Introduction
High mobility group box 1 (HMGB1, formerly HMG1) was
originally described as a non-histone chromatin-associated nuclear
protein (1–4). The HMGB1 sequence is highly conserved
among species, with murine HMGB1 differing in only two amino acids
to that in humans. HMGB1 consists of two tandem L-shaped domains,
HMGB boxes A and B, which are ~75 amino acids in length, and a
highly acidic carboxyl terminus, which is 30 amino acids in length.
HMG proteins are small, DNA-binding proteins that are important in
transcriptional regulation (5). In
HMGB1-deficient mice, mortality occurs within a few hours of birth,
demonstrating the important role of this protein in cellular
function. In cellular systems, HMGB1 is considered to have two
separate functions It is an intracellular regulator of
transcription and has an extracellular role, in which it promotes
metastasis (6–9). Overexpression of the HMGB1 protein is
linked to the following cancer-associated characteristics:
Unlimited proliferation, angiogenesis, resistance to apoptosis, the
production of growth factors by cells, lack of susceptibility to
growth inhibitors, inflammation and metastasis (10). It has also been demonstrated that
the protein is involved in cell invasion, tumor growth and
metastasis (11). Treatment with
anti-HMGB1 antibodies in mice leads to the suppression of
metastasis in Lewis lung tumor cells implanted beneath the skin
(12). The HMGB1 protein has been
detected in various types of tumor. Compared with normal tissues,
the HMGB1 protein is commonly overexpressed in gastric and
colorectal adenocarcinoma (13).
The HMGB1 protein is also upregulated in melanomas (14) and high levels of the protein are
observed in leukemia cells (15).
There is marked intertumoral variation in the expression of HMGB1
in different types of breast cancer (16). Although HMGB1 is overexpressed in
the majority of types of tumor, certain tumors contain no HMGB1
protein, including adrenal gland carcinoma, in which no HMGB1
expression is observed (17). It
is hypothesized that the HMGB1 protein affects cell invasion, tumor
growth and metastasis by high-affinity binding to the receptor for
advanced glycation end products (RAGE) (12,18).
HMGB1, which has been identified as a RAGE ligand, is secreted by
certain types of cell and is important in inflammation, cell
migration, differentiation and tumorigenesis (19,20).
The binding of HMGB1 to RAGE activates key cell signaling pathways,
including those of mitogen-activated protein kinases (MAPKs) and
nuclear factor (NF) (11). HMGB1
may act as a mediator of angiogenesis, increasing the expression of
angiogenic growth factors, including vascular endothelial growth
factor (21). HMGB1 has been
observed to lead to endothelial cell migration and sprouting in
vitro (22), mediate the
upregulation of vascular endothelial growth factor C and enhance
lymphangiogenesis (23).
Overexpression of HMGB1 suppresses the activity of caspase-9 and
capsase-3, suggesting that it interferes with the apoptotic
mechanism at the level of apoptosomal caspase-9 activation. The
apoptosis-repressing HMGB1 and cellular inhibitor of apoptosis 2
(c-IAP2) proteins are upregulated in the pathogenesis of colon
carcinoma (24). Clinical studies
have demonstrated that HMGB1-knockdown inhibits the metastatic
potential of cancer cells (25).
Therefore, the HMGB1 ligand or its receptor are important targets
as a possible application in cancer therapeutics (26). Ulinastatin (UTI) is a Kunitz-type
protease inhibitor (27) and an
effective calcium influx inhibitor of the cell transporter system
(28). Kobayashi et al
demonstrated that there are specific UTI binding sites on the
surfaces of certain tumor cells and exogenously applied UTI binds
to these sites. This potentially leads to the buildup of a
substantial quantity of UTI on the tumor cell surface (29). There is clear evidence that UTI is
important in preventing tumor cell invasion and metastasis
(30,31). UTI also inhibits tumor necrosis
factor α (TNF-α)-mediated translocation, protein kinase C (PKC)
activation (32) and the phorbol
myristate acetate (PMA)-dependent activation of the PKC and MAPK
cascade (31). Kobayashi et
al observed that UTI inhibited tumor invasion and metastasis,
possibly through the suppression of cell-associated plasmin
activity and the mRNA and protein expression of urokinase
plasminogen (uPA). In endotoxemic rats, UTI suppresses excessive
superoxide (O2−) generation, systemic
inflammation, lipid peroxidation and HMGB1 (33). However, the potential role of UTI
in the regulation of HMGB1 in cancer remains to be elucidated. The
present study aimed to determine the effect of the exogenous UTI in
the expression of HMGB1 and investigate the effect of UTI
inhibition on the activity and release of HMGB1.
Materials and methods
Cell culture
The human colon cancer cell line LoVo, was provided
by Dr Ma (Key Laboratory of Antibody Engineering, Department of
Education, Guangdong, China). The cell line was negative for
mycoplasma contamination. Cells were maintained in Dulbecco’s
modified Eagle’s medium (DMEM) supplemented with penicillin (100
U/ml), streptomycin (100 U/ml) and 10% heat-inactivated fetal
bovine serum (Gibco Life Technologies, Grand Island, NY, USA) at
37°C in 5% CO2.
Drug assay
The cells were divided into the four following
groups for the drug assay: Control (no UTI), UTI1 (400 U/ml UTI),
UTI2 (800 U/ml UTI) and UTI3 (1,600 U/ml UTI). Prior to
stimulation, the cells were washed three times with NaCl/propidium
iodide (Sigma Co., St. Louis, MO, USA) and incubated overnight in
complete medium containing 1% fetal bovine serum. The test drug was
added and the incubation was continued for different durations (24,
48 or 72 h). Subsequently, the medium was aspirated and the cells
were harvested and washed thoroughly with phosphate-buffered saline
(PBS). The cell viability immediately prior to harvesting remained
>90%.
Cell growth
The cultured cells were harvested from 80% confluent
monolayers by brief trypsinization with 0.1% trypsin and 0.1%
ethylenediaminetetraacetic acid (Gibco Life Technologies, Carlsbad,
CA, USA). The cells (2,000 cells/well) were seeded onto 96-well
tissue culture plates (Corning Inc., New York, NY USA) and were
cultured for 12 h in regular medium. The cells were washed twice
with PBS and treated with UTI. The cell growth was monitored
following 24, 48 and 72 h by MTT assay (36). All experiments were repeated three
times under the same conditions.
Invasion assay
Using a Boyden chamber system, the capability of
tumor cells to invade was assessed using previously described
methods (34). A polycarbonate
membrane with 8 μm pores was coated with a reconstituted basement
membrane gel (Matrigel; BD Biosciences, Franklin Lakes, USA). DMEM
containing 10% fetal bovine serum was placed in the lower
compartment of the chamber as a chemoattractant. The UTI-treated
LoVo cells (1×105) were resuspended in complete medium
(200 μl) and seeded in the upper chamber. Following incubation for
24 h at 37°C, the cells that had migrated to the lower chamber were
fixed and stained using 0.1% crystal violet (Sigma) and counted
using fluorescence microscopy (Olympus IX71; Olympus, Tokyo, Japan;
magnification, ×100).
Western blot analysis
The HMGB1 NF-κB protein was detected by
immunoblotting. The conditioned media were individually harvested
and the remaining monolayers were scraped and lysed in lysis buffer
containing 50 mM 4-(2-Hydroxyethyl) piperazine-1-ethanesulfonic
acid, 0.5 mM NaCl, 0.05% Tween-20, 1% Triton X-100, 1 mM
phenylmethanesulfonyl fluoride, 10 μg/ml E-64 and 10 μg/ml
leupeptin, to prepare the cell lysates. Equal quantities of
cellular protein (50 μg/lane) underwent 12% SDS-PAGE and were
transferred to polyvinylidene difluoride membranes (Roche,
Mannheim, Germany). The membrane was probed for 12 h with
anti-HMGB1 rabbit polyclonal antibody (1:1,000; Abcam, Cambridge,
UK) or anti-NF-κB rabbit polyclonal antibody (1:1,000; Abcam),
Horseradish peroxidase-conjugated anti-rabbit immunoglobulin G
(1:5,000; Abcam) was used as secondary antibody and was incubated
for 2 h. Immunoreactivity was detected using an Enhanced
Chemiluminescence kit (Thermo Fisher Scientific, Waltham, MA, USA)
and visualized using Image Gauge software (Fujifilm, Tokyo,
Japan).
Immunofluorescence image analysis
The cells were cultured in 96-well plates and fixed
using 10% paraformaldehyde in PBS for 10 min at room temperature.
The cells were then washed with PBS and incubated for 5 min at 4°C
with permeabilization buffer, containing 0.1% Triton X-100 in PBS.
The samples were blocked using 2% bovine serum albumin (BSA) in PBS
for 30 min and incubated with rabbit anti-HMGB1 (1:100) for 24 h at
4°C. Following washing three times with 0.2% BSA in PBS, an Alexa
Fluor 488 secondary antibody (1:50; Invitrogen Life Technologies,
Carslbad, CA, USA) was added for 1 h at room temperature. Mounting
medium containing Hoechst (1:50; Vector Laboratories, Inc., CA,
USA) was used for 30 min at room temperature. Fluorescent-labeled
cells were observed using an inverted microscope (Olympus, Tokyo,
Japan) and images were captured using XV Image processing software
(Mathworks, Natick, MA, USA).
Caspase activity assay
Caspase activity was quantified using a Caspase-3
Fluorometric Protease assay kit (BioVision, Inc, Milpitas, CA, USA)
according to the manufacturer’s instructions. All components
mentioned in this paragrph are part of the Caspase Assay kit. The
assay was based on the cleavage of the fluorogenic peptide
DEVD-AFC, a caspase-3 substrate, which was added to the cell
lysates prepared from the apoptotic cells and the non-apoptotic
controls. The cells (1×106) were counted, pelleted and
resuspended in 50 μl chilled cell lysis buffer prior to incubation
on ice for 10 min. A reaction buffer (2X; 50 μl) containing 10 mM
dithiothreitol was added to each sample, followed by 5 μl 1 mM
DEVE-AFC substrate to a final concentration of 50 μM and incubated
at 37°C for 1.5 h. The samples were transferred onto a 96-well
plate for detection of caspase activity and were read using an
automatic fluorometer microplate reader (SpectraMax M5; Molecular
Devices, Sunnyvale, CA, USA) equipped with a 400 nm excitation
filter and 505 nm emission filter.
Reverse transcription and quantitative
polymerase chain reaction (qPCR)
The total RNA extracts (2 μg) from the LoVo cells or
control cells were reverse-transcribed using Expand Reverse
Transcriptase (Roche Diagnostics, Basel, Switzerland) according to
the manufacturer’s instructions. The cDNA was then used for qPCR.
Primers were designed using the Primer Express program
(PerkinElmer, Inc., Waltham, MA, USA). Quantification of the target
cDNA was performed using an ABI PRISM 7500HT Sequence Detection
system (Applied BiosystemsLife Technologies, Foster City, CA, USA),
using SDS 2.1 software (Applied Biosystems Inc., Carlsbad, CA,
USA). All reactions were performed three times in triplicate. The
transcripts were detected using SYBR Green I (Applied Biosystems)
according to the manufacturer’s instructions and were normalized to
the internal control β-actin and optimal reaction conditions for
target gene amplification were according to the manufacturer’s
instructions. Primers were designed using the primer design
software, Primer 5.0 (Shanghai Biotechnology Co., Ltd., Shanghai,
China). The primer sequences used were as follows: Forward,
5′-TGAGCTCCATAGAGACAGCG-3′, and reverse,
5′-TGACATTTTGCCTCTCGGCT-3′.
Statistical analysis
Data are expressed as the means ± standard
deviation. All statistical analyses were performed using SPSS 18.0
software (SPSS, Inc., Chicago, IL, USA). The Mann-Whitney U test
was used to compare the different groups. P<0.05 was considered
to indicate a statistically significant difference.
Results
LoVo cell proliferation
The proliferation of UTI-treated LoVo cells were
significantly inhibited compared with the control group
(P<0.05). The inhibitory effect was enhanced following extended
treatment, which indicated a time-dependent effect (Fig. 1). Similarly, the inhibitory effect
increased significantly as the UTI concentration increased.
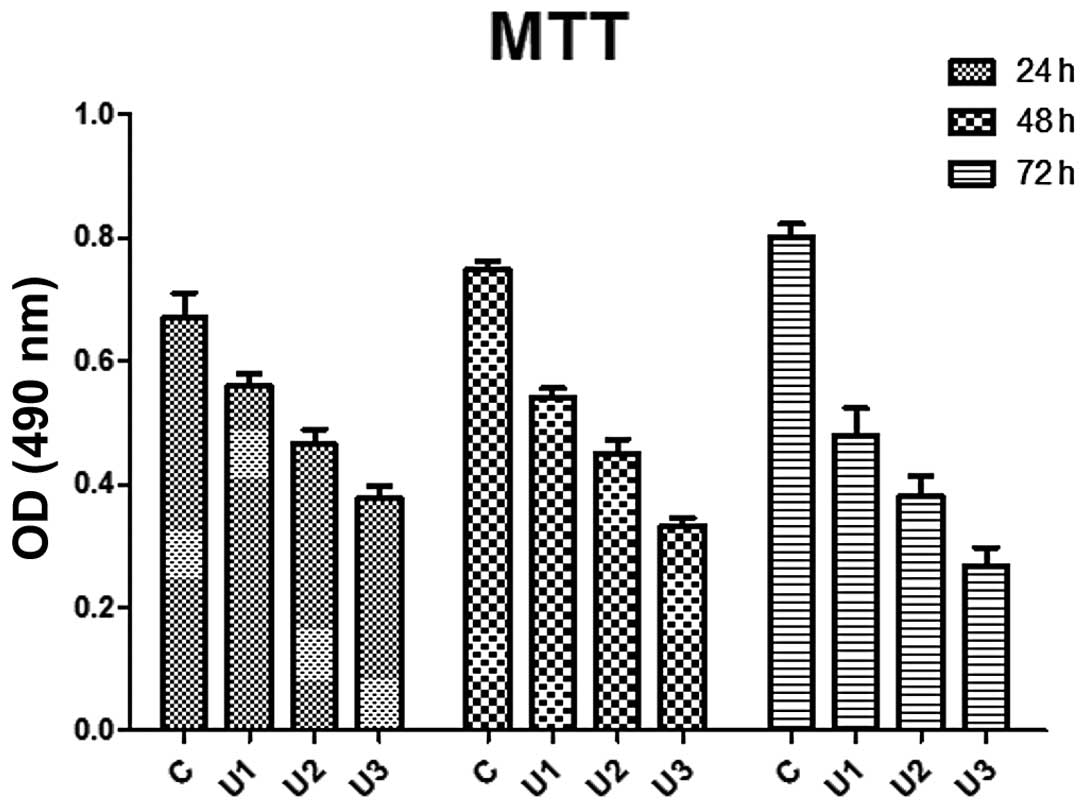 | Figure 1Effect of UTI on LoVo cell growth.
Compared with the control, the growth rates of the UTI1-3 LoVo
cells were reduced by 16.29, 30.62 and 43.73%; 27.69, 39.95 and
55.62%; and 40.24, 52.52% and 65.62%, respectively, after 24, 48,
and 72 h. The cells were reseeded into 96-well culture plates and
growth was assessed by MTT assay. Each value is expressed as the
mean of three experiments and the error bars denote the standard
deviation. C, control; U1, cells treated with 400 U/ml UTI; U2,
cells treated with 800 U/ml UTI; U3, cells treated with 1,600 U/ml
UTI; UTI, ulinastatin; OD, optical density. |
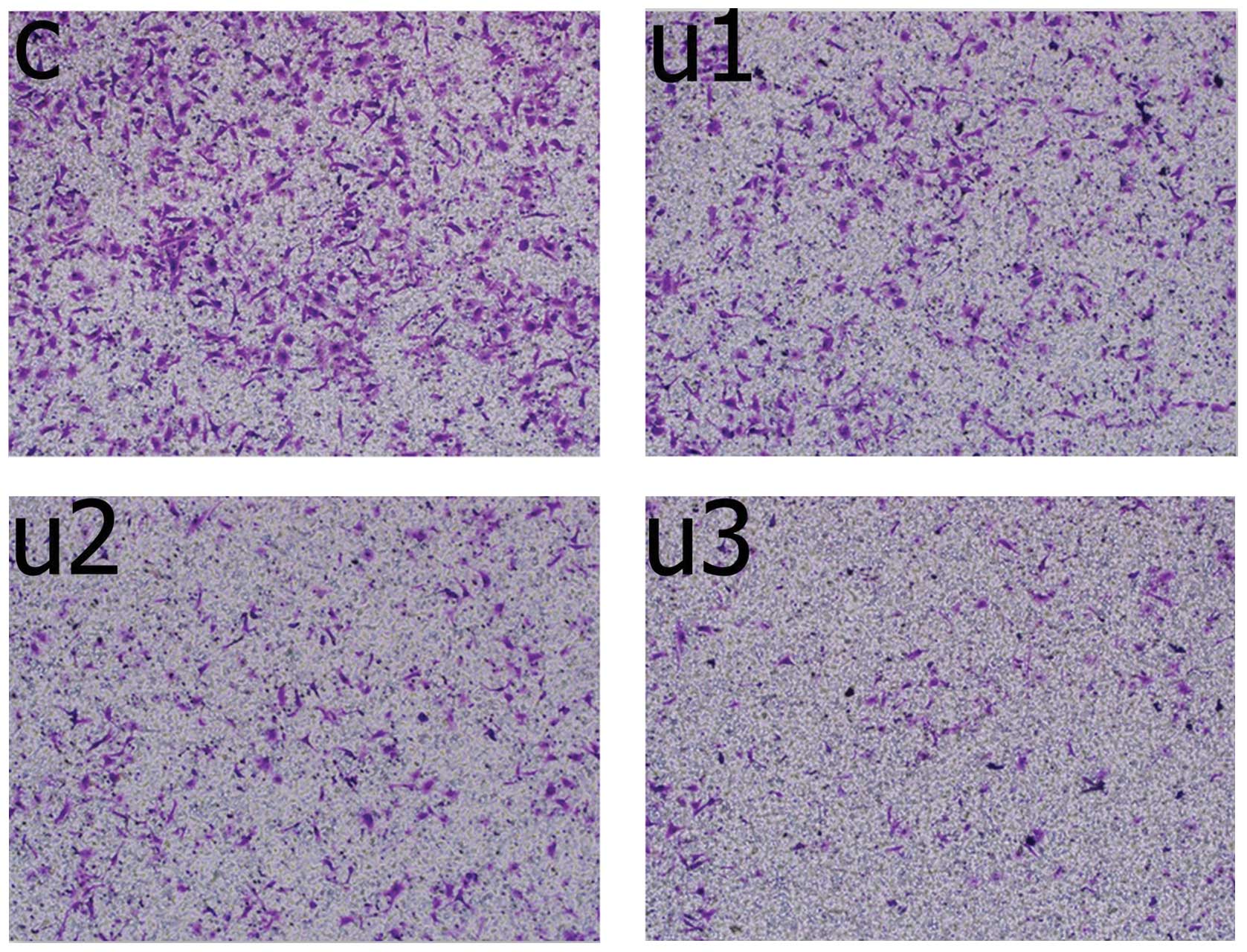
In vitro invasion
Following treatment (48 h), the number of UTI1 cells
that had invaded the membrane were significantly lower (95±6
cells/well), as compared with the untreated cells (153±11
cells/well, P=0.0001, unpaired Mann-Whitney U test; Fig. 2). The number of invading UTI2 cells
was significantly lower (38±5 cells/well), as compared with the
UTI1 cells (P=0.0001, unpaired Mann-Whitney U test). The number of
invading UTI3 cells was significantly lower (15±3 cells/well), as
compared with the UTI2 cells (P=0.003, unpaired Mann-Whitney U
test).
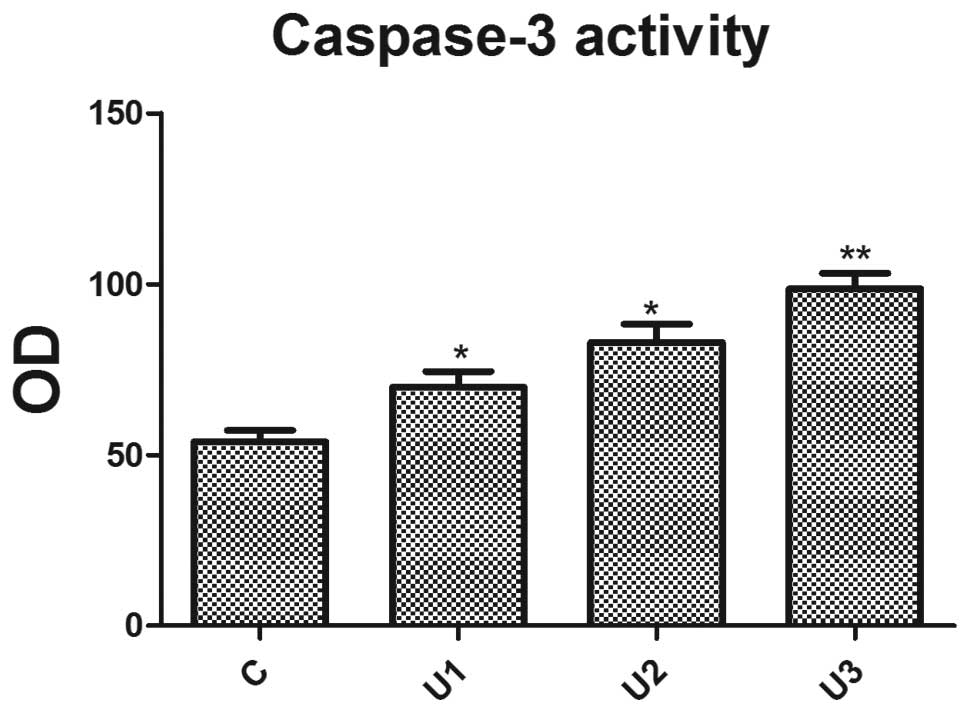
Caspase-3 activity
Using a fluorescent substrate peptide, the caspase-3
activity in cell lysates, prepared from UTI-treated LoVo cells, was
measured. UTI effectively enhanced caspase-3 activity (Fig. 3) and a significant difference
(P<0.05) was observed between the UTI groups.
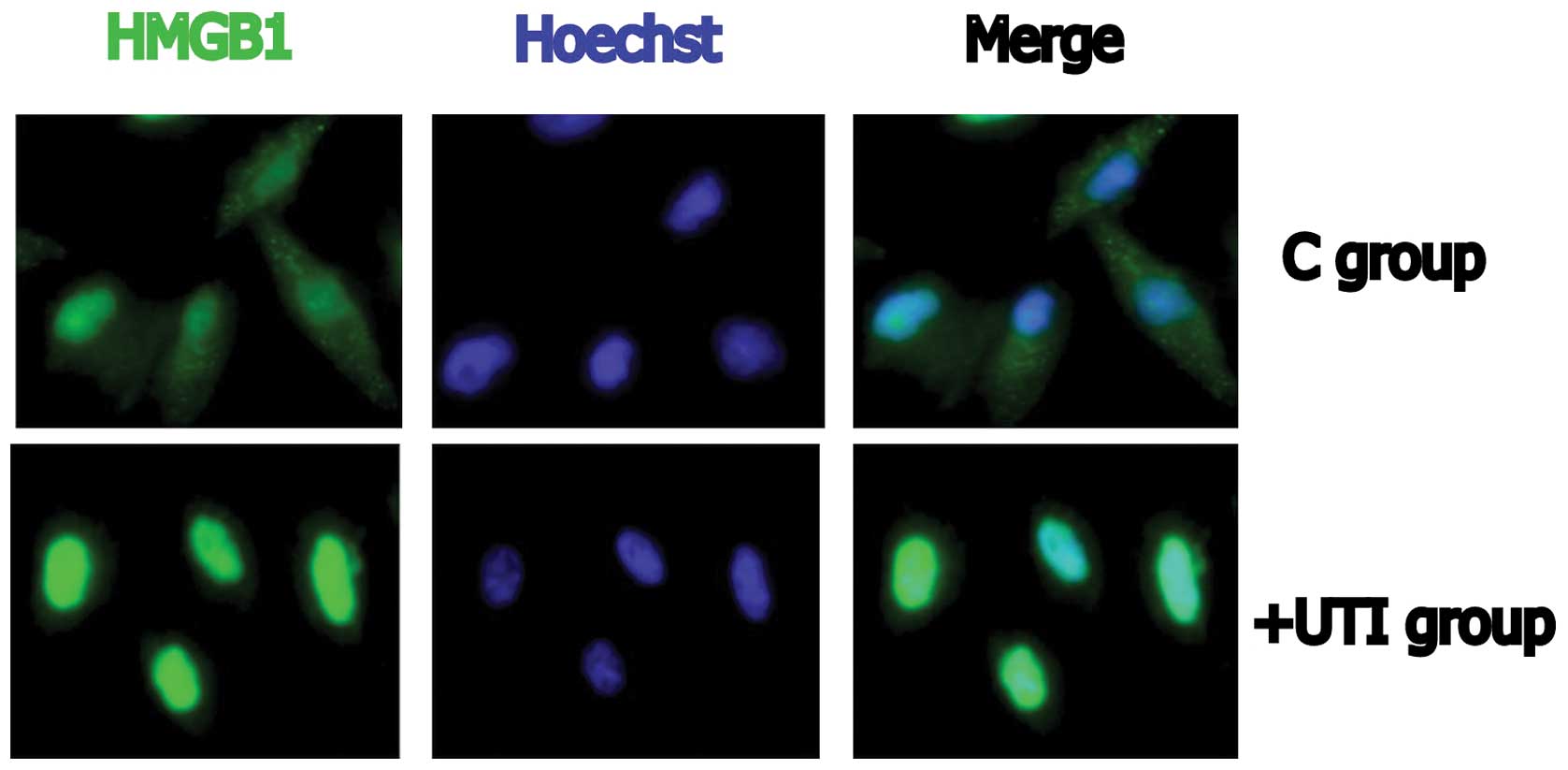
Immunofluorescence imaging
Cytoplasmic HMGB1 was observed in the control cells
(Fig. 4). In the UTI-treated
cells, HMGB1 was present mainly in the nucleus, indicating that the
distribution of HMGB1 had been altered. All data are representative
of two or three experiments. Fig.
4 depicts the UTI1 cells.
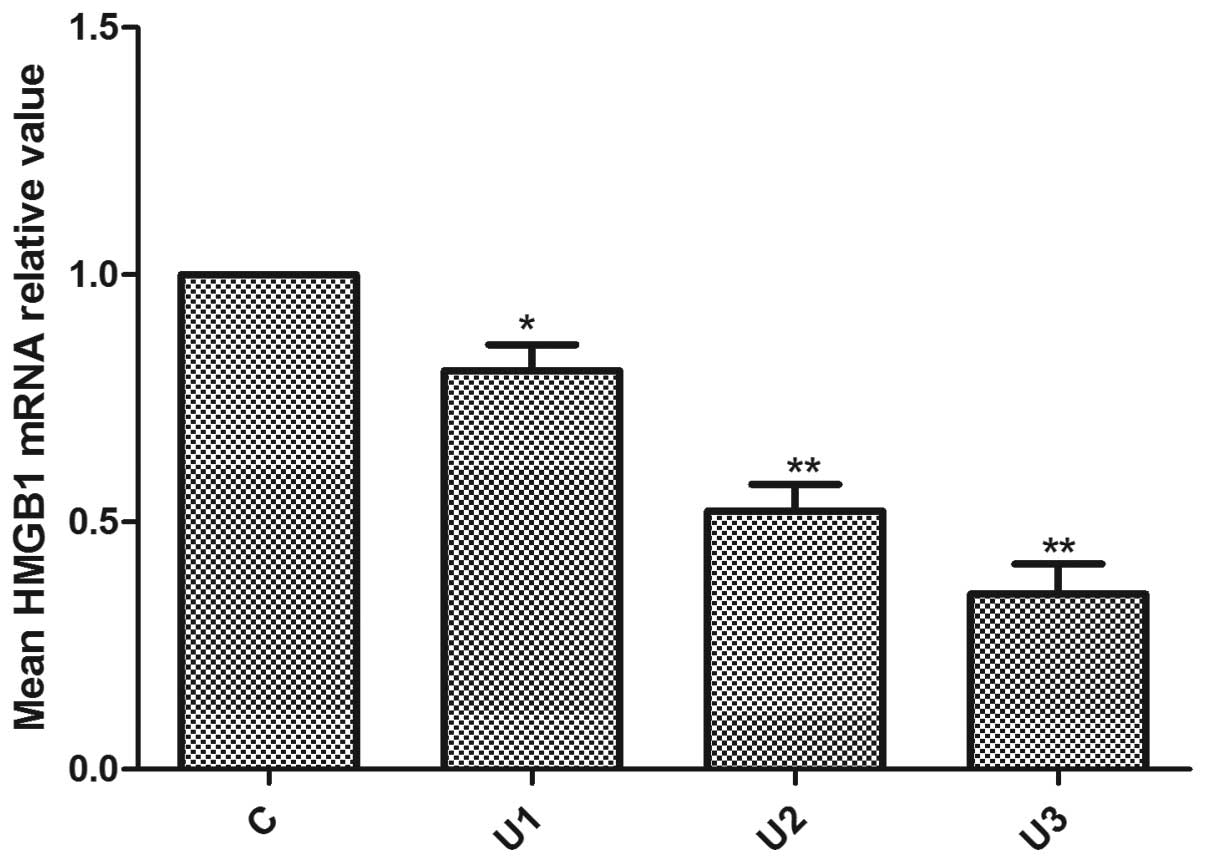
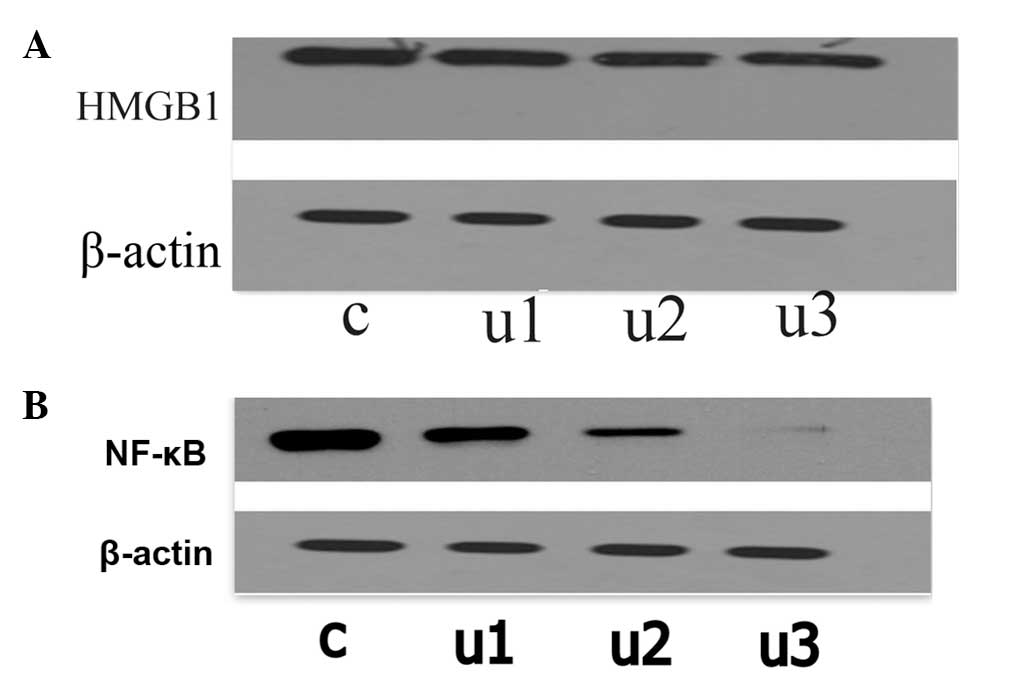
Effects of UTI on the expression of
HMGB1
The LoVo cells were exposed to UTI for 48 h and the
mRNA and protein expression of HMGB1 were examined by qPCR
(Fig. 5) and immunoblotting
(Fig. 6A). The mRNA levels of
HMGB1 were reduced following UTI treatment in comparison with the
control group. The mRNA level of HMGB1 in the control cells
remained unaltered. The protein levels of HMGB1 the in UTI1, UTI2
and UTI3 cells were reduced by 14.48, 31.69 and 43.56%,
respectively, compared with the control cells.
Effects of UTI on the protein expression
of NF-κB
The LoVo cells were exposed to UTI for 48 h and the
protein expression of NF-κB was examined using immunoblotting
(Fig. 6B). The results
demonstrated that UTI significantly inhibited the protein
expression of NF-κB compared with the control group
(P<0.05).
Discussion
HMGB1 is a nuclear protein that is involved in the
process of carcinogenesis (35).
HMGB1 is upregulated in certain types of tumor, including colon
adenoma and carcinoma and HMHB1 overexpression is observed in
prostate cancer and malignant melanoma cells (14,36,37).
HMGB1 is also overexpressed in colorectal cancer cells (HCT116,
HT-29, SW480 and DLD-1), derived from primary lesions and LoVo and
SW620 cells, derived from metastatic lymph nodes (38). Overexpression of HMGB1 promotes
cell motility, invasiveness, proliferation and angiogenesis in
cancer progression (39) and
results in the release or secretion of higher quantities of the
protein in the tumor microenvironment. This confers a selective
advantage to cancer cells, promoting more effective angiogenesis
and facilitating metastatic spread (35). Overexpression of HMGB1 inhibits
apoptosis while increasing the activity of NF-κB and leads to the
overexpression of the anti-apoptotic NF-κB target gene product
c-IAP2 in vitro. Further analysis has revealed a correlation
between increased levels of HMGB1 and increased quantities of
c-IAP2 in colon tumors. The overexpression of HMGB1 also inhibits
the activity of caspase-9 and caspase-3 (24). Released HMGB1 activates
tumor-associated macrophages and cancer cells, resulting in the
secretion of proinflammatory cytokines, including TNF-α,
interferon-c and interleukin (IL)-1b (40). This further stimulates the division
of cancer cells and endothelial cells, the latter leading to
neoangiogenesis (41,42). It has been demonstrated that UTI
inhibits the release TNF-α, IL-1 and IL-6, prompting investigation
of the therapeutic use of UTI in the inhibition of HMGB1 activity.
UTI significantly prevents the pulmonary metastasis of mouse Lewis
lung carcinoma 3LL cells significantly (43) and it has been suggested that UTI is
important in inhibiting the invasion and metastasis of tumor cells
(44), possibly by the direct
inhibition of cell-associated plasmin activity and by inhibiting
the mRNA expression of uPA and its receptor uPAR (45). UTI also inhibits the upregulated
expression of uPA and uPAR, possibly through MAPK-dependent
signaling cascades, in ovarian carcinoma cells in vitro and
in vivo (46) and UTI also
downregulates the expression of CXC chemokine receptor 4 and matrix
metalloproteinase-9 (47). In the
present study, treatment with UTI significantly inhibited LoVo cell
proliferation. RT-qPCR and western blot analysis demonstrated that
UTI inhibited the expression of HMGB1 mRNA and protein,
respectively, decreasing their expression in the UTI-treated LoVo
cells, compared with the untreated control cells. A possible reason
for this is that NF-κB activation is required for the migration of
cells to sites of tissue damage in response to the danger signal
HMGB1 (48) and UTI may decrease
NF-κB signal transduction (49).
The present study also demonstrated that UTI inhibited NF-κB.
Brezniceanu et al (50)
observed that the overexpression of HMGB1 inhibits Bak-induced cell
death in the human colon carcinoma cell line, RKO. In the present
study, UTI enhanced caspase-3 activity, possibly by inhibiting the
expression of HMGB1. Another possibility is that UTI may have
inhibited MAPK/extracellular signal-regulated signal kinase (ERK)
kinase (MEK) 1/2 (45). Degryse
et al (18) demonstrated
that phosphorylated ERKs promote smooth muscle cell migration in
response to HMGB1. ERK1/2 and MEK1/2 are rapidly phosphorized by
HMGB1 in 3T3 fibroblasts and U0126, a MAPK/MEK 1/2-specific
inhibitor, suppresses the migration induced by HMGB1 (18). HMGB1 stimulates ERK activity and it
has been established that PMA induces ERK activity in a number of
systems (51). It has been
suggested that the effect of PMA on the expression of other genes
is a result of activation of the classic pathway, the
RAS/Raf-1/MEK/ERK signaling cascade (52). An alternative pathway involves the
sequential activation of Rac1, MEK1, c-Jun N-terminal kinase (JNK)
and the JNK subset of MAPK (50).
UTI exerts its effects possibly through inhibition of the upstream
components of ERK activation in the MAPK cascade, which are a set
of signaling molecules that teleologically alter gene expression
(45).
The present study also demonstrated that UTI altered
the distribution of HMGB1. Cytoplasmic HMGB1 was entirely absent or
present at low levels in normal tissues and normal fibroblast cell
lines, whereas high levels of cytoplasmic HMGB1 were observed in
the tumor cell. The decreased affinity of HMGB1 to bind to DNA may
be associated with its transport into the cytoplasm. The
cytoplasmic transport of HMGB1 results from its phosphorylation
(53). Kang et al (54)observed that the secretion of HMGB1
is correlated with an increase in the invasiveness of cancer cells.
Following transport into the cytoplasm, phosphorylated HMGB1 is
secreted from the cell and the activation of genes associated with
cell migration by phosphorylated HMGB1 affects tumor progression
(54). In addition, the
phosphorylation of serine 35, 39 and 42 of the nuclear localization
signal 1 region in HMGB1 is essential for HMGB1 transport into the
cytoplasm and these serine residues are consistent with the
predicted PKC binding site (54).
The PKC family is comprised of at least 12 serine-threonine kinases
and these are divided into three major groups (55,56).
The most important cancer-associated targets of PKC are ERK1/2,
glycogen synthase kinase-3 beta, NF-κB and P-glycoprotein (57,58).
The addition of phosphate groups to the HMGB1 protein decreases its
DNA-binding activity and inhibits its accumulation in the nucleus.
The nuclear or cytoplasmic localisation of HMGBl proteins may rely
on their affinity for DNA (59).
Therefore, the hypophosphorylated HMGB1 protein in cancer cells may
increase its activitiy as a nuclear DNA-binding protein (14,45).
The present study demonstrated that UTI significantly decreased the
movement of HMGB1 from the nucleus to the cytoplasm, which may have
been caused by exogenous UTI-induced inhibition of the increased
membrane-associated PKC and decreased cytosolic PKC activity
(60). In conclusion, the present
study demonstrated that UTI inhibited the expression of HMGB1 in
LoVo cells.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 81302758) and the
President Fund of Nanfang Hospital (no. 2011B007).
References
|
1
|
Czura CJ, Wang H and Tracey KJ: Dual roles
for HMGB1: DNA binding and cytokine. J Endotoxin Res. 7:315–321.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Agresti A and Bianchi ME: HMGB proteins
and gene expression. Curr Opin Genet Dev. 2:170–178. 2003.
View Article : Google Scholar
|
|
3
|
Andersson U, Erlandsson-Harris H, Yang H
and Tracey KJ: HMGB1 as a DNA-binding cytokine. J Leukoc Biol.
6:1084–1091. 2002.
|
|
4
|
Muller S, Scaffidi P, Degryse B, et al:
New EMBO members’ review: the double life of HMGB1 chromatin
protein: architectural factor and extracellular signal. Embo J.
20:4337–4340. 2001. View Article : Google Scholar
|
|
5
|
Bustin M, Lehn DA and Landsman D:
Structural features of the HMG chromosomal proteins and their
genes. Biochim Biophys Acta. 1049:231–243. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Andersson U, Wang H, Palmblad K, et al:
High mobility group 1 protein (HMG-1) stimulates proinflammatory
cytokine synthesis in human monocytes. J Exp Med. 192:565–570.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang H, Wang H and Tracey KJ: HMG-1
rediscovered as a cytokine. Shock. 15:247–253. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Scaffidi P, Misteli T and Bianchi ME:
Release of chromatin protein HMGB1 by necrotic cells triggers
inflammation. Nature. 418:191–195. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Czura CJ and Tracey KJ: Targeting high
mobility group box 1 as a late-acting mediator of inflammation.
Crit Care Med. 31:S46–S50. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hanahan D and Weinberg RA: The hallmarks
of cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun KK, Ji C, Li X, et al: Overexpression
of high mobility group protein B1 correlates with the proliferation
and metastasis of lung adenocarcinoma cells. Mol Med Rep.
7:1678–1682. 2013.PubMed/NCBI
|
|
12
|
Taguchi A, Blood DC, Del TG, et al:
Blockade of RAGE-amphoterin signalling suppresses tumour growth and
metastases. Nature. 405:354–360. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiang YY, Wang DY, Tanaka M, et al:
Expression of high-mobility group-1 mRNA in human gastrointestinal
adenocarcinoma and corresponding non-cancerous mucosa. Int J
Cancer. 74:1–6. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Poser I, Golob M, Buettner R and
Bosserhoff AK: Upregulation of HMG1 leads to melanoma inhibitory
activity expression in malignant melanoma cells and contributes to
their malignancy phenotype. Mol Cell Biol. 23:2991–2998. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cabart P, Kalousek I, Jandová and Hrkai Z:
Differential expression of nuclear HMG1, HMG2 proteins and H1(zero)
histone in various blood cells. Cell Biochem Funct. 13:125–133.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Flohr AM, Rogalla P, Meiboom M, et al:
Variation of HMGB1 expression in breast cancer. Anticancer Res.
21:3881–3885. 2001.
|
|
17
|
Muller S, Ronfani L and Bianchi ME:
Regulated expression and subcellular localization of HMGB1, a
chromatin protein with a cytokine function. J Intern Med.
255:332–343. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Degryse B, Bonaldi T, Scaffidi P, et al:
The high mobility group (HMG) boxes of the nuclear protein HMG1
induce chemotaxis and cytoskeleton reorganization in rat smooth
muscle cells. J Cell Biol. 152:1197–1206. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guazzi S, Strangio A, Franzi AT and
Bianchi ME: HMGB1, an architectural chromatin protein and
extracellular signalling factor, has a spatially and temporally
restricted expression pattern in mouse brain. Gene Expr Patterns.
3:29–33. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hori O, Brett J, Slattery T, et al: The
receptor for advanced glycation end products (RAGE) is a cellular
binding site for amphoterin. Mediation of neurite outgrowth and
co-expression of rage and amphoterin in the developing nervous
system. J Biol Chem. 270:25752–25761. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ono M, Torisu H, Fukushi J, et al:
Biological implications of macrophage infiltration in human tumor
angiogenesis. Cancer Chemother Pharmacol. 43:69–71. 1999.
View Article : Google Scholar
|
|
22
|
Schlueter C, Weber H, Meyer B, et al:
Angiogenetic signaling through hypoxia: HMGB1: an angiogenetic
switch molecule. Am J Pathol. 166:1259–12563. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chuangui C, Peng T and Zhentao Y: The
expression of high mobility group box 1 is associated with lymph
node metastasis and poor prognosis in esophageal squamous cell
carcinoma. Pathol Oncol Res. 18:1021–1027. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Völp K, Brezniceanu ML, Bösser S, et al:
Increased expression of high mobility group box 1 (HMGB1) is
associated with an elevated level of the antiapoptotic c-IAP2
protein in human colon carcinomas. Gut. 55:234–242. 2006.
View Article : Google Scholar
|
|
25
|
Dong YD, Cui L, Peng CH, et al: Expression
and clinical significance of HMGB1 in human liver cancer: Knockdown
inhibits tumor growth and metastasis in vitro and in vivo. Oncol
Rep. 29:87–94. 2013.
|
|
26
|
Zeh HR 3rd and Lotze MT: Addicted to
death: invasive cancer and the immune response to unscheduled cell
death. J Immunother. 28:1–9. 2005. View Article : Google Scholar
|
|
27
|
Lindqvist A, Rouet P, Salier JP and
Akerström B: The alpha1-microglobulin/bikunin gene:
characterization in mouse and evolution. Gene. 234:329–336. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kanayama N, Halim A, Maehara K, et al:
Kunitz-type trypsin inhibitor prevents LPS-induced increase of
cytosolic free Ca2+ in human neutrophils and HUVEC
cells. Biochem Biophys Res Commun. 207:324–330. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kobayashi H, Gotoh J, Fujie M and Terao T:
Characterization of the cellular binding site for the urinary
trypsin inhibitor. J Biol Chem. 269:20642–20647. 1994.PubMed/NCBI
|
|
30
|
Kobayashi H, Shinohara H, Ohi H, et al:
Urinary trypsin inhibitor (UTI) and fragments derived from UTI by
limited proteolysis efficiently inhibit tumor cell invasion. Clin
Exp Metastasis. 12:117–128. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kobayashi H, Fujie M, Shinohara H, et al:
Effects of urinary trypsin inhibitor on the invasion of
reconstituted basement membranes by ovarian cancer cells. Int J
Cancer. 57:378–384. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kobayashi H, Sugino D and Terao T: Urinary
trypsin inhibitor, a Kunitz-type protease inhibitor, modulates
tumor necrosis factor-stimulated activation and translocation of
protein kinase C in U937 cells. Int J Oncol. 12:95–105.
1998.PubMed/NCBI
|
|
33
|
Tanaka R, Fujita M, Tsuruta R, et al:
Urinary trypsin inhibitor suppresses excessive generation of
superoxide anion radical, systemic inflammation, oxidative stress,
and endothelial injury in endotoxemic rats. Inflamm Res.
59:597–606. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Albini A, Iwamoto Y, Kleinman HK, et al: A
rapid in vitro assay for quantitating the invasive potential of
tumor cells. Cancer Res. 47:3239–3245. 1987.PubMed/NCBI
|
|
35
|
Ellerman JE, Brown CK, de Vera M, et al:
Masquerader: high mobility group box-1 and cancer. Clin Cancer Res.
13:2836–2848. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sasahira T, Akama Y, Fujii K and Kuniyasu
H: Expression of receptor for advanced glycation end products and
HMGB1/amphoterin in colorectal adenomas. Virchows Arch.
446:411–415. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ishiguro H, Nakaigawa N, Miyoshi Y, et al:
Receptor for advanced glycation end products (RAGE) and its ligand,
amphoterin are overexpressed and associated with prostate cancer
development. Prostate. 64:92–100. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yao Xingjun, Zhao Gang, Yang Hongfa, et
al: Overexpression of high-mobility group box 1 correlates with
tumor progression and poor prognosis in human colorectal carcinoma.
J Cancer Res Clin Oncol. 136:677–684. 2010. View Article : Google Scholar
|
|
39
|
Yao X, Zhao G, Yang H, et al:
Overexpression of high-mobility group box 1 correlates with tumor
progression and poor prognosis in human colorectal carcinoma. J
Cancer Res Clin Oncol. 136:677–684. 2010. View Article : Google Scholar
|
|
40
|
Srikrishna G and Freeze HH: Endogenous
damage-associated molecular pattern molecules at the crossroads of
inflammation and cancer. Neoplasia. 11:615–28. 2009.PubMed/NCBI
|
|
41
|
Srikrishna G and Freeze HH: Endogenous
damage-associated molecular pattern molecules at the crossroads of
inflammation and cancer. Neoplasia. 11:615–628. 2009.PubMed/NCBI
|
|
42
|
Le Bitoux MA and Stamenkovic I: Tumor-host
interactions: the role of inflammation. Histochem Cell Biol.
6:1079–1090. 2008. View Article : Google Scholar
|
|
43
|
Kobayashi H, Shinohara H, Fujie M, et al:
Inhibition of metastasis of Lewis lung carcinoma by urinary trypsin
inhibitor in experimental and spontaneous metastasis models. Int J
Cancer. 63:455–462. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shu H, Liu K, He Q, et al: Ulinastatin, a
protease inhibitor, may inhibit allogeneic blood
transfusion-associated pro-inflammatory cytokines and systemic
inflammatory response syndrome and improve postoperative recovery.
Blood Transfus. 109–118. 2014.
|
|
45
|
Kobayashi H, Suzuki M, Hirashima Y and
Terao T: The protease inhibitor bikunin, a novel anti-metastatic
agent. Biol Chem. 384:749–754. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sun ZJ, Yu T, Chen JS, et al: Effects of
ulinastatin and cyclophosphamide on the growth of xenograft breast
cancer and expression of CXC chemokine receptor 4 and matrix
metalloproteinase-9 in cancers. J Int Med Res. 38:967–976. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Palumbo R, Galvez BG, Pusterla T, et al:
Cells migrating to sites of tissue damage in response to the danger
signal HMGB1 require NF-kappaB activation. J Cell Biol. 179:33–40.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang H, Sun X, Gao F, et al: Effect of
ulinastatin on growth inhibition, apoptosis of breast carcinoma
cells is related to a decrease in signal conduction of JNk-2 and
NF-kappaB. J Exp Clin Cancer Res. 31:22012. View Article : Google Scholar
|
|
49
|
Brezniceanu ML, Völp K, Bösser S, et al:
HMGB1 inhibits cell death in yeast and mammalian cells and is
abundantly expressed in human breast carcinoma. Faseb J.
17:1295–1297. 2003.PubMed/NCBI
|
|
50
|
Jones LG, Ella KM, Bradshaw CD, et al:
Activations of mitogen-activated protein kinases and phospholipase
D in A7r5 vascular smooth muscle cells. J Biol Chem.
269:23790–23799. 1994.PubMed/NCBI
|
|
51
|
Nguyen DH, Catling AD, Webb DJ, et al:
Myosin light chain kinase functions downstream of Ras/ERK to
promote migration of urokinase-type plasminogen
activator-stimulated cells in an integrin-selective manner. J Cell
Biol. 146:149–164. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Olson MF, Ashworth A and Hall A: An
essential role for Rho, Rac, and Cdc42 GTPases in cell cycle
progression through G1. Science. 269:1270–1272. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kobayashi H, Suzuki M, Kanayama N, et al:
Suppression of urokinase receptor expression by bikunin is
associated with inhibition of upstream targets of extracellular
signal-regulated kinase-dependent cascade. Eur J Biochem.
269:3945–3957. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kang HJ, Lee H, Choi HJ, et al:
Non-histone nuclear factor HMGB1 is phosphorylated and secreted in
colon cancers. Lab Invest. 89:948–959. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Nishizuka Y: Intracellular signaling by
hydrolysis of phospholipids and activation of protein kinase C.
Science. 5082:607–614. 1992. View Article : Google Scholar
|
|
56
|
Paolucci L and Rozengurt E: Protein kinase
D in small cell lung cancer cells: rapid activation through protein
kinase C. Cancer Res. 3:572–577. 1999.
|
|
57
|
Goode N, Hughes K, Woodgett R and Parker
PJ: Differential regulation of glycogen synthase kinase-3 beta by
protein kinase C isotypes. J Biol Chem. 267:16878–16882.
1992.PubMed/NCBI
|
|
58
|
Burgering BM, de Vries-Smits AM, Medema
RH, et al: Epidermal growth factor induces phosphorylation of
extracellular signal-regulated kinase 2 via multiple pathways. Mol
Cell Biol. 13:7248–7256. 1993.PubMed/NCBI
|
|
59
|
Wisniewski JR, Schulze E and Sapetto B:
DNA binding and nuclear translocation of insect
high-mobility-group-protein-1 (HMG1) proteins are inhibited by
phosphorylation. Eur J Biochem. 225:687–693. 1994. View Article : Google Scholar
|
|
60
|
Kobayashi H, Suzuki M, Tanaka Y, et al:
Suppression of urokinase expression and invasiveness by urinary
trypsin inhibitor is mediated through inhibition of protein kinase
C- and MEK/ERK/c-Jun-dependent signaling pathways. J Biol Chem.
276:2015–2022. 2001. View Article : Google Scholar
|