Introduction
Cardiac hypertrophy is a pathology associated with
numerous heart conditions and contributes to increased morbidity
and mortality in patients (1,2). As
an adaptive response and a crucial compensatory mechanism to a
variety of intrinsic and extrinsic stimuli (3,4),
cardiac remodeling is a complex process involving numerous nervous
and signaling pathway activities (5,6). A
change in cardiac structure ultimately leads to changes in cardiac
function, which can result in coronary heart disease, congestive
heart failure, ventricular arrhythmia or sudden mortality (7).
Connexin43 (Cx43) is one of the major gap junction
(GJ) proteins and is distributed in the intercalated disc of the
myocardium. Cx43 mediates the cell-to-cell movement of ions,
metabolites and cell signaling molecules and plays an important
role in providing the basis for the electrical syncytial properties
of the heart (8). The reduction
and redistribution of Cx43 in the hypertrophic myocardium have been
implicated in the pathogenesis of ventricular arrhythmias (9).
Mitochondrial ATP-sensitive K+
(mitoKATP) channels are one of the factors involved in
the regulation of GJs. Activation of the KATP channel
inhibits GJ permeability and subsequently attenuates arrhythmias in
the acute ischemic myocardium (10). Numerous studies have demonstrated
the protective effect of Cx43 against ischemic injury by the
administration of a KATP channel agonist (11–13).
However, no favorable effects have been shown in transgenic animals
with a Cx43 deficiency (10).
Although KATP channel agonists provide beneficial
effects against ischemia and arrhythmia, it remains unclear whether
similar benefits can be observed by modulating Cx43 expression in
chronic cardiac hypertrophy. Therefore, the aims of the present
study were to investigate whether KATP channel agonist
administration attenuated isoproterenol (Iso)-induced cardiac
hypertrophy and modulated the expression of Cx43 in the
myocardium.
Materials and methods
Animals
Male Sprague Dawley rats were obtained from the
Experimental Animal Center of Anhui Province (Hefei, China). All
animals used in this study were maintained according to the Guide
for the Care and Use of Laboratory Animals (National Institutes of
Health publication No. 85-23, revised 1996). This study was
approved by the ethics committee of the Experimental Animal Center
of Anhui Province.
Rats were acclimated for one week prior to being
randomly divided into a normal control group and four cardiac
hypertrophy groups. The cardiac hypertrophy model was established
in rats by subcutaneous injection of Iso (5 mg/kg/day; Shanghai
Harvest Pharmaceutical Co., Ltd., Shanghai, China) for seven days.
The groups were treated as follows: i) Normal, received saline
injection and saline by gastric gavage; ii) vehicle, received Iso
injection and orally administered saline; iii) nicorandil, received
Iso injection and orally administered nicorandil (a specific
mitochondrial KATP channel agonist; Chugai
Pharmaceutical Co., Tokyo, Japan); iv) glibenclamide (Tianjin
Pacific Pharmaceutical Co., Ltd., Tianjin, China), received Iso
injection and orally administered glibenclamide (a KATP
channel blocker); v) nicorandil plus glibenclamide, received Iso
injection and a co-administration of nicorandil and glibenclamide.
Nicorandil and glibenclamide were administered orally by gastric
gavage at doses of 5 mg/kg/day, while the same volume of 0.9%
saline was administered in an identical manner. To prevent
hypoglycemic attacks during the administration of glibenclamide,
2.5% (wt/vol) sucrose in filtered water was supplied. Throughout
the study, rats were fed ad libitum. Glucose examinations
were performed once a week by the OneTouch® method
(OneTouch Ultra; Johnson & Johnson, New Brunswick, NJ, USA).
Body weight (BW) was measured every three days in order to adjust
the drug dosage.
ELISA
Following the final drug administration, rats were
fasted for 12 h and then weighed using an electronic balance. The
rats were subsequently sacrificed by cervical dislocation and blood
samples were collected from the abdominal aorta. The plasma was
harvested by centrifugation and B-type natriuretic peptide (BNP)
concentration was measured using a commercial ELISA kit (Shanghai
DoBio Biotech Co. Ltd., Shanghai, China) according to the
manufacturer’s instructions. Standard points and samples were
performed in either duplicate or triplicate.
Assessment of myocardial hypertrophy
The hearts were isolated following blood collection
to evaluate their mass. The pericardium and large vessels were
first removed, then washed with saline and dried on filter paper.
Total heart weight (HW) and left ventricular weight (LVW) were
determined using an electronic balance. The interventricular septum
remained as a part of the left ventricle (LV). The ratios of HW/BW
and LVW/BW were then calculated. Following the measurements, the LV
was sectioned into two slices for subsequent experiments.
Reverse transcription-polymerase chain
reaction (RT-PCR)
To investigate the mRNA expression of myocardial
Cx43, the myocardia were ground on ice and total RNA was extracted
using TRIzol® reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA). Total RNA concentration was determined and
reverse transcription was performed to obtain cDNA using a
RevertAid First Strand cDNA Synthesis kit (Thermo Fisher Scientific
Inc., Waltham, MA, USA). Gene-specific primers were designed using
Primer Premier 5 software (Premier Biosoft International, Palo
Alto, CA, USA) based on cDNA sequences from Genebank (https://www.ncbi.nlm.nih.gov/genbank/).
For Cx43, the primers were as follows: 5′-AAC CTA CAT CAT CAG CAT
CC-3′ (sense) and 5′-TGA TGA AGA TGG TTT TCT CC-3′ (antisense). For
GAPDH, the primers were: 5′-CAA GGT CAT CCA TGA CAA CAA CTT TG-3′
(sense) and 5′-GTC CAC CAC CCT GTT GCT GTA G-3′ (antisense). The
PCR products were separated by electrophoresis in 1.0% (w/v)
agarose gels, visualized by staining with ethidium bromide and
imaged using a UVP gel imaging system (JD-801; Jieda, Nanjing,
China). The mRNA expression level of Cx43 was normalized to that of
the reference mRNA, GAPDH.
Immunohistochemistry
To further detect the spatial distribution and
relative density of Cx43, immunohistochemical staining was
performed on the myocardium of the LV. Samples were fixed in
formalin prior to being embedded in paraffin. Sections, cut at 5
μm, were deparaffinized with xylene and rehydrated through an
ethanol series. The sections were then blocked with goat serum for
30 min and incubated with a rabbit polyclonal anti-Cx43 antibody
(Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at 4°C
overnight. The coverslips were washed three times with
phosphate-buffered saline for 5 min/wash and then incubated with a
fluorescent secondary antibody (Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China) at 37°C for 30 min. Ten
random fields from each section were visualized and imaged using an
Olympus BX51 microscope (Olympus Corp., Tokyo, Japan).
Statistical analyses
Data are presented as the mean ± standard deviation.
Statistical analyses were performed using GraphPad Prism 5.0
(GraphPad Software, Inc., La Jolla, CA, USA). Analysis between two
groups was performed using an unpaired Student’s t-test. A value of
P<0.05 was considered to indicate a statistically significant
difference between groups.
Results
General characteristics of rats following
hypertrophy induction and drug administration
Following Iso injection and drug administration,
rats in the four myocardium hypertrophy groups became less active
and exhibited a decrease in appetite, accompanied by a slow gain in
body weight. At the end of the experiment, six rats died, two in
both the vehicle and nicorandil plus glibenclamide groups, one in
both the nicorandil and the glibenclamide groups and none in the
control group. Hypoglycemia was not detected in any of the rats
throughout the experiment.
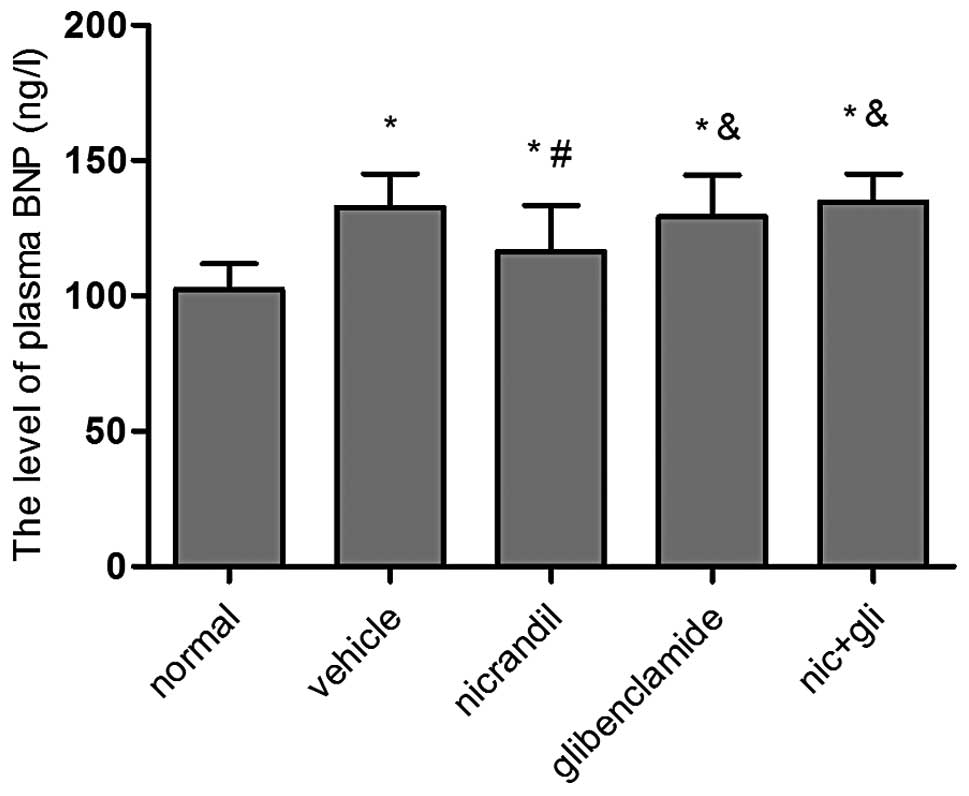
Nicorandil suppresses increases in plasma
BNP during cardiac hypertrophy
Cardiac hypertrophy is commonly associated with an
increased production of ventricular BNP (14). To evaluate the BNP expression level
following treatment, the plasma BNP concentrations at the end of
the experiment were assessed by ELISA. The data revealed that
plasma BNP levels were markedly higher in the vehicle group
(132.52±11.36) than those in the normal group (102.78±9.69)
(P<0.01). This increase was significantly suppressed in rats
treated with nicorandil compared with those treated with saline
(118.71±31.12 vs. 132.52±11.36) (P<0.05). However, plasma BNP
levels in the glibenclamide and co-administration groups were not
significantly different compared with those in the vehicle group
(P>0.05) (Fig. 1).
Nicorandil inhibits Iso-induced LV
hypertrophy
Hypertrophy was documented by determination of the
LVW/BW ratio. The BW of rats in all groups increased significantly
from the beginning of the experiment to the end of the
administration period (P<0.01 for each). However, the BW of the
rats in the four Iso-injection groups was significantly lower
relative to that of the rats in the normal control group. In
addition, the HW/BW and LVW/BW ratios were significantly higher in
the four treatment groups in comparison with those in the control
animals. Compared with the increase induced by Iso in the vehicle
group, the HW/BW and LVW/BW ratios of rats were significantly lower
in the nicorandil group (P<0.05). However, this effect was
eliminated by co-administration of nicorandil with glibenclamide.
The HW/BW and LVW/BW ratios in the glibenclamide group and the
combination group were not significantly different from those in
the saline-administered hypertrophy group (P>0.05) (Table I).
 | Table IHW/BW and LVW/BW values for rats in
each experimental group |
Table I
HW/BW and LVW/BW values for rats in
each experimental group
| Group | HW/BW (mg/g) | LVW/BW (mg/g) |
|---|
| Normal | 2.39±0.16 | 1.84±0.12 |
| Vehicle | 3.09±0.24a | 2.37±0.26a |
| Nic | 2.67±0.25a,b | 1.98±0.22a,b |
| Gli | 3.16±0.32a,c | 2.42±0.25a,c |
| Nic+Gli | 3.08±0.21a,c | 2.39±0.36a,c |
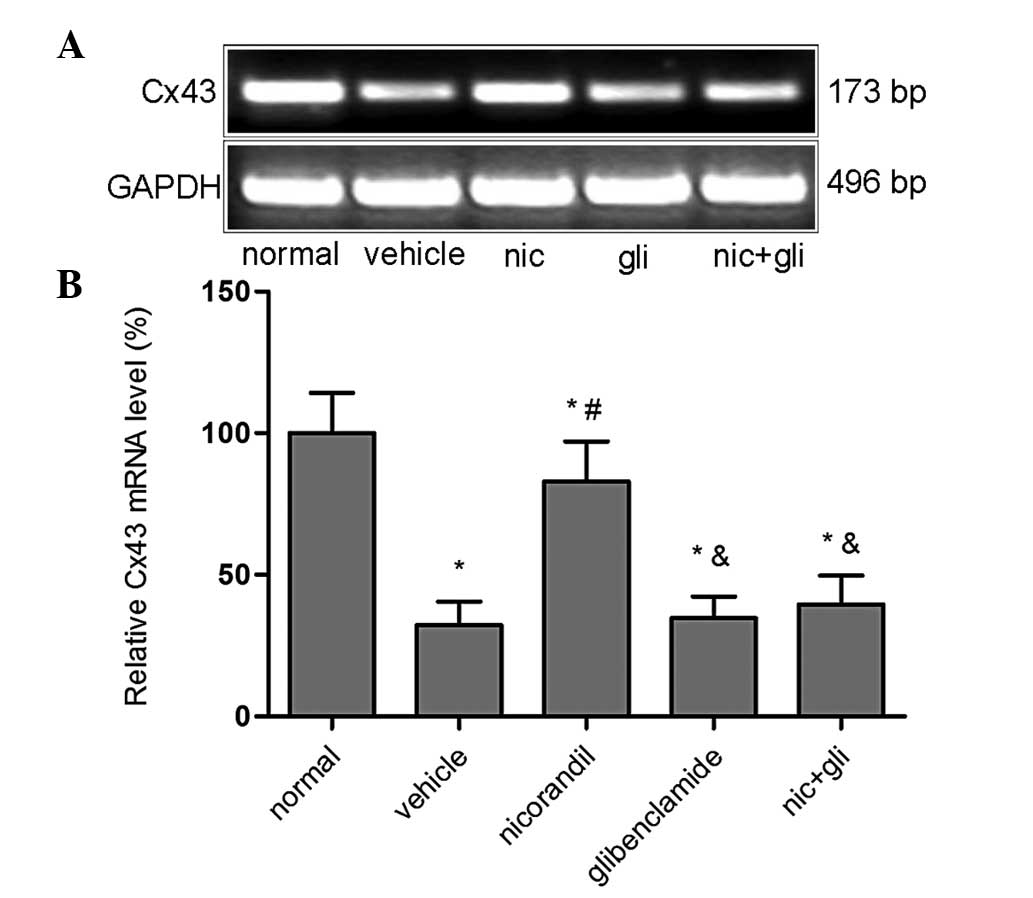
Cx43 mRNA expression
The expression of Cx43 mRNA was detected by RT-PCR
(Fig. 2). PCR amplification of the
cDNA revealed that the Cx43 mRNA levels in the LV of the nicorandil
group exhibited a significant increase compared with those in the
vehicle group (82.99±14.10 vs. 32.33±8.18%; P<0.01); however,
they remained lower than those in the normal group (P<0.05). The
increased Cx43 mRNA level, which was mediated by the
KATP channel agonist nicorandil, was reversed to levels
similar to those of the vehicle group following administration of
the blocker glibenclamide. This was clearly shown in the groups
administered glibenclamide (34.67±7.61%) or glibenclamide plus
nicorandil (39.53±10.20%), where the levels of Cx43 mRNA were
significantly decreased compared with those in the nicorandil group
(82.99±14.10%) (P<0.01) and were reduced to the level of
saline-treated hypertrophic rats. The relative Cx43 mRNA was
expressed by setting the normal group as 100%.
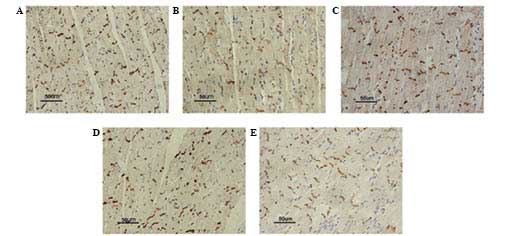
Immunohistochemical analyses of Cx43
To further investigate the spatial distribution and
expression of Cx43, immunohistochemical analysis was performed in
LV tissue sections. In the longitudinal axis of the myocardium
fibers, myocardial Cx43 was distributed in the form of a stripe in
the intercalated disc, stained brown-yellow, in the normal group.
Hypertrophy resulted in markedly diminished expression of Cx43 as
well as spatial redistribution. Immunohistochemical images showed
that the positively stained areas were different in size and color
intensity compared with those of the normal myocardium, and some
Cx43 expression was redistributed from the intercalated disc to the
entire cell membrane (Fig. 3).
Compared with the vehicle group, the expression of Cx43 in the
myocardium in the nicorandil group was significantly increased, and
the majority of Cx43 expression was confined to the intercalated
disc. Conversely, following co-administration of nicorandil plus
glibenclamide, Cx43 expression was reduced and the distribution
remained irregular; similar results were observed in the group
treated with glibenclamide alone.
Discussion
This study demonstrated that long-term oral
treatment with the KATP channel agonist nicorandil for
four weeks could prevent the development of LV remodeling and
preserve myocardial Cx43 expression levels and distribution in
Iso-induced rat models of myocardial hypertrophy. The beneficial
effects of nicorandil were eliminated following the administration
of the KATP channel blocker glibenclamide. It was
observed that the mass of the hypertrophic myocardium and the
expression of Cx43 in the glibenclamide and co-treatment groups
exhibited similarities to hearts in the saline-treated hypertrophy
group, which further confirmed the crucial role of KATP
channel agonists in the reduction of hypertrophy and protection of
myocardial Cx43 expression.
Iso-induced hypertrophy is an accessible and
standardized model to study the effects of various drugs on cardiac
hypertrophy (15). The duration of
the present experiment was set at four weeks, since the majority of
the myocardial remodeling in the rat (70–80%) occurs within three
weeks (16). Several factors that
may affect hypertrophy or Cx43 expression had to be excluded, for
example hemodynamics. Previous studies have demonstrated that an
oral dosage of nicorandil (5 mg/kg/day) does not exert any
hemodynamic effects (5), but the
reduction of hypertrophy in animals has been previously assumed to
be due to the blood pressure-lowing effect of the drug (17). In addition, the effect of insulin
levels had to be excluded. Lee et al (18) reported that Cx43 expression in
infarcted rats administered nicorandil was markedly upregulated
compared with the expression in those treated with vehicle.
However, the insulin levels in these two groups were not
significantly different, suggesting that insulin levels were not a
factor affecting the expression of Cx43 (18).
As a predictor of myocardial hypertrophy and heart
failure, BNP levels increase in the early stages of the disease and
are strongly associated with the incidence of cardiovascular
mortality in patients with coronary artery disease (19). The Task Force for the Diagnosis and
Treatment of Chronic Heart Failure of the European Society of
Cardiology recommended BNP or N-terminal-proBNP assays as a
necessary step for the diagnosis of heart failure or LV dysfunction
(20). In the present study, the
increased concentration of plasma BNP in rats with cardiac
hypertrophy was attenuated following treatment with nicorandil
compared with those treated with saline. By contrast, the plasma
BNP concentration showed no statistically significant differences
following the administration of glibenclamide or glibenclamide plus
nicorandil as compared with the vehicle group. These results
indicated the crucial role of the KATP channel agonist
in protecting the heart against hypertrophic injury, in part by
decreasing BNP expression levels. In addition, a previous clinical
study reported that nicorandil treatment may reduce the BNP
expression levels by reducing the central blood pressure in
patients with chronic kidney disease (21).
The HW/BW and LVW/BW ratios were used to reflect the
degree of cardiac hypertrophy. A ratio of LVW/BW >2.35 mg/g was
regarded as an indicator of a successful hypertrophy model. In the
vehicle group, the ratio of LVW/BW was 2.37±0.26, which confirmed
the success of hypertrophy induction by Iso injection. Similar to
the aforementioned changes in BNP level, HW/BW and LVW/BW ratios
were significantly decreased following treatment with the
KATP channel agonist nicorandil. The reduction in the
LVW/BW and HW/BW ratios was reversed following administration of
nicorandil, thus verifying the effect of KATP against
cardiac hypertrophy.
The mechanical and electrical activation of the
heart is in part dependent on the spatial distribution of GJs
(22). Changes in GJ patterning
and expression level can result in the development of arrhythmias
in numerous cardiovascular diseases (23–25).
The alteration in the expression level and spatial distribution of
Cx43 in the hypertrophic myocardium suggests that this protein is
influenced by ventricular remodeling (18). Naitoh et al (26) proposed that the mitoKATP
channel was one of the mechanisms regulating GJ permeability, thus
leading to cardioprotective effects against ischemia. Previous
studies have reported that decreasing Cx43 expression in the
myocardium results in high susceptibility to arrythmogenesis
(18,25). However, the restoration of
expression levels and distribution of Cx43 improves conductivity,
decreases the spatial heterogeneity of repolarization and reduces
the susceptibility of the remodeled heart to fatal arrhythmias
(27). The beneficial effects of
the KATP channel agonist on Cx43 observed in this study
and the elimination of this effect following the addition of the
KATP channel blocker together confirmed the crucial role
of KATP channel agonists in maintaining normal Cx43
expression.
As a nitrate-like KATP channel agonist,
nicorandil has been widely used in Japan, Europe and Korea.
Clinical reports from Japan and Europe have shown that nicorandil
has equivalent anti-angina effects to nitrates, calcium-channel
blockers and β-blockers (28–30).
A double-blind, multicenter, active-controlled, randomized clinical
trial previously assessed the safety and efficacy of nicorandil in
patients with stable angina pectoris (AP) in China, suggesting that
nicorandil is an effective drug for AP (31). Numerous clinical and experimental
studies in non-hypertrophied myocardium have demonstrated a
protective effect of nicorandil against ischemic injury through
activation of the potassium channel (32–34).
Sakai (17) reported that
nicorandil exerted blood pressure-lowing effects, which
subsequently reduced cardiac hypertrophy in animals (17). Regardless of the precise mechanism
of nicorandil in cardiovascular diseases, our previous study also
demonstrated the protective effects of nicorandil against
myocardial ischemia-reperfusion injury, which were due in part to
the opening of mitoKATP channels (13). It was found in the present study
that long-term oral administration of nicorandil is beneficial for
the reduction of cardiac hypertrophy and modulation of myocardial
Cx43 in Iso-induced rat models of cardiac hypertrophy. Since
nicorandil is expected to have a wider range of clinical use in
cardiovascular diseases, other potential mechanisms remain to be
further investigated.
Acknowledgements
This study was supported by a research grant from
the Research Grants Council of Anhui Province (Project nos.
11040606M155 and 2012jyzd09w).
References
|
1
|
Weber KT, Anversa P, Armstrong PW, et al:
Remodeling and reparation of the cardiovascular system. J Am Coll
Cardiol. 20:3–16. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Verdecchia P, Carini G and Circo A; MAVI
(MAssa Ventricolare sinistra nell'Ipertensione) Study Group. Left
ventricular mass and cardiovascular morbidity in essential
hypertension: the MAVI study. J Am Coll Cardiol. 38:1829–1835.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choudhary R, Mishra KP and Subramanyam C:
Prevention of isoproterenol-induced cardiac hypertrophy by eugenol,
an antioxidant. Indian J Clin Biochem. 21:107–113. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rajesh KG, Sasaguri S, Suzuki R, Xing Y
and Maeda H: Ischemic preconditioning prevents reperfusion heart
injury in cardiac hypertrophy by activation of mitochondrial KATP
channels. Int J Cardiol. 96:41–49. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee TM, Lin MS and Chang NC: Effect of
ATP-sensitive potassium channel agonists on ventricular remodeling
in healed rat infarcts. J Am Coll Cardio. 51:1309–1318. 2008.
View Article : Google Scholar
|
|
6
|
Ennis IL, Escudero EM, Console GM, et al:
Regression of isoproterenol-induced cardiac hypertrophy by
Na+/H+ exchanger inhibition. Hypertension.
41:1324–1329. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Frohlich ED: State of the Art lecture.
Risk mechanisms in hypertensive heart disease. Hypertension.
34:782–789. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Joyner RW: Effects of the discrete pattern
of electrical coupling on propagation through an electrical
syncytium. Circ Res. 50:192–200. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Danik SB, Liu F, Zhang J, Suk HJ, Morley
GE, Fishman GI and Gutstein DE: Modulation of cardiac gap junction
expression and arrhythmic susceptibility. Circ Res. 95:1035–1041.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boengler K, Dodoni G, Rodriguez-Sinovas A,
et al: Connexin 43 in cardiomyocyte mitochondria and its increase
by ischemic preconditioning. Cardiovasc Res. 67:234–244. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roell W, Lewalter T, Sasse P, et al:
Engraftment of connexin 43-expressing cells prevents post-infarct
arrhythmia. Nature. 450:819–824. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rottlaender D, Boengler K, Wolny M, et al:
Connexin 43 acts as a cytoprotective mediator of signal
transduction by stimulating mitochondrial KATP channels in mouse
cardiomyocytes. J Clin Invest. 120:1441–1453. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang A, Chen F, Xie Y, Guo Z and Yu Y:
Protective mechanism of nicorandil on rat myocardial
ischemia-reperfusion. J Cardiovasc Med (Hagerstown). 13:511–515.
2012. View Article : Google Scholar
|
|
14
|
de Lemos JA, McGuire DK and Drazner MH:
B-type natriuretic peptide in cardiovascular disease. Lancet.
362:316–322. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang Y, Wang M, Le X, et al: Antioxidant
and cardioprotective effects of Danshensu
(3-(3,4-dihydroxyphenyl)-2-hydroxy-propanoic acid from Salvia
miltiorrhiza) on isoproterenol-induced myocardial hypertrophy in
rats. Phytomedicine. 18:1024–1030. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pfeffer MA and Braunwald E: Ventricular
remodeling after myocardial infarction. Experimental observations
and clinical implications. Circulation. 81:1161–1172. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sakai K: Nicorandil: animal pharmacology.
Am J Cardiol. 63:2J–10J. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee TM, Lin CC, Lien HY and Chen CC: K ATP
channel agonists preserve connexin43 protein in infarcted rats by a
protein kinase C-dependent pathway. J Cell Mol Med. 16:776–788.
2012. View Article : Google Scholar
|
|
19
|
Guéant Rodriguez RM, Spada R, Pooya S, et
al: Homocysteine predicts increased NT-pro-BNP through impaired
fatty acid oxidation. Int J Cardiol. 167:768–775. 2013. View Article : Google Scholar
|
|
20
|
Swedberg K, Cleland J, Dargie H, et al:
Task Force for the Diagnosis and Treatment of Chronic Heart Failure
of the European Society of Cardiology: Guidelines for the diagnosis
and treatment of chronic heart failure: executive summary (update
2005): The Task Force for the Diagnosis and Treatment of Chronic
Heart Failure of the European Society of Cardiology. Eur Heart J.
26:1115–1140. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kimura T, Kitamura H, Inoue K, et al:
Effects of nicorandil on the reduction of BNP levels in patients
with chronic kidney disease. Clin Exp Nephrol. 15:854–860. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kanno S and Saffitz JE: The role of
myocardial gap junctions in electrical conduction and
arrhythmogenesis. Cardiovasc Pathol. 10:169–177. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Egashira K, Nishii K, Nakamura K, Kumai M,
Morimoto S and Shibata Y: Conduction abnormality in gap junction
protein connexin45-deficient embryonic stem cell-derived cardiac
myocytes. Anat Rec A Discov Mol Cell Evol Biol. 280:973–979. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y and Hill JA: Electrophysiological
remodeling in heart failure. J Mol Cell Cardiol. 48:619–632. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ohara T, Ohara K, Cao JM, et al: Increased
wave break during ventricular fibrillation in the epicardial border
zone of hearts with healed myocardial infarction. Circulation.
103:1465–1472. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Naitoh K, Ichikawa Y, Miura T, et al:
MitoKATP channel activation suppresses gap junction
permeability in the ischemic myocardium by an ERK-dependent
mechanism. Cardiovasc Res. 70:374–383. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Amino M, Yoshioka K, Tanabe T, et al:
Heavy ion radiation up-regulates Cx43 and ameliorates
arrhythmogenic substrates in hearts after myocardial infarction.
Cardiovasc Res. 72:412–421. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Döring G: Antianginal and anti-ischemic
efficacy of nicorandil in comparison with isosorbide-5-mononitrate
and isosorbide dinitrate: results from two multicenter,
double-blind, randomized studies with stable coronary heart disease
patients. J Cardiovasc Pharmacol. 20(Suppl 3): S74–S81. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
The SWAN Study Group. Comparison of the
antiischaemic and antianginal effects of nicorandil and amlodipine
in patients with symptomatic stable angina pectoris: the SWAN
study. J Clin Basic Cardiol. 2:213–217. 1999.
|
|
30
|
Di Somma S, Liguori V, Petitto M,
Carotenuto A, Bokor D, de Divitiis O and de Divitiis M: A
double-blind comparison of nicorandil and metoprolol in stable
effort angina pectoris. Cardiovasc Drugs Ther. 7:119–123. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu WL, Shan YD, Guo JX, et al:
Double-blind, multicenter, active-controlled, randomized clinical
trial to assess the safety and efficacy of orally administered
nicorandil in patients with stable angina pectoris in China. Circ
J. 71:826–833. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Baines CP, Liu GS, Birincioglu M, Critz
SD, Cohen MV and Downey JM: Ischemic preconditioning depends on
interaction between mitochondrial KATP channels and actin
cytoskeleton. Am J Physiol. 276:H1361–H1368. 1999.PubMed/NCBI
|
|
33
|
Sugimoto K, Ito H, Iwakura K, et al:
Intravenous nicorandil in conjunction with coronary reperfusion
therapy is associated with better clinical and functional outcomes
in patients with acute myocardial infarction. Circ J. 67:295–300.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Garlid KD, Paucek P, Yarov-Yarovoy V, et
al: Cardioprotective effect of diazoxide and its interaction with
mitochondrial ATP-sensitive K+ channels. Possible
mechanism of cardioprotection. Circ Res. 81:1072–1082. 1997.
View Article : Google Scholar : PubMed/NCBI
|