Introduction
Mesenchymal stem cells (MSCs) are multipotent adult
stem cells, found intrinsically throughout the body following
development. They multiply by cell division to replenish dying
cells and repair malfunctioning tissues (1). MSCs are capable of differentiating
into osteoblasts, adipocytes, chondrocytes, fibroblasts, neuronal
tissues, myocytes and tenocytes (2). They are considered to reside in
specific areas of each tissue and remain inactive for long periods
of time, until they are activated by signals indicating that more
cells are required to maintain tissue integrity, or by signals from
sites of tissue injury. Different cytokines and growth factors are
recruited in stem cell fate regulation, including quiescence,
self-renewal, differentiation, apoptosis and mobilization from
their original niche (3–5).
MSCs have been found in numerous tissue types of
mesenchymal origin, predominantly in bone marrow, but also in
adipose tissues, skeletal muscle, connective tissues, teeth and
visceral organs (6). MSCs from
different tissue sources share certain global characteristics but
variations do exist among them (7). No specific antigenic phenotypes for
MSCs are found, but they share the features of endothelial,
epithelial and muscle cells, including CD29, CD44, CD90 and CD105
(8). MSCs do not express the
typical hematopoietic antigens, such as CD45 and CD34. It has been
reported that there were differences in yield, expansion and
multipotent differentiation potential among MSCs isolated from bone
marrow, synovium, periosteum, adipose tissue and muscle (9,10).
MSCs isolated from alveolar bone demonstrated less chondrogenic and
adipogenic potential than those isolated from iliac bone (11). Improved understanding of the
characteristics of stem cells from different sources may facilitate
the identification of an improved cellular source for tissue
engineering. For example, Rui et al (12) found that tendon-derived stem cells
(TDSCs) possess higher BMP2 receptor expression to facilitate
osteogenic differentiation when compared with bone marrow-derived
MSCs (BMSCs), and therefore, implicating TDSCs to be an attractive
source for tendon-bone junction healing. Numerous comparisons have
been made for adipose tissue-derived stem cells (ADSCs) and BMSCs,
illustrating that ADSCs were viable alternatives, even as a more
preferable source for cell therapy or pre-clinical drug testing
than BMSCs (13–15).
While the tendons attach muscle to bones and
ligaments connect bone to bone, forming and maintaining joints, the
fascia is a collective tissue that essentially holds the entire
body together. The fascia is also defined as a ‘web of tissue’ that
surrounds every muscle, bone and organ in the body and holds
everything in place. It is essential for the body’s self-healing
process, as once the epidermis is penetrated, it is the fascia that
staves off infection and further damage to the interior of the
body. Similar to all other tissues in the human body, the fascia
becomes inflamed when damaged, causing discomfort and pain.
However, similar to anterior cruciate ligament injuries, a torn
fascia (i.e. plantar fascia injury) is always associated with a
slow and poor recovery. There is high prospective to apply tissue
engineering strategies to improve the fascia healing process by
using the stem cells within. Previously, Tao et al (16) suggested a novel term called
‘fasciology’, hypothesizing that the fascial network distributed
throughout the body constructs a supporting-storing system to
nurture surrounding internal organs. The essence of Traditional
Chinese Medicine meridians and acupuncture may be explained in the
view of fascial anatomy. Over the last decade, this novel theory
has been conceptually verified through finding evidence from the
mechanism of acupuncture and Traditional Chinese Medicine,
evolutionary biology, holistic therapies and complementary medicine
(17). Li et al (18) also discovered that cells isolated
from the fascia of the gluteus maximus possessed chondrogenic
potential, which was different from neighboring muscle-derived stem
cells. However, despite these findings, to the best of our
knowledge, there is no comprehensive report to date that has
characterized the other stem cell properties of those cells
isolated from rat fascia structures.
Based on the aforementioned studies reporting that
the fascia is an intact structure that functions to connect muscles
and organs, it was hypothesized that resident fascia-derived stem
cells (FDSCs) should possess high chondrogenic, low osteogenic and
adipogenic differentiation potential and responsiveness to the
induction signals for collagen-rich fascial structure regeneration.
Therefore, FDSCs may represent an improved alternative cell source
compared with conventional ADSCs and BMSCs for musculoskeletal
tissue repair and tissue engineering. The present study aimed to
compare the stem cell marker expression, immunophenotypic profile,
proliferative capacity and multilineage differentiation potential
of rat FDSCs, ADSCs and BMSCs in vitro.
Materials and methods
Isolation and culture of rat FDSCs, ADSCs
and BMSCs
The Animal Research Ethics Committee of the Chinese
University of Hong Kong (Hong Kong, China) approved all of the
experiments. Eight male Sprague-Dawley rats (10 weeks old) weighing
250 g were used in the present study. FDSCs, ADSCs and BMSCs were
isolated from the same animals. The procedures for the isolation of
FDSCs, ADSCs and BMSCs are described as follows. FDSCs were
isolated from the fascia of the left gluteus maximus of the rats,
which were carefully detached from the muscle using surgical
scissors. ADSCs were isolated from the inguinal fat pad. Both FDSCs
and ADSCs were enzymatically isolated from their extracellular
matrix using type I collagenase (3 mg/ml; Sigma-Aldrich, St. Louis,
MO, USA) and passed through a 70-mm cell strainer
(Becton-Dickinson, Franklin Lakes, NJ, USA) to yield single-cell
suspensions. The BMSCs were isolated from the bone of femora by
centrifugation as described previously (19). Isolated FDSCs, ADSCs and BMSCs were
cultured in a growth medium [α-MEM (Invitrogen Life Technologies,
Carlsbad, CA, USA) containing 10% fetal bovine serum and 1%
penicillin/streptomycin (Invitrogen Life Technologies)] and seeded
at a density of 2×105/cm2 at 37°C in 95%
humidified air and 5% CO2. On day 7, all non-adherent
cells were removed followed by a medium change twice a week. The
monolayer of adherent cells was trypsinized by 0.25% trypsin-EDTA
when it reached half-confluence and reseeded at a density of
1×104/cm2 [passage 1 (P1)]. Passage 2 (P2)
culture was used for all characterization and in vitro
assays.
Colony formation unit assay, cell
proliferation and viability assay
The colony-forming unit (CFU) assay is used to
quantify functional stem cells. Briefly, 500 FDSCs, ADSCs and BMSCs
at P2 were seeded in 100-mm sterile petri dishes and cultured for
14 days. The colonies formed were stained with 1% crystal violet
(Sigma-Aldrich) in methanol for 30 min. For the cell proliferation
assay, FDSCs, ADSCs and BMSCs at P2 were seeded in a 96-well plate
at a density of 5,000 cells/well and incubated at 37°C, 5%
CO2. At day 2, cell proliferation was assessed using a
5-bromo-2-deoxyuridine (BrdU) assay kit (Roche Applied Science,
Penzberg, Germany) according to the manufacturer’s instructions.
The absorbance was measured at an optical density (OD) of 450 nm,
using a μQuant™ Microplate Spectrophotometer (BioTek Instruments,
Inc., Winooski, VT, USA). The relative cell viability in
metabolically active cells was also determined by measuring the
reduction of MTT dye (Sigma, St. Louis, MO, USA) to blue formazan
crystals at an OD of 540 nm, following a 3 h incubation at
37°C.
Immunophenotypic profile
The immunophenotypic identities of the FDSCs, ADSCs
and BMSCs were characterized by flow cytometry using the CANTO ll
flow cytometer with the FACs Diva software (BD Biosciences, San
Diego, CA, USA). All of the antibodies were obtained from AbD
Serotec (Raleigh, NC, USA) and used at 1:100 dilutions. The
antibodies used were against cell surface antigens, CD44 (cat. no.:
MCA643F), CD71 (cat. no.: MCA155PE), CD90 (cat. no.: MCA47PE),
CD106 (positive; cat. no.: MCA4633F), and hematopoietic markers
CD11b (cat. no.: MCA275FT) and CD45 (negative; cat. no.:
MCA43FT).
Stem cell marker analysis
The expression of pluripotency and self-renewal stem
cell markers octamer-binding transcription factor 4 (Oct4), sex
determining region Y (SRY)-box (Sox)2 and Krüppel-like factor 4
(Klf4) in FDSCs, ADSCs and BMSCs at P2 were compared using
quantitative polymerase chain reaction (qPCR). The amount of mRNA
was determined using the Quanti-Fast SYBR Green RT-PCR kit (Qiagen,
Hilden, Germany) with a validated primer set specific for the
target genes from Qiagen (as listed in Table I) in the CFX96 Real-Time PCR
Detection system (Bio-Rad, Hercules, CA, USA). The relative
expression of the qPCR product was calculated using the comparative
2−ΔΔCt method. The endogenous control
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was used for
normalization.
 | Table IPrimer sequences for self-renewal
stem cell markers and differentiation markers. |
Table I
Primer sequences for self-renewal
stem cell markers and differentiation markers.
| Symbol | Description | Primer sequence
(5′→3′) | Accession No. |
|---|
| Oct4 | POU class 5
homeobox 1 | (F)
GTCCCTAGGTGAGTCGTCCT
(R) TGGAAGCTTAGCCAGGTTCG | NM_001009178 |
| Sox2 | SRY-box 2 | (F)
GAGGAGGAGAGCGACTGTTT
(R) CTGGCGGAGAATAGTTGGGG | NM_001109181 |
| Klf4 | Kruppel-like factor
4 | (F)
GCCACCCACACTTGTGACTA
(R) TTCTCGCCTGTGTGAGTTCG | NM_053713 |
| Runx2 | Runt-related
transcription factor 2 | (F)
CACAAGTGCGGTGCAAACTT
(R) GCAGCCTTAAATATTACTGCATGG | NM_053470 |
| Alpl | Alkaline
phosphatase | (F)
GATGGTATGGGCGTCTCCAC
(R) TCTTGGAGAGAGCCACAAAGG | NM_013059 |
| OPN | Osteopontin | (F)
CCGAGGTGATAGCTTGGCTT
(R) CTCTTCATGCGGGAGGTGAG | NM_012881 |
| ON | Osteonectin | (F)
ACCTGGACTACATCGGACCA
(R) ACCAGGACGTTTTTGAGCCA | NM_012656 |
| C/EBPα | CCAAT/enhancer
binding protein α | (F)
GGCCATTCGCGACCC
(R) ACTCCATGGGGGAGTTAGAGT | NM_012524 |
| PPARγ | Peroxisome
proliferator-activated receptors γ | (F)
CCTGTTGACCCAGAGCATGG
(R) GGTCCACAGAGCTGATTCCG | NM_013124 |
| AP2 | Adipocyte fatty
acid-binding protein | (F)
TCGTCATCCGGTCAGAGAGT
(R) CCAGCTTGTCACCATCTCGT | U75581.1 |
| Adipsin | Complement factor
D | (F)
TGGGGCAATCACCAAGAACA
(R) CGAGATCCCCACGTAACCAC | NM_001077642 |
| Sox9 | SRY-box containing
gene 9 | (F)
TGGGAGCGACAACTTTACCA
(R) GAGGAGGAGGGAGGGAAAAC | XM_001081628 |
| Col1a2 | Collagen, type I, α
2 | (F)
GAGGCTTCTACAGGGCTGAC
(R) CTTAAGTCACGGCATGTGCG | NM_053356 |
| Col2a1 | Collagen, type II,
α 1 | (F)
GTTCACGTACACTGCCCTGA
(R) AAGGCGTGAGGTCTTCTGTG | NM_012929 |
| Agg | Aggrecan | (F)
GAAGTGGCGTCCAAACCAAC
(R) AGCTGGTAATTGCAGGGGAC | NM_022190.1 |
Assessment of differentiation
potential
For the differentiation studies, FDSCs, ADSCs and
BMSCs at P2 were seeded in six-well plates at a density of
3×105 cells/well. Following three days, the growth
medium was replaced with osteogenic medium (growth medium
supplemented with 100 nM dexamethasone, 50 μg/ml
ascorbate-2-phosphate and 10 mM β-glycerol phosphate), or
adipogenic medium (growth medium supplemented with 1 μM
dexamethasone, 50 μg/ml insulin, 0.5 mM methyl-isobutylxanthine and
100 μM indomethacin) with the medium changed twice a week for 14
days. The chondrogenetic potential of FDSCs was induced by the
StemPro® Chondrogenesis Differentiation kit (Invitrogen
Life Technologies), according to the manufacturer’s instructions.
Briefly, 1.6×107 cells were used to generate a micromass
culture for 28 days. The differentiated cells were visualized using
alizarin red S, oil red O and alcian blue staining for successful
osteogenesis, adipogenesis and chondrogenesis, respectively. To
compare the osteogenic, adipogenic and chondrogenic potential of
the FDSCs with ADSCs and BMSCs, the mRNA expression of the marker
genes was measured at day 7 (for ostegenesis and adipogenesis) and
day 14 (for chondrogenesis). The amount of mRNA was determined
using the Quanti-Fast SYBR Green RT-PCR kit (Qiagen) with a
validated primer set specific for the target genes from Qiagen (as
listed in Table I) in the CFX96
Real-Time PCR Detection System (Bio-Rad, United States). The
relative expression of the qPCR product was calculated using the
comparative 2−ΔΔCt method. The endogenous control GAPDH
mRNA was used for normalization.
Statistical analysis
The differences between groups were tested by
one-way analysis of variance, followed by a post-hoc Dunn’s test.
All statistical analyses were performed with SPSS 15.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference between values. Values are
expressed as the mean ± standard derivation.
Results
Cell morphology
FDSCs from the fascia of the left gluteus maximus of
the rats were isolated (n=3) and the cells were well attached on
culture vessels until confluent. Similar fibroblast-like cell
morphology was observed when compared with those for BMSCs and
ADSCs under similar growth conditions (Fig. 1). The cells were reseeded and
expanded to a number of passages (n>5) without slowing
proliferation, which proceeded with a doubling time of ~14–21
days.
Comparison of cell proliferation
The CFU assay identified that the size of the
colonies from FDSCs was generally bigger than that of BMSCs and
similar to that of ADSCs (Fig.
2A). An increased number of colonies were observed for the
FDSCs compared with the other two cell types. In addition, FDSCs
exhibited significantly higher proliferation potential than BMSCs
under different serum concentrations, as indicated by the BrdU and
MTT assays (Fig. 2B and C).
However, in the 10% FBS medium, both ADSCs and FDSCs exhibited
similar proliferation patterns. In the lower serum-containing
growth medium (0.5%), FDSCs attained a markedly higher
proliferation than that of ADSCs in both BrdU and MTT assays (both
P<0.05).
Immunophenotypic profile
Flow cytometry using antibodies against specific
surface antigens of stem cells was performed. The immunophenotypic
profile of BMSCs was CD11b−, CD31−,
CD34−, CD44+, CD45−,
CD71+, CD90+ and CD106+ (Table II). FDSCs demonstrated an
identical immunophenotypic profile to that of ADSCs, which was
similar to that of BMSCs except for no of expression of CD106.
 | Table IIImmunophenotypic profile of three
stem cell populations. Surface markers are indicated as positive
(+) and negative (−). |
Table II
Immunophenotypic profile of three
stem cell populations. Surface markers are indicated as positive
(+) and negative (−).
| Surface
markers | BMSCs | ADSCs | FDSCs |
|---|
| CD11b | − | − | − |
| CD31 | − | − | − |
| CD34 | − | − | − |
| CD44 | + | + | + |
| CD45 | − | − | − |
| CD71 | + | + | + |
| CD90 | + | + | + |
| CD106 | + | − | − |
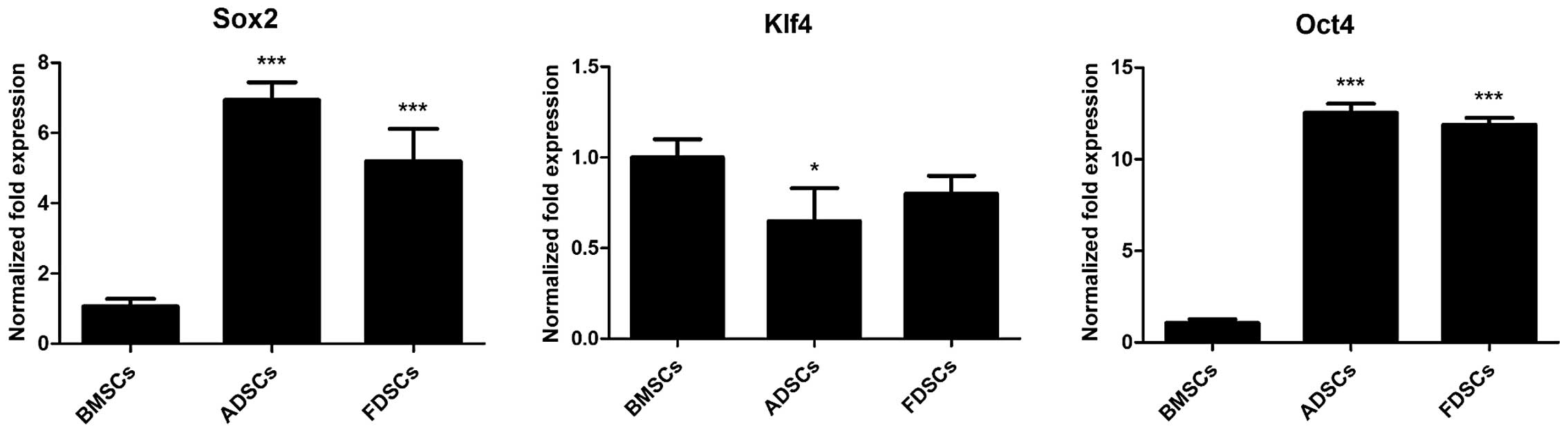
Comparison of stem cell marker
expression
Stem cell marker expression was assessed by qPCR.
Both FDSCs and ADSCs exhibited significantly higher expression of
Sox2 and Oct4 than BMSCs by 5- to 13-fold (P<0.001; Fig. 3). The expression of Sox2 in FDSCs
was evidently lower than that in ADSCs (P<0.001). However, a
lower expression of Klf4 in ADSCs (P<0.05) and FDSCs as compared
with that in BMSCs was observed.
Comparison of differentiation
potential
All three cell populations demonstrated the ability
of in vitro differentiation (Fig. 4). MSCs exhibited the highest
osteogenic potential, as demonstrated by marked staining with
alizarin red S. All of the cell populations demonstrated an
adipogenic potential with a higher number of adipocytes formed in
the ADSCs group, demonstrated by oil red O staining. Chondrogenic
potential (proteoglycan deposition) was demonstrated by alcian blue
staining and FDSCs demonstrated improved staining among the three
cell populations.
Comparison of gene expression
The expression levels of osteogenic markers
runt-related transcription factor 2 (Runx2), alkaline phosphatase
(ALP), osteopontin (OPN) and osteonectin (ON) in both ADSCs and
FDSCs (all P<0.001) were found to be notably lower than those in
the BMSCs (Fig. 5A). The
expression pattern of these genes in FDSCs was similar to that of
ADSCs. Among the three cell populations, ADSCs exhibited the
significantly highest expression of the adipogenic markers
CCAAT/enhancer binding protein α (C/EBPα), peroxisome
proliferator-activated receptor γ (PPARγ), AP2 and adipsin,
particularly on AP2 expression which was 25-fold higher (all
P<0.001; Fig. 5B). Meanwhile,
increased expression of adipocyte fatty acid binding protein (AP2)
and adipsin only were observed in FDSCs (P<0.001). Markedly
higher expression (~80-fold to that in the BMSCs) of chondrogenic
markers collagen, type I, α 2 (Col1a2), together with collagen,
type II, α 1 (Col2a1; >5-fold), were also observed in the FDSCs
(P<0.001; Fig. 5C). High
expression of these genes was observed in ADSCs, but their
expression was approximately half of that of the FDSCs
(P<0.001). The expression of the other chondrogenic markers Sox9
(P<0.001) and aggrecan (AGG) (P<0.05) was similar in the
ADSCs and FDSCs and their expression was marginally higher than
that in the BMSCs.
 | Figure 5mRNA expression of osteogenic,
adipogenic and chondrogenic markers was analyzed by quantitative
polymerase chain reaction. (A) Expression of all the osteogenic
markers Runx2, ALP, OPN and ON were significantly decreased in
ADSCs and FDSCs (***P<0.001). (B) The highest
expression of all adipogenic markers, C/EBPα, PPARγ, AP2 and
Adipsin, was found in ADSCs (***P<0.001),
particularly for AP2 expression (>25-fold increase). In FDSCs,
significantly higher expression of AP2 and Adipsin only as compared
with that in BMSCs were observed (***P<0.001). (C)
Increased expression of the chondrogenic markers Sox9, Col1a2,
Col2a1 (***P<0.001), and AGG (*P<0.05)
were observed in both ADSCs and FDSCs, while the expression of
Col1a2 and Col2a1 was significantly increased in FDSCs. The values
were calculated with reference to GAPDH and the results are
expressed as the mean values ± standard deviation of data from
experiments in triplicate. FDSCs, fascia-derived stem cells; ADSCs,
adipose-derived stem cells; BMSCs, bone marrow-derived mesenchymal
stem cells; Runx2, runt-related transcription factor 2; ALP,
alkaline phosphatase; OPN, osteopontin; ON, osteonectin; C/EBPα,
CCAAT/enhancer binding protein α; PPARγ, peroxisome
proliferator-activated receptor γ; AP2, adipocyte fatty acid
binding protein; Sox2, sex-determining region Y-box 2; Col1a2,
collagen, type I, α 2; Col2a1, collagen, type II, α 1; AGG,
aggrecan. |
Discussion
The fascia first received attention as an important
structure in the 1930s, but few studies were performed
investigating its significance for several decades (20). With technological advancements,
however, including imaging and anatomical technologies, the fascia
structure has been attracting increasing attention. A growing
number of studies and evidence have demonstrated that multipotent
stem cells should be resided in the fascia (21). Skeletal muscle perimysium, a sheath
of connective tissue that segregates skeletal muscle fascicles and
fibers, has a similar histology, structure and function to fascia,
but is different in the scale of muscle structure. It was further
hypothesized that non-myogenic cells within skeletal muscle, likely
associated with endomysium and perimysium, may possess chondrogenic
potential. However, there is no known physical method to isolate
these tissues from skeletal muscle, considering its
super-structural complexity. Therefore, the presence of
chondrogenic cells in the skeletal muscle of a Fischer 344 rat
gluteus maximus muscle was also investigated by isolating a
heterogeneous population of muscle-derived cells, which were then
examined for the presence of cells with chondrogenic potential
(18). The present study
successfully isolated cells from the superficial fascia of the
limbs of adult rats. To the best of our knowledge, the present
study was the first to isolate and characterize rat FDSCs in
vitro. These fascia-derived cells had universal MSC
characteristics, including clonogenicity, high proliferative
potential at reduced serum conditions, MSC marker expression and
multidifferentiation potential, including osteogenesis,
adipogenesis and chondrogenesis.
The present study compared the immunophenotypic
profiles of FDSCs, ADSCs and BMSCs, and found a similar expression
pattern of CD44, CD71 and CD90, as well as a difference in the
expression of CD106. The BMSCs expressed CD106, which was not
detected in ADSCs and FDSCs. The presence of CD106 is controversial
in ADSCs. Schäffler and Büchler (22) defined the surface marker set for
ADSCs, which included CD106. However, De Ugarte et al
(23) and Zuk et al
(24) found that CD106 was absent
in ADSCs. The present study also demonstrated that there was
minimal contamination with hematopoietic and endothelial cells in
the culturing system, as evidenced by the weak expression of CD34,
CD45 and CD31 in the cultured cells. FDSC propagation (P2)
demonstrated the high purity of the cells exhibiting markers
similar to BMSCs and ADSCs and negativity for hematopoietic markers
suggested that they possibly attained stem cell phenotype
characteristics.
The proliferation capacity of stem cells is
important with regard to their application in cell therapy. A
number of previous studies have indicated that stem cells from
different sources exhibited differences in proliferation and
differentiation potential, implying that selecting the appropriate
cell source for musculoskeletal tissue engineering is significant
(25). In the present study, the
proliferation capacity of FDSCs was compared with that of ADSCs and
BMSCs in media containing 0.5 and 10% of FBS, using the BrdU and
MTT assays. In medium containing 10% FBS, it was identified that
the FDSCs had a similar proliferative response to that of the
ADSCs, but with a higher proliferation capacity than the BMSCs.
This observation was consistent with previous studies demonstrating
that ADSCs exhibited a higher proliferation capacity than BMSCs
(26,27). Proliferation studies were also
performed, using 0.5% FBS medium. Of note, it was identified that
FDSCs exhibited a higher proliferation than both the BMSCs and
ADSCs. This may imply that the nutrient supply in the
microenvironment may alter the stem cells’ proliferation capacity.
Potier et al (28) reported
that serum starvation and deprivation of growth factors may promote
premature aging in MSCs and studies of MSCs in a hypoxic
environment, indicating that serum starvation may be associated
with marked cell death. By contrast, the present study
substantiated that FDSCs function effectively under conditions of
serum deprivation. Further investigation is required to determine
whether FDSCs exhibit and maintain a hypoxic environment within the
appropriate peripheral musculoskeletal tissues with poor
vasculature in vivo, to determine its possible clinical
applications.
The FDSCs isolated in the present study expressed a
number of key embryonic self-renewal stem cell marker genes,
including Oct4, Sox2 and Klf-4. It has been demonstrated that Oct4
transcription factors are critical for stem cell fate selection, in
addition to their roles in maintaining the pluripotency and
self-renewal capacity in mesenchymal stem cells (29). Oct4 has often been used as a marker
of stemness, as differentiated cells demonstrated reduced
expression of this marker. Several studies have suggested that Oct4
is essential in sustaining self-renewal capacity of adult somatic
stem cells (30,31). In addition, Oct4 binds to DNA
cooperatively with Sox2 at non-palindromic sequences to activate
transcription of key pluripotency factors. It has been demonstrated
that differentiation signals modulate the expression of Oct4 and
Sox2, such that the induction of Oct4 suppressed neural ectodermal
differentiation and promoted mesendodermal differentiation, whereas
induction of Sox2 inhibited mesendodermal differentiation and
promoted neural ectodermal differentiation (32). Klf-4 DNA-binding protein has been
recently found to be important in regulating MSC transcriptional
activity and controlling cell fate (33). In the present study, the FDSCs and
ADSCs demonstrated high levels of expression of Oct4 and Sox2 as
compared with that in BMSCs. The higher expression of Oct4 in FDSCs
as compared with that in BMSCs and the comparatively lower
expression of Sox2 in FDSCs than that in ADSCs, as observed in the
present study, may favor mesendodermal lineage choice of FDSCs when
compared with both cell types.
In the present study, it was confirmed that FDSCs,
ADSCs and BMSCs have the potential to differentiate into
osteogenic, adipogenic and chondrogenic lineages. However, BMSCs
have greater osteogenesis potential, while ADSC have a greater
adipogenic potential and FDSCs have a greater chondrogenic
potential, as evidenced by the increment of expression of their
corresponding differentiation markers when compared with those of
the other two stem cell populations. In previous years, stem cells
have generated increasing interest considering their potential
therapeutic use. Previous studies have provided clear evidence that
multipotent adult stem cells exist in numerous organs and tissues,
including bone marrow, muscle, fat, periosteum and synovial
membrane from both rodents and humans (9,10,15).
A number of studies have suggested that different stem cells may
share common properties for single targeted stem cell therapy
(34). For instance, previous
studies have suggested that equal or comparable osteogenic capacity
were found between ADSCs and BMSCs (24,35).
Therefore, ADSCs are attractive for musculoskeletal tissue
engineering, since adipose tissue possesses abundant and easily
accessible MSCs. However, the present study, in parallel with
certain recent data (36, 37), suggested that the differentiation
potential of the stem cells from different origin may not be
identical. Stem cells from different sources may represent distinct
cell populations that are at different lineage-specific commitment
with distinct biological properties (38). Such differences in differentiation
potential may be due to the inherent differences between FDSCs,
ADSCs and BMSCs. Therefore, selecting an improved stem cell source
for therapeutic use and tissue engineering is required.
In conclusion, a population of stem cells was
isolated from the fascia tissue of rats, which exhibited universal
stem cell characteristics, including clonogenicity, proliferative
capacity, multipotent potential and MSC and ESC marker expression.
In addition, these FDSCs were more chondrogenic when compared with
ADSCs and BMSCs. The feasibility of isolating stem cells from rat
fascia tissues may provide new opportunities for investigating
FDSCs for tissue engineering and improving the understanding of the
role and mobility of FDSCs in musculoskeletal tissue healing.
Additional comparative and functional in vitro and in
vivo studies are required to verify these findings to finally
provide a better understanding of the biological differences of
MSCs from different sources and to identify the most suitable MSCs
for treatment of specific diseases. Furthermore, the discovery of
FDSCs provided a possible functional role of the fascia structure,
namely that of an active support-storage system to their
surrounding internal organs.
Acknowledgements
This study was financially supported by the National
Natural Science Foundation of China (grant no. 81200651). This
research project was also supported in part by the grants of the
State Key Laboratory of Phytochemistry and Plant Resources in West
China (CUHK) from HKSAR and CUHK, the Focused Innovations Scheme
(Major Area Scheme A - Phase 2) of the Chinese University of Hong
Kong and internal funding from the Institute Guangzhou of Advanced
Technology, Chinese Academy of Science, Guangzhou.
References
|
1
|
Mundra V, Gerling IC and Mahato RI:
Mesenchymal stem cell-based therapy. Mol Pharm. 10:77–89. 2013.
View Article : Google Scholar :
|
|
2
|
Gimble JM, Guilak F, Nuttall ME, et al: In
vitro differentiation potential of mesenchymal stem cells. Transfus
Med Hemother. 35:228–238. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Toupadakis CA, Granick JL, Sagy M, et al:
Mobilization of endogenous stem cell populations enhances fracture
healing in a murine femoral fracture model. Cytotherapy.
15:1136–1147. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Corallini F, Secchiero P, Beltrami AP, et
al: TNF-alpha modulates the migratory response of mesenchymal stem
cells to TRAIL. Cell Mol Life Sci. 67:1307–1314. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wong HL, Siu WS, Shum WT, et al:
Application of Chinese herbal medicines to revitalize adult stem
cells for tissue regeneration. Chin J Integr Med. 18:903–908. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krampera M, Franchini M, Pizzolo G and
Aprili G: Mesenchymal stem cells: from biology to clinical use.
Blood Transfus. 5:120–129. 2007.PubMed/NCBI
|
|
7
|
Zhang HT, Liu ZL, Yao XQ, Yang ZJ and Xu
RX: Neural differentiation ability of mesenchymal stromal cells
from bone marrow and adipose tissue: a comparative study.
Cytotherapy. 14:1203–1214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hass R, Kasper C, Böhm S and Jacobs R:
Different populations and sources of human mesenchymal stem cells
(MSC): A comparison of adult and neonatal tissue-derived MSC. Cell
Commun Signal. 9:122011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yoshimura H, Muneta T, Nimura A, et al:
Comparison of rat mesenchymal stem cells derived from bone marrow,
synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res.
327:449–462. 2007. View Article : Google Scholar
|
|
10
|
Fan J, Varshney RR, Ren L, Cai D and Wang
DA: Synovium-derived mesenchymal stem cells: a new cell source for
musculoskeletal regeneration. Tissue Eng Part B Rev. 15:75–86.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matsubara T, Suardita K, Ishii M, et al:
Alveolar bone marrow as a cell source for regenerative medicine:
differences between alveolar and iliac bone marrow stromal cells. J
Bone Miner Res. 20:399–409. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rui YF, Lui PP, Ni M, et al: Mechanical
loading increased BMP-2 expression which promoted osteogenic
differentiation of tendon-derived stem cells. J Orthop Res.
29:390–396. 2011. View Article : Google Scholar
|
|
13
|
Ikegame Y, Yamashita K, Hayashi S, et al:
Comparison of mesenchymal stem cells from adipose tissue and bone
marrow for ischemic stroke therapy. Cytotherapy. 13:675–685. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vidal MA, Walker NJ, Napoli E and
Borjesson DL: Evaluation of senescence in mesenchymal stem cells
isolated from equine bone marrow, adipose tissue, and umbilical
cord tissue. Stem Cells Dev. 21:273–283. 2012. View Article : Google Scholar
|
|
15
|
Taléns-Visconti R, Bonora A, Jover R, et
al: Hepatogenic differentiation of human mesenchymal stem cells
from adipose tissue in comparison with bone marrow mesenchymal stem
cells. World J Gastroenterol. 12:5834–5845. 2006.PubMed/NCBI
|
|
16
|
Tao H, Yu MC, Yang HY, et al: Correlations
between fasciology and yin yang doctrine. J Acupunct Meridian Stud.
4:141–146. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang J, Wang CL, Shen BL, Yang LL and Yuan
L: Explanation of essence and substance basis of channels and
collaterals with fasciology. Zhongguo Zhen Jiu. 27:583–585.
2007.(In Chinese). PubMed/NCBI
|
|
18
|
Li G, Zheng B, Meszaros LB, et al:
Identification and characterization of chondrogenic progenitor
cells in the fascia of postnatal skeletal muscle. J Mol Cell Biol.
3:369–377. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ko CH, Siu WS, Wong HL, et al: Pro-bone
and antifat effects of green tea and its polyphenol,
epigallocatechin, in rat mesenchymal stem cells in vitro. J Agric
Food Chem. 59:9870–9876. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stecco C, Tiengo C, Stecco A, et al:
Fascia redefined: anatomical features and technical relevance in
fascial flap surgery. Surg Radiol Anat. 35:369–376. 2013.
View Article : Google Scholar
|
|
21
|
Schleip R, Jäger H and Klingler W: What is
‘fascia’? A review of different nomenclatures. J Bodyw Mov Ther.
16:496–502. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schäffler A and Büchler C: Concise review:
adipose tissue-derived stromal cells--basic and clinical
implications for novel cell-based therapies. Stem Cells.
25:818–827. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
De Ugarte DA, Alfonso Z, Zuk PA, et al:
Differential expression of stem cell mobilization-associated
molecules on multi-lineage cells from adipose tissue and bone
marrow. Immunol Lett. 89:267–270. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zuk PA, Zhu M, Ashjian P, et al: Human
adipose tissue is a source of multipotent stem cells. Mol Biol
Cell. 13:4279–4295. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Steinert AF, Rackwitz L, Gilbert F, Nöth U
and Tuan RS: Concise review: the clinical application of
mesenchymal stem cells for musculoskeletal regeneration: current
status and perspectives. Stem Cells Transl Med. 1:237–247. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kern S, Eichler H, Stoeve J, Klüter H and
Bieback K: Comparative analysis of mesenchymal stem cells from bone
marrow, umbilical cord blood, or adipose tissue. Stem Cells.
24:1294–1301. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peng L, Jia Z, Yin X, et al: Comparative
analysis of mesenchymal stem cells from bone marrow, cartilage, and
adipose tissue. Stem Cells Dev. 17:761–773. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Potier E, Ferreira E, Meunier A, et al:
Prolonged hypoxia concomitant with serum deprivation induces
massive human mesenchymal stem cell death. Tissue Eng.
13:1325–1331. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Greco SJ, Liu K and Rameshwar P:
Functional similarities among genes regulated by OCT4 in human
mesenchymal and embryonic stem cells. Stem Cells. 25:3143–3154.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Z, Tian X, Yuan Y, et al: Effect of
cell culture using chitosan membranes on stemness marker genes in
mesenchymal stem cells. Mol Med Rep. 7:1945–1949. 2013.PubMed/NCBI
|
|
31
|
Rasini V, Dominici M, Kluba T, et al:
Mesenchymal stromal/stem cells markers in the human bone marrow.
Cytotherapy. 15:292–306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thomson M, Liu SJ, Zou LN, et al:
Pluripotency factors in embryonic stem cells regulate
differentiation into germ layers. Cell. 145:875–889. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Saulnier N, Puglisi MA, Lattanzi W, et al:
Gene profiling of bone marrow- and adipose tissue-derived stromal
cells: a key role of Kruppel-like factor 4 in cell fate regulation.
Cytotherapy. 13:329–340. 2011. View Article : Google Scholar
|
|
34
|
Wu X, Ren J and Li J: Fibrin glue as the
cell-delivery vehicle for mesenchymal stromal cells in regenerative
medicine. Cytotherapy. 14:555–562. 2012. View Article : Google Scholar
|
|
35
|
De Ugarte DA, Morizono K, Elbarbary A, et
al: Comparison of multi-lineage cells from human adipose tissue and
bone marrow. Cells Tissues Organs. 174:101–109. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang W, Zhang X, Wang S, et al:
Comparison of the use of adipose tissue-derived and bone
marrow-derived stem cells for rapid bone regeneration. J Dent Res.
92:1136–1141. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tan Q, Lui PP, Rui YF and Wong YM:
Comparison of potentials of stem cells isolated from tendon and
bone marrow for musculoskeletal tissue engineering. Tissue Eng Part
A. 18:840–851. 2012. View Article : Google Scholar :
|
|
38
|
Wegmeyer H, Bröske AM, Leddin M, et al:
Mesenchymal stromal cell characteristics vary depending on their
origin. Stem Cells Dev. 22:2606–2618. 2013. View Article : Google Scholar : PubMed/NCBI
|