within 2 weeks),
while the control rats were injected with an equal dose of saline.
Rats were considered kindled when seizure attacks (score ≥3)
occurred after each PTZ injection for five consecutive days using
Racine’s scale system (17). At
time points of 3 days and 1, 2, 4 and 6 weeks after the first
injection, the rats were sacrificed by immediate decapitation under
deep anesthesia, with the exception of the rats used for
immunohistochemical anaylsis, which were perfused first.
All animals were treated humanely and this study
conformed to the Guide for the Care and Use of Laboratory Animals
published by the National Institutes of Health (18). All animal use protocols were
approved by the Animal Ethics Committee of Central South University
(Changsha, China).
Behavior monitoring
The rats were observed for the occurrence of
PTZ-induced seizures for at least 2 h immediately following the PTZ
injection, each day, until kindling or sacrifice occurred. The
convulsive behavior was evaluated as previously described (17): 0, no behavioral changes; 1, facial
movements, ear and whisker twitching; 2, myoclonic convulsions
without rearing; 3, myoclonic convulsions with rearing; 4, clonic
convulsion with loss of posture; 5, generalized tonic-clonic
seizures.
Timm staining
At different time points, the rats were deeply
anesthetized with 10% chloral hydrate and perfused intracardially
with 300 ml saline, followed by 200 ml 0.1 M phosphate buffer (pH
7.2–7.6) containing 0.4% sodium sulfide and 200 ml 4%
paraformaldehyde at 4°C. The brains were removed, fixed in 4%
paraformaldehyde for 24 h, then transferred to 0.1 M phosphate
buffer containing 30% sucrose, and finally cut into 30-μm coronal
sections.
The sections were stained in the dark for 90 min in
a solution containing 60 ml 50% gum arabic, 10 ml 2 M citrate
buffer, 30 ml 0.5 M hydroquinone and 0.5 ml 17% silver nitrate.
After washing in water, the slides were restained with Nissl
solution (Beyotime Co., Shanghai, China). Following this, the
slides were routinely dehydrated, cleaned and mounted with gum.
Immunohistochemistry
At different time points, the rats were deeply
anesthetized with 10% chloral hydrate and perfused intracardially
with 300 ml saline and 200 ml 4% paraformaldehyde in 0.1 M
phosphate buffer at 4°C, and then decapitated. The brains were
removed and placed in 4% paraformaldehyde overnight, then
transferred into 0.1 M phosphate buffer containing 20% and 30%
sucrose. Subsequently, serial sections were cut at a thickness of
20 μm for analysis. The tissue sections were subjected to
conventional rewarming and heat-induced antigen retrieval in 10 mM
sodium citrate buffer at boiling point for 24 min, with
supplementation of cool sodium citrate buffer every 6 min.
Peroxidase and lipids were eliminated by the admixture of 1%
hydrogen and methanol at 4°C for 30 min. After rinsing in 0.01 M
phosphate-buffered saline (PBS), the sections were blocked using a
5% goat serum reagent at room temperature for 2 h and incubated
with anti-RGMa (rabbit anti-rat polyclonal antibody; 1:50; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and anti-FAK Tyr397
(rabbit anti-rat polyclonal antibody; 1:50; Santa Cruz
Biotechnology, Inc.) overnight at 4°C. Subsequently,
immunohistochemistry was performed according to the kit protocol of
the Biotin-Streptavidin Horseradish Peroxidase (HRP) Detection
system, using normal goat serum for inhibition, biotin-labeled goat
anti-rabbit immunoglobulin (Ig)G and streptavidin/HRP (Zhongshan
Golden Bridge Biotechnology Co., Ltd., Zhongshan, China).
Immunofluorescence
The rewarming and antigen recovery of brain tissue
sections were performed as above. Each section was permeabilized
with 1% Triton X-100 (Sigma) in Tris-buffered saline with Tween 20
(TBST). After blocking with 10% goat serum (Zhongshan Golden Bridge
Biotechnology Co., Ltd.) at room temperature for 2 h, each sample
was incubated with anti-RGMa and anti-FAK Tyr397 at 4°C overnight.
After rinsing in 0.01 M PBS, the sections were incubated with goat
anti-rabbit IgG (1:1,000; Invitrogen Life Technologies, Carlsbad,
CA, USA) in the dark for 2 h at room temperature.
Western blot analysis
At different time points, rats in the control and
PTZ groups were deeply anesthetized with chloral hydrate and
immediately decapitated for western blot analysis. Tissues were
snap-frozen in liquid nitrogen, and proteins isolated from the
hippocampus were extracted using RIPA lysate buffer (Beyotime Co.).
All proteins were denatured (95°C, 10 min) and chilled on ice (5
min), then electrophoresis was performed (10% SDS-PAGE). The
proteins were transferred onto 0.45- or 0.22-μm polyvinylidene
difluoride membranes (Pall Corporation, Port Washington, NY, USA),
washed three times with TBST, and then blocked with 5% skimmed milk
in TBS (room temperature, 2 h). The membranes were incubated at 4°C
overnight with the appropriate primary antibodies (anti-RGMa rabbit
anti-rat polyclonal antibody, 1:800; anti-FAK Tyr397 rabbit
anti-rat polyclonal antibody, 1:500; anti-Ras rabbit anti-rat
polyclonal antibody, 1:500; anti-GAPDH rabbit anti-rat polyclonal
antibody, 1:1,000). Unbound antibodies were washed, and the
membranes were subsequently incubated with HRP-labeled goat
anti-rabbit IgG secondary antibodies (1:2,000 dilutions; Beyotime
Co.) for 1 h (room temperature). After washing (3×15 min), the
immunoreactive bands were visualized by enhanced chemiluminescence
(Bio-Rad imaging system; Bio-Rad, Hercules, CA, USA) and quantified
using Image Lab software (Bio-Rad).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Intergroup differences in Timm scores were compared using the
Mann-Whitney U test, while intragroup differences were compared
using the Kruskal-Wallis H test and then the Nemenyi test for
pairwise comparison. Differences among multiple groups were
assessed by a one-way analysis of variance, and differences between
two groups were evaluated using the independent samples t-test.
P<0.05 was considered to indicate a statistically significant
difference. All statistical analyses were performed using the
Statistical Package for the Social Sciences, version 17.0 (SPSS,
Inc., Chicago, IL, USA).
Results
Behavioral outcomes
With the exception of three rats that died as a
result of persistent generalized tonic-clonic seizure at 3 days or
at 2 weeks, and one rat that was not kindled, the PTZ-treated rats
developed seizure activity of different degrees after continuous
PTZ injections for 21–28 days (an average of 23.6±2.0 days). The
PTZ-induced seizure activity usually occurred 5–10 min after
injection with a duration of 5–20 min. No epileptiform activity was
observed behaviorally in the control rats.
Severity of MFS in the CA3 region is
correlated with the evolution of seizure behavior
The distribution of Timm granules in the stratum
oriens of CA3 was rated on a scale of 0 to 5 according to published
criteria (19). In control rats,
there was no significant difference in Timm scores in the CA3
region (P>0.05). There were significant differences in the Timm
scores in the CA3 region at each time point between the PTZ group
and control group (P<0.05). The degrees of MFS in the CA3 were
consistent with grades of seizure in the PTZ group and reached a
peak at 4 weeks (Fig. 1).
Conversely, Timm scores in the inner molecular layer were 0–1
throughout the experiment in the PTZ group, with no difference in
the control group (data not shown).
Expression of RGMa is significantly
downregulated during PTZ kindling progression
RGMa expression was mainly observed in the neuronal
membrane in the CA3 region of the hippocampus. Compared with the
control group, the expression of RGMa in pyramidal cells of the CA3
region was significantly downregulated (P<0.05) in the PTZ
group; it decreased markedly from 3 days and maintained the low
expression at 6 weeks (Figs. 2 and
5A). No obvious distinction was
observed in the CA3 region at different time points in the control
group. The western blot analysis demonstrated similar results
(Figs. 3A and 5C).
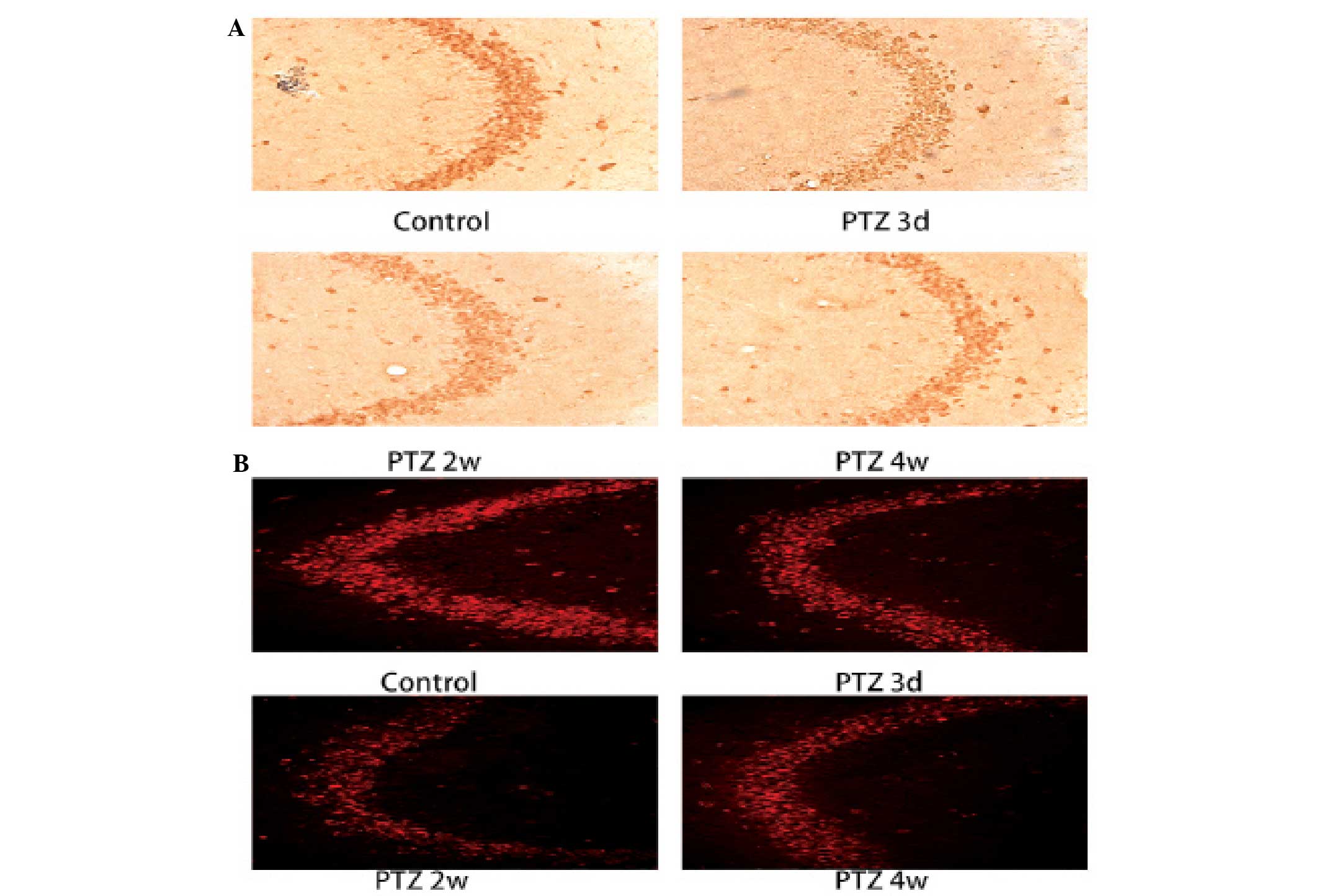
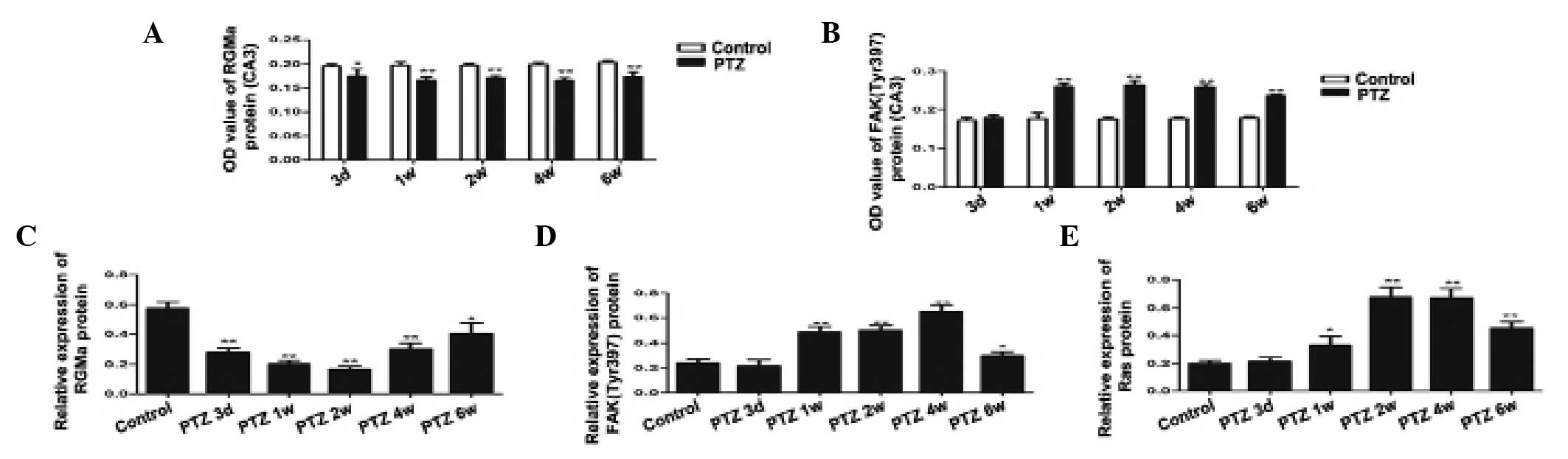 | Figure 5Expression of (A) RGMa and (B) FAK
(Tyr397) in the CA3 area of the hippocampus, as assessed by
immunohistochemical staining in the control and the PTZ groups.
Compared with the control group, RGMa expression was significantly
downregulated as early as 3 days post PTZ injection and the low
expressionwas maintained at 6 weeks; FAK (Tyr397) was significantly
increased in 1 week, reached a peak at 2 weeks and exhibited a
decline at 6 weeks. Expression of (C) RGMa, (D) FAK (Tyr397) and
(E) Ras in the hippocampus by western blot analysis. GAPDH was used
as the loading control. Quantification of the western blot results
revealed that RGMa expression decreased at all time-points in the
PTZ group compared with the control and that the expression of FAK
(Tyr397) increased in the PTZ compared with the control group. Ras
was significantly increased after 1 week, reached a peak at 4 weeks
and declined at 6 weeks.. The data are expressed as the mean ± SD.
*P<0.05, compared with the control; and
**P<0.01, compared with the control. RGMa, repulsive
guidance molecule A; FAK, focal adhesion kinase; PTZ,
pentylenetetrazole. |
Expression of FAK (Tyr397) and Ras is
significantly upregulated during PTZ kindling progression
From the western blot analysis, the expression of
FAK (Tyr397) and Ras were significantly upregulated (P<0.05) in
the PTZ group. The expression of FAK (Tyr397) increased markedly
from 1 week and reached a peak at 4 weeks, then declined at 6 weeks
(Figs. 3B and 5D). The expression of Ras increased from
1 week and reached a peak at 4 weeks, then began to decline at 6
weeks (Figs. 3C and 5E). The immunohistochemistry and
immunofluorescence results were in accordance with the western
blotting (Figs. 4 and 5B). Figs. 4A
and B show that the expression of FAK (Tyr397) accumulated
mainly in the cytoplasm in the CA3 region. No obvious distinction
was observed in the CA3 region at different time points in the
control group.
Discussion
This study examined the potential roles of RGMa and
its potential downstream molecules on epileptogenesis and MFS using
the PTZ kindling rat model. It was found that MFS preceded the
appearance of spontaneous recurrent seizures (SRS) in the PTZ
kindling model. The RGMa-FAK-Ras pathway was correlated with the
progression of MFS.
With regard to the fact that MFS preceded the
appearance of SRS, it is known that the occurrence and development
of epilepsy are usually associated with neuronal loss (20), MFS (21) and synaptic reorganization in the
hippocampus (22). The present
study demonstrated that the degree of aberrant MFS was consistent
with the severity of the seizure and that MFS can precede the
occurrence of SRS. Although, there are also some reservations.
Other studies have demonstrated that MFS is not associated with the
progression of spontaneous seizures (23–24).
However, in the current study, MSF was observed on day 3, which was
before the appearance of SRS, indicating that MFS was more likely
to be the cause of SRS.
The present study found that RGMa plays a potential
role in neuronal reorganization in the kindling rat hippocampus. In
the development of the neural circuit, specific axonal guidance and
growth factors are essential for guiding the axon to the proper
projection area. These factors can suppress the spontaneous or
aberrant axonal growth to maintain the established neural
connections and participate in the regulation of synaptic
plasticity. Four major classes of axon guidance and growth
molecules, the ephrins, netrins, slits and semaphorins, have been
reported to play a role in synaptic reorganization in the adult
brain and thereby promote epileptogenesis (5–7). In
this way, the axonal growth and guidance-related signal molecules
have major therapeutic significance for epilepsy.
RGMa is a novel type of GPI-anchored glycoprotein
with no significant homology to any other known guidance and growth
molecules. It is not only an axonal guidance molecule but also a
novel axonal growth inhibitor. GPI-anchored molecules encompass a
large group of proteins of great functional diversity. These
molecules can act through contact attraction or repulsion to
regulate axonal pathfinding and growth. RGMa plays a role in axonal
morphogenesis by regulating neurite extension and branching
(25). At present, the research on
RGMa is more focused on spinal cord injury and cerebral vascular
diseases. (10,26–28)
These studies indicate the essential role of RGMa in axonal growth,
pathfinding and synaptic plasticity. The correlation between RGMa
and epileptogenesis and MFS remains unknown. One case report has
demonstrated a deletion of the RGMa gene in a child with epilepsy
and mental deficiency (29), and
Brinks et al (30) reported
that RGMa is involved in the layer-specific innervation of
perforant fibers to the dentate gyrus. The principal finding of the
present study was that RGMa was significantly downregulated in the
hippocampus at time points consistent with the development of
aberrant MFS in a model of PTZ-induced TLE. Therefore, our present
findings provide new insights into the morphogenesis of mossy
fibers: RGMa signaling negatively regulates the branching of mossy
fibers. These results are consistent with the previous studies
showing that RGMa inhibits neurite branching and outgrowth by
binding to its receptor neogenin (10,31).
However, the molecular mechanisms of RGMa in MFS remain to be
explored.
The current study found that RGMa may participate in
the plastic changes through the FAK-Ras pathway. Both the axon
growth and guidance are eventually realized through induced
cytoskeletal rearrangement. Studies have shown that RGMa induces
cytoskeletal rearrangement by regulating the activation of Ras and
RhoA (14,32). Both Ras and RhoA belong to the Ras
superfamily GTPase proteins, which orchestrate actin filament
assembly and disassembly by controlling actin polymerization,
branching and depolymerization (33,34).
RhoA has been demonstrated to be associated with epilepsy (35); however, to date, research into Ras
has focused on survival, growth, proliferation and differentiation
of cells (36–38). Studies have shown that Ras may
regulate axonal extension and guidance during development (12–13).
However, it is unknown whether Ras functions as a mediator of
kindling-induced structural changes, and how RGMa regulates the
activation of Ras.
A potential candidate to mediate the flow of
information from the extracellular environment to the cytoskeleton,
and thereby to control axonal development, is FAK. FAK expression
is enriched in cell bodies and growth cones (39); several extracellular cues, such as
axonal growth and guidance factors, have been shown to function
upstream of FAK (40–41), suggesting that it might regulate
the interactions between growing neurites and the extracellular
matrix. It can interact with a complex molecular network via
multiple phosphorylation sites. While the best characterized FAK
phosphorylation event is the autophosphorylation at Tyr397, this
phosphorylation event is the first and most important step in FAK
activation. Autophosphorylation of FAK at Tyr397 leads to the
formation of phosphotyrosine docking sites for several classes of
signaling molecules and phosphorylation at additional tyrosine
residues (42), suggesting that
autophosphorylation of FAK at Tyr397 may act as an essential
intracellular adaptor. A previous study has revealed that RGMa
could inactivate Ras via FAK dephosphorylation to induce growth
cone collapse (14). Therefore,
RGMa may also participate in the plastic changes that occur during
TLE development through the RGMa-FAK-Ras pathway.
In the present study, it was found that FAK (Tyr397)
and Ras were significantly upregulated in the hippocampus at time
points consistent with the development of aberrant MFS. Therefore,
we hypothesize that following a decrease of RGMa expression in the
hippocampus, the degree of FAK phosphorylation at Tyr397 increases
then upregulates the expression of Ras, which ultimately
facilitates the pathfinding and synaptic specificity of MFS via
cytoskeletal rearrangement, providing a structural basis for
enhanced excitation and epileptogenesis in the hippocampus.
In conclusion, this study demonstrated that MFS is
not the outcome of SRS. The results of this study indicate, for the
first time, that RGMa may be involved in MFS and synaptic
reorganization through the RGMa-FAK-Ras pathway. Understanding the
molecular mechanisms underlying MFS may lead to therapeutic
interventions that protect the brain from recurrent spontaneous
seizures.
Acknowledgements
This study was supported by the Fundamental Research
Funds for the Central Universities of Central South University
(2013zzts093).
References
|
1
|
Andrade-Valença LP, Valença MM, Velasco
TR, Carlotti CG Jr, Assirati JA, Galvis-Alonso OY, Neder L, Cendes
F and Leite JP: Mesial temporal lobe epilepsy: clinical and
neuropathologic findings of familial and sporadic forms. Epilepsia.
6:1046–1054. 2008. View Article : Google Scholar
|
|
2
|
Lamont SR, Stanwell BJ, Hill R, Reid IC
and Stewart CA: Ketamine pre-treatment dissociates the effects of
electroconvulsive stimulation on mossy fiber sprouting and cellular
proliferation in the dentate gyrus. Brain Res. 1053:27–32. 2008.
View Article : Google Scholar
|
|
3
|
Kuo LW, Lee CY, Chen JH, Wedeen VJ, Chen
CC, Liou HH and Tseng WY: Mossy fiber sprouting in
pilocarpine-induced status epilepticus rat hippocampus: a
correlative study of diffusion spectrum imaging and histology.
Neuroimage. 41:789–800. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Koyama R and Ikegaya Y: Mossy fiber
sprouting as a potential therapeutic target for epilepsy. Curr
Neurovasc Res. 1:3–10. 2004. View Article : Google Scholar
|
|
5
|
Lin H, Huang Y, Wang Y and Jia J:
Spatiotemporal profile of N-cadherin expression in the mossy fiber
sprouting and synaptic plasticity following seizures. Mol Cell
Biochem. 358:201–205. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fang M, Liu GW, Pan YM, Shen L, Li CS, Xi
ZQ, Xiao F, Wang L, Chen D and Wang XF: Abnormal expression and
spatiotemporal change of Slit2 in neurons and astrocytes in
temporal lobe epileptic foci: a study of epileptic patients and
experimental animals. Brain Res. 1324:14–23. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pan Y, Liu G, Fang M, Shen L, Wang L, Han
Y, Shen D and Wang X: Abnormal expression of netrin-G2 in temporal
lobe epilepsy neurons in humans and a rat model. Exp Neurol.
224:340–346. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Monnier PP, Sierra A, Macchi P,
Deitinghoff L, Andersen JS, Mann M, Flad M, Hornberger MR, Stahl B,
Bonhoeffer F and Mueller BK: RGM is a repulsive guidance molecule
for retinal axons. Nature. 419:392–395. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oldekamp J, Krämer N, Alvarez-Bolado G and
Skutella T: Expression pattern of the repulsive guidance molecules
RGM A, B and C during mouse development. Gene Expr Patterns.
4:283–288. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hata K, Fujitani M, Yasuda Y, Doya H,
Saito T, Yamagishi S, Mueller BK and Yamashita T: RGMa inhibition
promotes axonal growth and recovery after spinal cord injury. J
Cell Biol. 173:47–58. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang T, Wu X, Yin C, Klebe D, Zhang JH and
Qin X: CRMP-2 is involved in axon growth inhibition induced by RGMa
in vitro and in vivo. Mol Neurobiol. 47:903–913. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fivaz M, Bandara S, Inoue T and Meyer T:
Robust neuronal symmetry breaking by Ras-triggered local positive
feedback. Curr Biol. 18:44–50. 2008. View Article : Google Scholar
|
|
13
|
Hall A and Lalli G: Rho and Ras GTPases in
axon growth, guidance, and branching. Cold Spring Harb Perspect
Biol. 2:a0018182010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Endo M and Yamashita T: Inactivation of
Ras by p120GAP via focal adhesion kinase dephosphorylation mediates
RGMa-induced growth cone collapse. J Neurosci. 29:6649–6662. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Giachello CN, Premoselli F, Montarolo PG
and Ghirardi M: Pentylenetetrazol-induced epileptiform activity
affects basal synaptic transmission and short-term plasticity in
monosynaptic connections. PLoS One. 8:e569682013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ruethrich H, Grecksch G, Becker A and Krug
M: Potentiation effects in the dentate gyrus of
pentylenetetrazol-kindled rats. Physiol Behav. 60:455–462. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Racine RJ: Modification of seizure
activity by electrical stimulation. II. Motor seizure.
Electroencephalogr Clin Neurophysiol. 32:281–294. 1974. View Article : Google Scholar
|
|
18
|
National Institues of Health. Guide for
the care and use of laboratory animals. The National Academies
Press; Washington, DC: pp. 1–127. 1996
|
|
19
|
Holmes GL, Sarkisian M, Ben-Ari Y and
Chevassus-Au-Louis N: Mossy fiber sprouting after recurrent
seizures during early development in rats. J Comp Neurol.
404:537–553. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cavazos JE and Cross DJ: The role of
synaptic reorganization in mesial temporal lobe epilepsy. Epilepsy
Behav. 8:483–493. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zeng LH, Rensing NR and Wong M: The
mammalian target of rapamycin signaling pathway mediates
epileptogenesis in a model of temporal lobe epilepsy. J Neurosci.
29:6964–6972. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Colciaghi, Finardi A, Nobili P, Locatelli
D, Spigolon G and Battaglia GS: Progressive brain damage, synaptic
reorganization and NMDA activation in a model of epileptogenic
cortical dysplasia. PLoS One. 9:e898982014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lew FH and Buckmaster PS: Is there a
critical period for mossy fiber sprouting in a mouse model of
temporal lobe epilepsy. Epilepsia. 52:2326–2332. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nissinen J, Lukasiuk K and Pitkanen A: Is
mossy fiber sprouting present at the time of the first spontaneous
seizures in rat experimental temporal lobe epilepsy? Hippocampus.
11:299–310. 2001. View Article : Google Scholar
|
|
25
|
Yoshida J, Kubo T and Yamashita T:
Inhibition of branching and spine maturation by repulsive guidance
molecule in cultured cortical neurons. Biochem Biophys Res Commun.
372:725–729. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schwab JM, Conrad S, Monnier PP, et al:
Spinal cord injury-induced lesional expression of the repulsive
guidance molecule (RGM). Eur J Neurosci. 21:1569–1576. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schwab JM, Conrad S, Monnier PP, Julien S,
Mueller BK and Schluesener HJ: Central nervous system
injury-induced repulsive guidance molecule expression in the adult
human brain. Arch Neurol. 62:1561–1568. 2005.PubMed/NCBI
|
|
28
|
Feng J, Wang T, Li Q, Wu X and Qin X: RNA
interference against repulsive guidance molecule A improves axon
sprout and neural function recovery of rats after MCAO/reperfusion.
Exp Neurol. 238:235–242. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Capelli LP, Krepischi AC, Gurgel-Giannetti
J, Mendes MF, Rodrigues T, Varela MC, Koiffmann CP and Rosenberg C:
Deletion of the RMGA and CHD2 genes in a child with epilepsy and
mental deficiency. Eur J Med Genet. 55:132–134. 2012. View Article : Google Scholar
|
|
30
|
Brinks H, Conrad S, Vogt J, Oldekamp J,
Sierra A, Deitinghoff L, Bechmann I, Alvarez-Bolado G, Heimrich B,
Monnier PP, Mueller BK and Skutella T: The repulsive guidance
molecule RGMa is involved in the formation of afferent connections
in the dentate gyrus. J Neurosci. 24:3862–3869. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kubo T, Endo M, Hata K, Taniguchi J,
Kitajo K, Tomura S, Yamaguchi A, Mueller BK and Yamashita T: Myosin
IIA is required for neurite outgrowth inhibition produced by
repulsive guidance molecule. J Neurochem. 15:113–126. 2008.
View Article : Google Scholar
|
|
32
|
Conrad S, Genth H, Hofmann F, Just I and
Skutella T: Neogenin-RGMa signaling at the growth cone is bone
morphogenetic protein-independent and involves RhoA, ROCK, and PKC.
J Biol Chem. 282:16423–16433. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang J, Wang YH, Hou YY, Xi T, Liu Y and
Liu JG: The small GTPase RhoA, but not Rac1, is essential for
conditioned aversive memory formation through regulation of actin
rearrangements in rat dorsal hippocampus. Acta Pharmacol Sin.
34:811–818. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Begum R, Nur-E-Kamal MS and Zaman MA: The
role of Rho GTPases in the regulation of the rearrangement of actin
cytoskeleton and cell movement. Exp Mol Med. 36:358–366. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yuan J, Wang LY, Li JM, Cao NJ, Wang L,
Feng GB, Xue T, Lu Y and Wang XF: Altered expression of the small
guanosine triphosphatase RhoA in human temporal lobe epilepsy. J
Mol Neurosci. 42:53–58. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Burrows JF, Kelvin AA, McFarlane C, Burden
RE, McGrattan MJ, De la Vega M, Govender U, Quinn DJ, Dib K, Gadina
M, Scott CJ and Johnston JA: USP17 regulates Ras activation and
cell proliferation by blocking RCE1 activity. J Biol Chem.
284:9587–9595. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Malik NM, Gilroy DW and Kabouridis PS:
Regulation of growth and survival of activated T cells by
cell-transducing inhibitors of Ras. FEBS Lett. 583:61–69. 2009.
View Article : Google Scholar :
|
|
38
|
Scita G, Tenca P, Frittoli E, Tocchetti A,
Innocenti M, Giardina G and Di Fiore PP: Signaling from Ras to Rac
and beyond: not just a matter of GEFs. EMBO J. 19:2393–2398. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Contestabile A, Bonanomi D, Burgaya F,
Girault JA and Valtorta F: Localization of focal adhesion kinase
isoforms in cells of the central nervous system. Int J Dev
Neurosci. 21:83–93. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chacón MR, Fernández G and Rico B: Focal
adhesion kinase functions downstream of Sema3A signaling during
axonal remodelling. Mol Cell Neurosci. 44:30–42. 2010. View Article : Google Scholar
|
|
41
|
Woo S, Rowan DJ and Gomez TM: Retinotopic
mapping requires focal adhesion kinase-mediated regulation of
growth cone adhesion. J Neurosci. 29:13981–13991. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Parsons JT: Focal adhesion kinase: the
first ten years. J Cell Sci. 116:1409–1416. 2003. View Article : Google Scholar : PubMed/NCBI
|