Introduction
The incidence of colorectal cancer has risen
steadily and currently ranks among the top three types of cancers
affecting males and females in Europe (1). As of 2004, in China, the mortality
rate of colorectal cancer was 5.29/100,000 in males and
3.86/100,000 in females, placing it among the sixth most
life-threatening types of cancer affecting the Chinese population
(2). Currently, a combination of
surgery, radiotherapy and chemotherapy is used to treat colorectal
cancer (3).
There is increasing evidence that Chinese herbal
medicine may assist in treating certain types of cancer. Herbal
medicine has been found to inhibit the growth of tumor cells,
improve disease symptoms and reduce the side-effects of
radiotherapy and chemotherapy. Studies of antitumor herbal medicine
have revealed a variety of effects on cells, including the
inhibition of tumor cell proliferation, promotion of apoptosis and
alterations in the expression of tumor-associated genes. Lingqi
capsules (LQC), which include Ganoderma lucidum,
Astragalus membranaceus, ginseng and Pseudobulbus Cremastrae
seu Pleiones are a compound preparation, which are intended to
tonify qi, nourish yin, eliminate pathogens, detoxify and disperse
masses (4–5). Treatment with LQC has been shown to
inhibit the proliferation and growth of lung adenocarcinoma cells
and tumor metastasis (6–8). Furthermore, the pathogenetic
mechanism of tumors is that the number of apoptotic cells is
insufficient to control the excessive proliferation of abnormal
cells (9,10). In the present study, the colorectal
cancer cell line, LoVo, was used to investigate the effect and
mechanism of LQC on tumor growth in vitro and in vivo
in an animal model.
Materials and methods
Materials
LQCs were produced by the Drug Manufacturing Room of
the First Affiliated Hospital of Harbin Medical University (Harbin,
China). Each capsule contained 0.4 g Astragalus
membranaceus, 0. 3g Ganoderma lucidum, 0.2 g Curcuma
zedoaria, 0.2 g Poria cocos, 0.15 g ginseng, 0.15 g
Cyrtomium fortune, 0.1 g Chinese yam, 0.05 g fruit of the
Chinese wolfberry, 0.05 g Paris polyphylla and 0.05 g
Pseudobulbus Cremastrae seu Pleiones, dissolved in saline. The
Wistar rats and nude mice were provided by the Drug Safety
Evaluation Center of Heilongjiang University of Traditional Chinese
Medicine (Heilongjiang, China). The LoVo colorectal cancer cells
were provided by the Provincial Cancer Institute of Heilongjiang
(Heilongjiang, China).
Materials and methods
Preparation of LQC serum
Animal welfare and experimental procedures were
performed in strict accordance with animal welfare and other
related ethical regulations, and approved by the Institutional
Animal Care Committee of Drug Safety Evaluation Center at
Heilongjiang University of Chinese Medicine (Harbin, China). Wistar
rats were randomly divided into five groups with four individuals
in each: the low-, medium- and high-dose LQC groups, the negative
control group and the positive control group, treated with
cyclophosphamide tablets (Haizheng Pharmaceutical Co., Taizhou,
China). The rats in the low-, medium- and high-dose LQC groups were
gavaged with 8.91, 17.82 and 35.64 g/kg/day LQC, respectively. The
rats in the negative control group received saline. Animals in the
positive control group received 0.027 g/kg/day cyclophosphamide.
The rats were gavaged continuously for 7 days and blood samples
were obtained from the inferior vena cava 2 h after the final
gavage. The LQC serum was separated using 0.22-μm microporous
filter sterilization and the processed samples were stored in a
refrigerator at −20°C.
LQC treatment of cultured cells
The LoVo cells were trypsinized and counted during
the logarithmic growth phase. Subsequently, 1×105
cells/ml were inoculated on a 6-well plate and cultured overnight
in 7.5% CO2. Following cell adherence, 50 μl serum
containing low-, medium- or high-dose LQC was added to the cells.
The positive control group received serum from the rats treated
with cyclophosphamide and the negative control group received serum
from rats treated with an equal volume of saline. The cells were
then incubated for 24, 48 and 72 h, at 37°C in 5% CO2.
The cell morphology and the quantity, color and transparency of the
culture medium, as well as adhesion, adherence, stretching, moving
and other activities of the cells were observed under an inverted
microscope (Olympus CKX31; Olympus Corporation, Tokyo, Japan).
Detection of LoVo cell proliferation by
MTT assay
Cells in the logarithmic growth phase were collected
and inoculated into a 96-well plate (100 μl of 1×105
cells/well) and were then cultured overnight in an incubator at
37°C and 5% CO2 (volume fraction). RPMI-1640 (50 μl) was
added to each well, while serum from the rats treated with
cyclophosphamide tablets was added to the positive control group
and an equal volume of serum from the saline-treated rats was added
to the negative control group. Each group had six parallel wells.
The cells were cultured for 24, 48 and 72 h, at 37°C in 5%
CO2, following addition of the serum. MTT (20 μl; 5
mg/ml) was then added to each well and the medium was discarded
after 4 h. Dimethyl sulfoxide (150 μl) was added to each well to
terminate the reaction and decoloration and agitation were applied
for 10 min. Optical absorbance was determined at 490 nm using a
microplate reader (450 SN11693; Bio-Rad, Hercules, CA, USA) and the
rate of cell growth inhibition (%) was calculated using the
following formula: 1 − (absorbance value of the experimental
group/absorbance value of the negative control group) × 100.
Tumor modeling in nude mice
The nude mice were randomly divided into five
groups: low-, medium- and high-dose LQC groups (1.25, 2.5 and 5.0
g/kg, respectively), a negative control group (0.2 ml normal
saline) and a positive control group (39 mg/kg cyclophosphamide),
with four mice in each group. The nude mice were subcutaneously
inoculated in the armpit of the right front limb with 0.2 ml LoVo
cells following routine skin disinfection. Treatment began 24 h
after inoculation and the mice in the five groups were gavaged
continuously for 21 days. The mice were sacrificed through orbital
venous blood drawing on the second day following drug
administration (day 22). The skin from the surgical sites was then
sterilized using alcohol and stripped of tumor nodules. Fibrous and
necrotic tissue was then removed and weighed on an analytical
balance.
Detection of apoptosis in the
tumor-bearing mice
The tumor tissues were cut on an ice tray using
ophthalmic scissors, placed on a 300-mesh copper grid, washed with
phosphate-buffered saline (PBS) solution and prepared as a single
cell suspension by rubbing the grid. The precipitate was removed
and the concentration of the cells was adjusted to 2×106
cells/ml. A 1-ml sample of the cell suspension was added to 1.6 ml
RNase (1 mg/ml) and incubated at 37°C in a water bath for 30 min.
Following centrifugation AT 2,3000 × g for 10 min, the precipitate
was dissolved in 0.4 ml PBS. Subsequently, 200 μl propidium iodide
(PI; Sigma, St. Louis, MO, USA) dye containing 200 mg/ml PI, 1%
Triton, 0.9% NaCl and 50 mg/ml RNase) were added at 4°C and the
cells were stained for 30 min. Detection was performed using flow
cytometry at 488 nm excitation (BD FACSAria III, BD Biosciences,
Franklin Lakes, NJ, USA). The number of PI-positive cells was
automatically determined using computer software (CellQuest, BD
Biosciences). A DNA content distribution diagram was generated to
determine the apoptotic rate.
Detection of the protein expression of
HGF and c-Met by western blotting
Tumor tissue blocks (10 mg) were placed in a
homogenizer (Bio-Gen PRO200, PRO Scientific, Oxford, CT, USA)and
cut into small pieces using clean tissue scissors. Single detergent
lysates (400 μl; 50 mM Tris (pH 7.4), 150 mM NaCl, 1% NP-40, 0.5%
sodium deoxycholate) were added for homogenization and the
homogenizer was placed on ice to repeatedly grind the tissue. After
30 min, the lysate was transferred to 1.5-ml Eppendorf tubes and
centrifuged at 12,000 rpm at 4°C for 5 min. The supernatant was
collected by centrifugation at 13,400 × g, and sub-packaged in
0.5-ml tubes stored at −20°C. β-actin (anti-β-actin antibody, Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA) was used as the
internal reference. The proteins were separated by 5% SDS-PAGE and
then electrophoretically transferred onto polyvinylidene difluoride
(PVDF) membranes (Pall Corporation, Port Washington, NY, USA). The
PVDF membrane was sealed using confining liquid [5% skim milk and
TBST (20 mM Tris-HCl, pH 7.4 (25°C), 150 mM NaCl and 0.05%
Tween®-20)] for 1 h and mouse anti-human monoclonal
antibodies against HGF or c-Met were added (1:100; Sigma) prior to
the membrane being placed on an agitator overnight at 4°C.
Horseradish peroxidase-labeled goat anti-mouse immunoglobulin G
polyclonal antibody was used as the secondary antibody (1:1,000;
Sigma), which was added prior to incubation of the membrane at 37°C
for 2 h. Following washing with Tris-buffered saline with Tween,
chemiluminescence agent (Thermo SuperSingle West Femto Trial kit;
Thermo Fisher Scientific, Waltham, MA, USA) was added and the
protein expression levels were determined by
electrochemiluminescence detection. Image-Pro Plus software (Media
Cybernetics, Inc., Bethesda, MD, USA) was used to calculate the
average gray values of the HGF and c-Met proteins relative to that
of β-actin.
Detection of the mRNA expression of HGF
and c-Met by reverse transcription quantitative polymerase chain
reaction (RT-qPCR)
Liquid nitrogen and a mortar were used to grind 1.0
g tumor tissue for RNA extraction using TRIzol reagent (Invitrogen
Life Technologies, Carlsbad, CA, USA). A spectrophotometer (Solid
Spec-3700DUV; Shimadzu Corporation, Kyoto, Japan)was then used to
detect the concentration of total RNA. Based on the published
sequences in GenBank, the following primers were used: HGF,
forward 5′-AATGCAGCCAGCATCCATCG-3′ and reverse
5′-CCACCATTATCCCCCTCACAT-3′ and c-MET, forward 5′-ACCTCAGCAA
TGTCAGCACCA-3′ and reverse 5′-GGCCATGTGATGTCATTCTGG-3′. RT-qPCR was
performed using PrimeScript RT kits and the RT-qPCR reaction liquid
was prepared according to the manufacturer’s instructions (Takara
Bio, Inc., Shiga, Japan). The RT-qPCR reaction conditions were as
follows: RT at 37°C for 15 min and 85°C for 15 sec. The cDNA
samples were obtained and the template replaced with sterile
deionized water as a negative control. The qPCR reaction liquid was
prepared on ice according to the SYBR Premix Ex Taq kit
instructions (Takara Bio, Inc.). The qPCR reaction was performed as
follows: denaturing at 95°C for 10 sec, 9°C for 5 sec, 60°C for 34
sec and amplifying to 40 cycles followed by collecting signals at
60°C for 34 sec. SYBR melting curve analysis was performed using
the RT-qPCR products, with all samples set in triplicate, and
sterile deionized water was used to replace the template as a
negative control. The average Ct value of the X gene and the
β-actin gene of the replicate samples was obtained and the
reference gene β-actin was used to correct the average Ct value of
the genes in the untreated drug samples and treated drug samples.
The calculations used were as follows: ΔCt untreated drug sample =
X gene mean Ct value - β-actin gene mean Ct value; and ΔCt treated
drug sample = X gene mean Ct value - β-actin gene mean Ct value.
The ΔCt of the untreated drug samples and treated drug samples were
normalized as follows: ΔΔCt = ΔCt treated drug sample − ΔCt
untreated drug sample. Finally, the difference in expression of the
X gene between the treated and untreated drug samples was
calculated as 2−ΔΔCt.
Statistical analysis
Analysis of the results was performed using SPSS
statistical software, version 17.0 (SPSS, Inc., Chicago, IL, USA).
The values are expressed as the mean ± standard deviation.
Comparison between different groups was performed by univariate
analysis of variance and pair-wise comparison using
Student-Newman-Keuls method. The tests were two-sided with an α
level of 0.05. P<0.05 was considered to indicate a statistically
significant difference.
Results
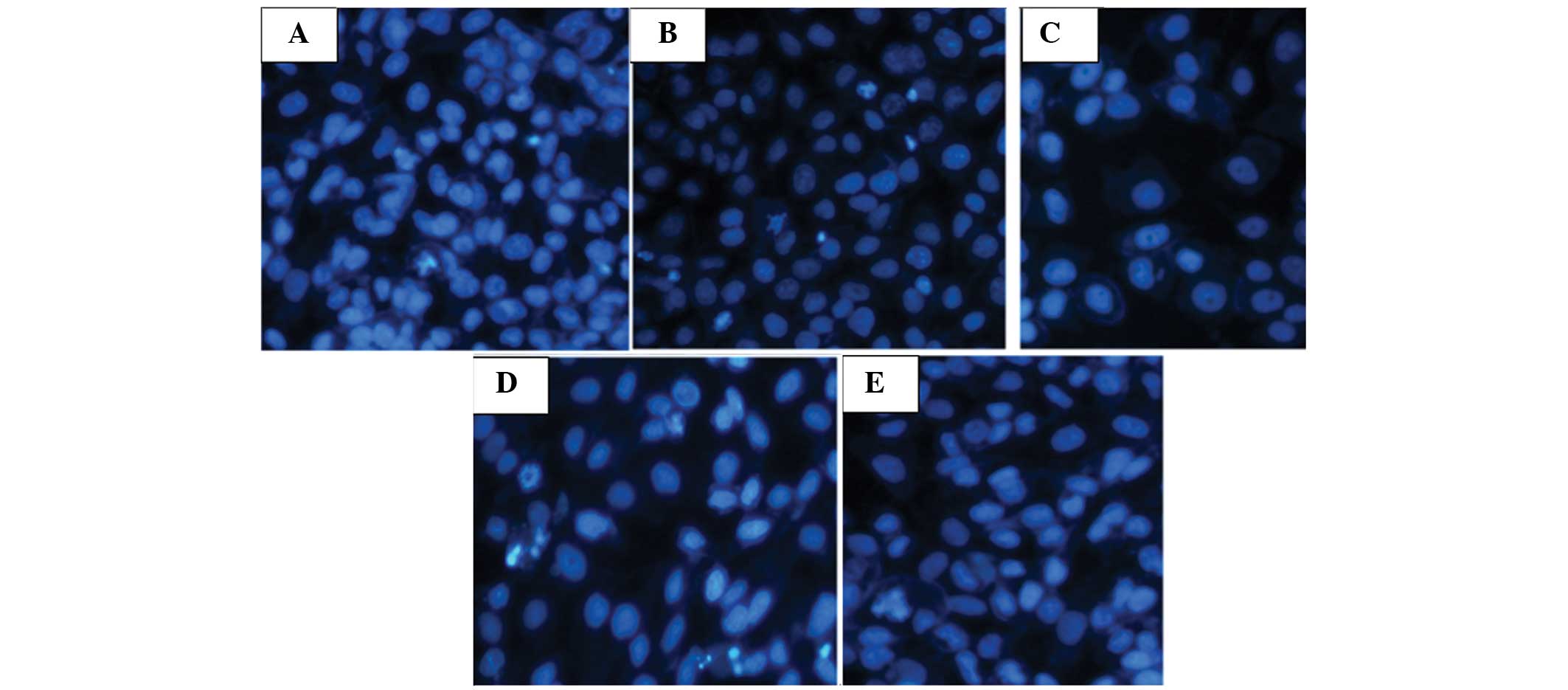
Cell morphology
The cells in the low-dose group became round and
were smaller in size with slight germination on the surface
(Fig. 1A). The cells became
irregular and a small number were exfoliated, floating in the
culture medium. There were also a small number of cells that were
swollen, ruptured and necrotic. The cells in the medium-dose group
demonstrated reduced cell proliferation, decreased density and
exhibited morphological changes and merging of cells (Fig. 1B). In addition, the cells were
significantly swollen, ruptured and necrotic. In the high-dose
group, the cells had deeper staining, smaller volumes and became
round with a condensed purple nucleus and a number of nuclei were
not clearly visible (Fig. 1C). In
the positive control group, the cells exhibited a lavender
cytoplasm, purple oval nuclei and dark purple nucleoli (Fig. 1D). By contrast, the cells in the
negative control group grew adherently with plump cytoplasms. The
adjacent cells integrated into each other, grew in multi-layers,
mainly as spindles or ovals, and exhibited rapid proliferation
(Fig. 1E).
LoVo cell proliferation is inhibited by
LQC serum
Following treatment with different concentrations of
LQC serum (1.25, 2.5 and 5.0 g/kg) for 24, 48 and 72 h,
proliferation of the LoVo cells was significantly inhibited
(Fig. 2). Furthermore, as the
serum drug concentration and prolonged exposure time was prolonged,
the inhibitory effect of the LQC serum was significantly enhanced,
demonstrating a time- and dose-dependent association
(P<0.05).
LQC treatment decreases tumor size in
mice
The tumor xenografts were introduced into nude mice
using LoVo cells to produce tumors. Mice were treated with LQ serum
and the tumor tissues were harvested to assess the effects of the
treatment on tumor progression. Compared with the negative
controls, tumor weights from the nude mice receiving drug treatment
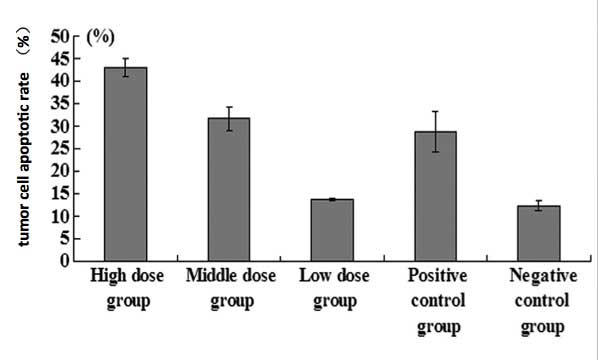
were significantly lower. Apoptotic rates in the high- and
medium-dose LQC groups and in the positive control group were
significantly increased compared with those in the negative control
group (P<0.05; Figs. 3 and
4).
Protein and mRNA expression of HGF and
c-Met are altered by LQ treatment
The protein levels of HGF and c-Met were measured in
tumors obtained from nude mice injected with LoVo cells. Compared
with the negative controls, the protein expression levels of HGF in
the high-, medium- and low-dose treatment groups and in the
positive control group were significantly decreased, while the
expression of c-Met protein was significantly increased (P<0.05;
Figs. 5 and 6).
The mRNA levels of HGF and c-Met were also measured
in the tumors obtained from nude mice injected with LoVo cells.
Compared with the negative controls, the mRNA expression of mRNA in
the high-, medium- and low-dose treatment groups and in the
positive control group were significantly decreased, while the mRNA
expression of c-Met was significantly increased (P<0.05), as
shown in Figs. 7 and 8.
Discussion
In the present study, LQCs were used to treat LoVo
cells as a model of colorectal cancer to investigate the inhibitory
effect on tumor cells. The in vitro results demonstrated
that serum from rats treated with LQCs effectively inhibited the
proliferation of LoVo cells, particularly at medium and high doses,
which had comparable inhibitory effects to cyclophosphamide. In
addition, the inhibitory effect of LQ serum treatment was most
marked after 72 h, suggesting that LQCs have an effect on
colorectal cancer cells that is enhanced with increased dosage and
duration of exposure. Furthermore, in vivo experiments
revealed that LQCs promote cell apoptosis, delay tumor growth and
reduce tumor weight.
HGF is a pluripotent cell growth factor produced by
stromal cells with a variety of biological effects (11). HGF is also a cytokine with a
significant ability to stimulate liver cell regeneration through
the promotion of karyokinesis, affecting motility and morphological
variation in a number of epithelial cells, including those in the
alveolar epithelium and gastrointestinal mucosa (12,13).
HGF has a variety of biological effects, which are mainly achieved
through its receptor, c-Met (14).
c-Met is a tyrosine-kinase receptor and is involved in the
regulation of cytoskeletal rearrangement, cell proliferation,
differentiation and motility (15,16).
Numerous studies have demonstrated that the HGF/c-Met signal
transduction pathway is important in the invasion and metastasis of
tumor cells and in angiogenesis (17,18).
Furthermore, the expression of HGF and c-Met in colorectal cancer
tissues is significantly higher compared with that in benign
colorectal adenoma and the normal colon tissues surrounding the
tumors. In addition, the lymphatic vessel density in colorectal
cancer tissues, in which HGF and c-Met expression are positive, is
significantly increased. As lymph node metastasis is closely
correlated with clinical stage, it is hypothesized that
overexpression of HGF and c-Met can promote the occurrence and
development of colorectal cancer and lymphatic metastasis (19).
In the present study, a significant reduction was
observed in the protein and mRNA expression of HGF in the tumor
cells of a nude mouse model of colorectal cancer treated with LQC.
By contrast, the protein and mRNA expression levels of c-Met were
significantly increased. The effect of a high-dose of LQC in
regulating the expression of HGF and c-Met was comparable to that
of cyclophosphamide, indicating that LQC may achieve its inhibitory
effect on tumors through downregulating the expression of HGF and
upregulating the expression of c-Met.
In conclusion, the present study demonstrated the
ability of LQC to significantly inhibit the growth of LoVo cells,
revealing dose-dependent activity and a significant antitumor
effect in LoVo-grafted nude mice via inhibition of the HGF/c-Met
signal transduction pathway. These results provide a basis for
designing and selecting therapeutic regimens for colorectal cancer,
drug screening and relevant investigation into the efficacy of
utilizing LQC as a treatment for colorectal cancer.
Acknowledgements
The study was supported by funds from the Natural
Science Foundation of Heilongjiang province (China).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li M and Gu J: Changing patterns of
colorectal cancer over the recent two decades in China. Zhonghua
Wei Chang Wai Ke Za Zhi. 7:214–217. 2004.(In Chinese).
|
|
3
|
Bilir C, Engin H and Temi YV: Effect of
gender on coagulation functions: a study in metastatic colorectal
cancer patients treated with bevacizumab, irinotecan,
5-Fluorouracil, and leucovorin. Adv Hematol. 2014:4734822014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yeh YC, Chen HY, Yang SH, et al: Hedyotis
diffusa combined with Scutellaria barbata are the core treatment of
Chinese herbal medicine used for breast cancer patients: A
population-based study. Evid Based Complement Alternat Med.
2014:2023782014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao L, Zhao AG, Zhao G, et al: Survival
benefit of traditional chinese herbal medicine (a herbal formula
for invigorating spleen) in gastric cancer patients with peritoneal
metastasis. Evid Based Complement Alternat Med. 2014:6254932014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xiao JY, Sun YM, Wang WK, et al: Effect of
Lingqi capsule on the content of IL-6 in peripheral blood of mice
bearing H22. China Pharmacist. 10:518–520. 2007.(In Chinese).
|
|
7
|
Wang HT and Su YM: Effect of Lingqi
capsule on expression VEGF, MMP-2 and MMP-9 in A549 cell. Zhonghua
Zhong Yi Yao Za Zhi. 24:1646–1648. 2009.(In Chinese).
|
|
8
|
Li H, Wang HG, Zhao X and Su YM: The
effect of Lingqi capsules on the Bcl-2 expression of apoptosis gene
of A549 cell lines. Zhong Yi Yao Xin Xi. 29:105–107. 2012.(In
Chinese).
|
|
9
|
Sankari SL, Masthan KM, Babu NA, et al:
Apoptosis in cancer - an update. Asian Pac J Cancer Prev.
13:4873–4878. 2012. View Article : Google Scholar
|
|
10
|
Tang JL, Zhang HH and Lai MD: Role of
autophagy and apoptosis in tumor. Zhonghua Bing Li Xue Za Zhi.
41:573–576. 2012.(In Chinese). PubMed/NCBI
|
|
11
|
Kim MD, Kim SS, Cha HY, et al: Therapeutic
effect of hepatocyte growth factor-secreting mesenchymal stem cells
in a rat model of liver fibrosis. Exp Mol Med. 46:e1102014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ohya W, Funakoshi H and Nakamura T:
Hepatocyte growth factor (HGF). Nihon Rinsho. 63:116–122. 2005.(In
Japanese).
|
|
13
|
Varkaris A, Corn PG, Gaur S, et al: The
role of HGF/c-Met signaling inprostate cancer progression and c-Met
inhibitors in clinical trials. Expert Opin Investig Drugs.
10:1677–1684. 2011. View Article : Google Scholar
|
|
14
|
Gao CF and Vande Woude GF: HGF/SF-Met
signaling in tumor progression. Cell Res. 15:49–51. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Faletto DL, Kaplan DR, Halverson DO, et
al: Signal transduction in c-met mediated motogenesis. EXS.
65:107–130. 1993.PubMed/NCBI
|
|
16
|
Cecchi F, Rabe DC and Bottaro DP:
Targeting the HGF/Met signaling pathway in cancer therapy. Expert
Opin Ther Targets. 16:553–572. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao CF and Vande Woude GF: HGF/SF-Met
signaling in tumor progression. Cell Res. 15:49–51. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Giordano S, di Renzo MF, Olivero M, et al:
The C-met/HGF receptor in human tumours. Eur J Cancer Prev.
1:45–49. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kataoka H, Itoh H, Hamasuna R, et al:
Pericellular activation of hepatocyte growthfactor/scatter factor
(HGF/SF) in colorectal carcinomas: roles of HGF activator (HGFA)
and HGFA inhibitor type 1 (HAI-1). Hum Cell. 14:83–93.
2001.PubMed/NCBI
|