Introduction
Breast cancer is a significant health concern
amongst females. Gene therapy as a treatment for cancer has
received increasing attention following the development of
efficient molecular techniques (1,2).
Studies have demonstrated that the development of novel blood
vessels surrounding tumors provide nutrition and remove metabolic
products, which promotes tumor growth and induces tumor metastasis
(3). Inhibition of angiogenesis
results in the apoptosis or necrosis of tumor cells (4). Angiogenesis inhibitors target
vascular endothelial cells, which are genetically stable and
invulnerable to mutation (5). In
addition, endogenous angiogenesis inhibitors are highly selective
and specifically inhibit the proliferation of endothelial cells
within lesions (6). Therefore, the
use of such inhibitors has become an effective strategy for the
treatment of various types of cancer (7).
Tumstatin was discovered as an endogenous
angiogenesis inhibitor, exhibiting marked anti-tumor activity in
2003 (8). Tumstatin exerts its
effects by inhibiting protein synthesis in vascular endothelial
cells and inducing apoptosis, thereby inhibiting angiogenesis. In
addition, tumstatin inhibits the proliferation of tumor cells and
promotes their apoptosis (9,10).
Furthermore, tumstatin is able to inhibit tumor growth and the
associated pathological angiogenesis without affecting
physiological angiogenesis (11).
Studies regarding the safety and effectiveness of
active fragments of tumstatin have attracted attention worldwide as
tumstatin was demonstrated to be a pathogenic antigen with a high
molecular weight, which may result in glomerulonephritis and lung
hemorrhage (12). In previous
studies by our group, amino acids 185–203 at the C terminus were
successfully transformed into the T21 peptide, which demonstrated
anti-angiogenic activity, and amino acids 75–95 at the N-terminus
were transformed into the T19 peptide, which exhibited anti-cancer
activity. The lower molecular weights of these fragments reduced
the immunogenicity and improved the safety of tumstatin. The T21
peptide was subsequently reconstructed with the
arginylglycylaspartic acid (RGD)-peptide sequence, which was shown
to inhibit the proliferation and migration of tumor cells. The two
active fragments of tumstatin, T21RGD and T19 peptide, were ligated
via a soft linker to form the recombinant T42 peptide, which was
constructed based on previous studies (13,14).
The activity of the constructed peptide was hypothesized to be
higher than that of any of the single fragments used in previous
studies. Previous studies demonstrated that the
tumstatin-associated T42 and quadruple (4x)T42 peptide had
significant anti-tumor activity (15,16)
and inhibited the growth and proliferation of the human cancer cell
line HepG2 while promoting apoptosis (9).
An adenovirus is a non-integrated double-stranded
DNA virus which is widely distributed throughout nature. Although
certain types of adenovirus cause acute gastrointestinal, pulmonary
or eye infection in humans (17),
the adenovirus used in gene therapy is comparatively safe and
carries a low risk of pathogenesis, teratogenesis or
carcinogenesis. As the most widely used vector in clinical trials
for gene therapy, the adenoviral vector has also been extensively
used in research. Adenovirus-mediated gene therapy is
conventionally conducted in vivo (18,19).
In previous experiments by our group, purified T42
peptide was applied to MCF-7 cells in vitro. T42 was found
to have an anti-tumor effect (data is not shown); however, the
metabolic rate of such small molecular proteins in the body was so
rapid that high, repeated doses were required. Therefore, it was
hypothesized that expression of the 4xT42 peptide gene may also
have anti-tumor effects and circumvent the requirement for high,
repeated doses. In the present study, it was demonstrated for the
first time, to the best of our knowledge, that adenoviral vectors
containing the T42 or quadruple T42 (4xT42) genes had antitumor
activity. By artificially synthesizing the T42 peptide gene, the
adenoviral vectors plasmid adenovirus-enhanced green fluorescent
protein-T42 (pAd-EGFP-T42) and pAd-EGFP-4xT42 were successfully
constructed using the isocaudarner ligation technique.
Subsequently, 293 cells were infected with pAd-EGFP-T42 and
pAd-EGFP-4xT42. To confirm the expression of the T42 and 4xT42
genes, GFP expression levels were monitored by fluorescence
microscopy and the expression of T42 was assessed by reverse
transcription polymerase chain reaction (RT-PCR). The anti-cancer
effects of pAd-EGFP-T42/pAd-EGFP-4xT42 on breast cancer cells in
vitro as well as in vivo were also preliminarily
investigated. In addition, the mechanism underlying the anticancer
activity was further investigated.
Materials and methods
Cells, drugs and animals
The human embryonic kidney 293 cell line and the
human breast cancer cell line MCF-7 were obtained from the American
Type Culture Collection (Manassas, VA, USA). The cells were
maintained in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen
Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal
bovine serum (FBS; Sijiqing Biological Engineering Materials Co.,
Hangzhou, China) at 37°C in 5% CO2. Female BALB/c
immunodeficient nude mice that were 6–8 weeks old and weighed 18–20
g were used. The mice were maintained under controlled conditions
of humidity (50 ± 10%), light (12/12 h light/dark cycle) and
temperature (23 ± 2°C), had ad libitum access to water and
were fed a pelleted basal diet. All animal handling and
experimental procedures were performed in accordance with the Guide
for the Care and Use of Laboratory Animals published by the
National Institutes of Health (NIH Published No. 85-23, revised
1996; Bethesda, MA, USA). The present study was approved by the
Medical Ethics Committee of Heilongjiang province (Daqing,
China),
Construction, collection, amplification
and identification of pAd-EGFP-T42, pAd-EGFP-4xT42 and
pAd-EGFP
The T42 gene was synthesized artificially according
to the reference sequence of protein
MPFLFCNVNDVCNFASRGDYSGGASPFLECHGRGTCN YYSNS (AXYBIO BIO-TECH CO.,
Changsha, China). Recognition sites for the restriction enzymes
BamHI and BglII-EcoRI were added to the 5′ and
3′ ends of the sequence, respectively. PCR followed by restriction
digestion and subsequent clone screening was used to generate
pEC3.1(+)-EGFP-T42 and pEC3.1(+)-EGFP-4xT42 from the recombinant
plasmids pEC3.1(+)-T42 and pEC3.1(+)-4xT42. The pAd-EGFP-T42,
pAd-EGFP-4xT42 and pAd-EGFP plasmids were constructed by
transferring the EGFP-4xT42, EGFP-T42 and EGFP expression cassettes
into pAd-BL-Dest adenoviral vectors (Invitrogen Life Technologies)
using homologous recombination in vitro by LR reaction
(20). The vector was then
linearized by single enzyme digestion with restriction endonuclease
Pac I (New England Biolabs, Inc., Ipswich, MA, USA) for
transfection. The linearized vector was transfected into 293 cells
and the GFP expression levels were examined to determine whether
the pAd)-EGFP-T42, pAd-EGFP-4xT42 and pAd-EGFP had been
successfully recombined. The successfully transfected 293 cells
were collected when a marked cytopathogenic effect was observed and
>50% of the cells were floating. The cells were subjected to
three rounds of freeze-thaw cycles and centrifugation at 12,000 × g
for 10 min at 4°C, and the viral fluid was collected. The
adenovirus was purified using the Adenovirus Purification Maxi kit
(ClonTech Laboratories, Inc., Mountain View, CA, USA). RT-PCR was
subsequently used to determine the viral load (20), using a kit purchased from Takara
Bio, Inc. (Otsu, Japan). To measure the viral titer, 20 μl viral
sample was serially diluted and cultivated for two days in a
24-well plate. The number of cells expressing GFP fluorescence was
counted under a fluorescence microscope (Nikon-TE2000-U; Nikon
Corporation, Tokyo, Japan). The viral titer was calculated using
the following equation: (Number of cells with fluorescence) ×
(dilution factor)/(quantity of viruses inoculated).
Plaque assay
The plaque assay utilized a confluent monolayer of
293 cells plated in six-well plates (NEST Science Co., Ltd.,
Shanghai, China). The samples were subjected to ten-fold serial
dilutions in DMEM containing glutamine, 5% heat-inactivated FBS and
0.05% gentamicin (Invitrogen Life Technologies). Diluted samples
(0.2 ml) were added to each well of cells in duplicate or
triplicate. Following sample inoculation, the plates were placed in
an incubator (Thermo Scientific 3111; Thermo Fisher Scientific,
Waltham, MA, USA) for 1 h at 37°C with 5% CO2. Every 15
min, the plates were agitated, allowing the sample dilution to move
over the monolayer and ensuring full coverage by the inoculum.
Following 1 h of incubation, the wells were covered with 2 ml 1:1
primary overlay comprising 1% agarose (SeaKem ME; 0.5% final
concentration) and 2X basal medium Eagle with Earle’s salts
[4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 10% FBS and
0.05% gentamicin]. The plates were incubated at 37°C with 5%
CO2 for seven days. At seven days post-inoculation, 2 ml
secondary staining overlay [primary overlay and 5% Neutral Red
stain (Santa Cruz Biotechnology, Inc., Dallas, TX, USA)] was added
to each well. At 24 and 48 h, the plaques were counted to determine
the sample viral titer in plaque forming units (Pfu)/ml. The
minimum detection limit of the plaque assay was calculated as
<1.666 Pfu based on the observation of one plaque out of three
replicates (average=0.333) counted in a sample of 0.2 ml virus
added to cells. The maximum detection limit in the current format
was >5×108 Pfu/ml [based on the observation of more
than 100 plaques in the replicates of the six-fold dilution
(100×5×106)].
Multiplicity of infection
MCF-7 cells were seeded into 24-well plates at
5×104 cells/well. pAd-EGFP-T42, pAd-EGFP-4xT42 or
pAd-EGFP was added at MOIs of 0, 25, 50, 100 and 200, respectively
(six wells for each MOI). Following 4 h of incubation, cells were
washed twice with 500 μl PBS prior to the addition of 500 μl DMEM
containing 10% FBS to each well. Following 24 h of further
incubation, the transduction efficiency was evaluated by observing
the expression of eGFP.
MTT analysis
Four groups were evaluated in the present study:
pAd-EGFP-T42, pAd-EGFP-4xT42, pAd-EGFP control and a blank control
group. At 24 h prior to infection, MCF-7 cells that were in an
exponential growth phase were plated in six-well plates at a
density of 4×105 cells/well. The following day, the
cells in each group were transfected and incubated for 18 h.
Following incubation, the success of transfection was evaluated by
examination of the cells under a fluorescence microscope
(Nikon-TE2000-U), and the cells in each group were transferred to
96-well plates at a density of 2×104 cells/well with an
MOI of 100. An MTT assay was subsequently conducted in each group
of cells at 12, 24, 36 and 48 h following transfection. To perform
the MTT assay, 25 μl fresh MTT (5 mg/ml; Sigma-Aldrich, St Louis,
MO, USA) was added to each well. The cells were then incubated at
37°C for 4 h to allow the incorporation and conversion of MTT to
the formazan derivative. The formazan derivative was solubilized by
the addition of 100 μl DMSO (Sigma-Aldrich). The absorbance values
were measured at 570 nm using an EXL808 plate reader (Bio-Tek
Instruments, Inc., Winooski, VT, USA).
Flow cytometric analysis
MCF-7 cells were prepared and separated into four
groups according to an identical protocol to that of the MTT assay.
Twenty-four hours following confirmation of transfection, 100 MOI
recombinant adenovirus or control pAd-EGFP adenovirus was added to
each well and the plates were incubated for 4 h at 37°C and 5%
CO2. The cells were then resuspended in ice-cold 70%
ethanol and stored at −20°C prior to analysis. The cellular DNA was
stained with PBS containing 100 μg/ml propidium iodide (Beyotime
Institute of Biotechnology, Nantong, China), 0.6% Nonidet P-40 and
100 μg/ml RNase A (Beyotime Institute of Biotechnology). The
samples (105) were analyzed using a FACS BD Aria flow
cytometer at 488 nm (BD Biosciences, Franklin Lakes, NJ, USA). Ten
thousand cells were analyzed using three replicates. The results
were analyzed using the FACSDiva™ version 6.1.1 (BD Biosciences)
software.
Western blot analysis
MCF-7 cells were inoculated in six-well plates at
4×105 cells/well and treated as aforementioned. A lysis
buffer [150 mmol/l NaCl, 1% NP-40, 0.02% sodium azide, 10 μg/ml
phenylmethanesulfonylfluoride and 50 mmol/l Tris-HCl (pH 8.0)] was
added to the cells at 150 μl per tube. The lysate was incubated on
ice for 30 min and centrifuged at 12,000 xg for 4 min at 4°C. The
supernatant was collected and the protein concentration was
analyzed using a bicinchoninic acid protein assay kit (Beyotime
Institute of Biotechnology). Subsequently, western blot analysis
was performed. Briefly, protein samples were electrophoresed by 10%
SDS-PAGE and transferred onto a nitrocellulose membrane (Millipore,
Billerica, MA, USA). The membrane was incubated in fresh blocking
buffer at room temperature for 1 h and then probed with the
following antibodies [all diluted at 1:1,000, v/v; Beyotime
Institute of Biotechnology): Mouse anti-human polyclonal β-actin,
mouse anti-human polyclonal anti-caspase-3, mouse anti-human
monoclonal anti-B-cell lymphoma 2 (Bcl-2) and rabbit anti-human
polyclonal Bcl-2-associated X protein in blocking buffer [0.1%
(v/v) Tween 20 in Tris-buffered saline, pH 7.4, containing 5% (w/v)
skimmed milk] at 4°C overnight. Following washing three times with
Tris-buffered saline containing 0.1% (v/v) Tween 20 (TBST) buffer
for 5 min each, the membrane was incubated with the appropriate
horseradish peroxidase-conjugated secondary antibody [goat
anti-mouse immunoglobulin G (IgG), 1:5,000; Kangchen Biotechnology,
Shanghai, China and goat anti-rabbit IgG, 1:2,000; Santa Cruz
Biotechnology, Inc. Dallas, TX, USA] at room temperature for 1 h
and then washed again three times in TBST buffer. The membrane was
incubated with enhanced chemiluminescence substrate solution (Santa
Cruz Biotechnology, Inc.) for 5 min according to the manufacturer’s
instructions prior to visualization with autoradiography film
(Bio-Rad ChemiDoc XRS; Bio-Rad Laboratories, Hercules, CA,
USA).
Protective effects of pAd-EGFP-T42 and
pAd-EGFP-4xT42 on a xenograft mouse model
MCF-7 cells in the exponential phase of growth were
adjusted to a density of 5×107 cells/ml. Mice were
inoculated subcutaneously in the right breast fat pad with 0.1 m1
MCF-7 cell suspension and a 10-mm3 (volume=long diameter
× short diameter2 × π/6) tumor formed ~two weeks
subsequently. The nude mice were divided randomly into four groups
of ten mice. Then, 1.0×109 Pfu/kg pAd-EGFP-T42,
pAd-EGFP-4xT42 or pAd-EGFP was injected locally into the tumor on
days one, five and ten. For the control group, PBS (0.1 ml total)
was injected locally into the tumor on an identical treatment
schedule. The tumor was dissected for measurement 28 days after the
first injection and the growth inhibition ratio of the tumor was
calculated using the following equation: Growth inhibition ratio of
the tumor=[1−(size of tumor of each group on day 28-size on day
0)/(size of tumor of the blank control group on day 28-size on day
0)] ×100%.
Histological examination
The entire tumor was embedded in paraffin. Paraffin
sections were subsequently dewaxed and antigen retrieval was
performed by incubating the sections in 0.01 mol/l citrate buffer
(pH 6.0) at 92–98°C. The sections were then incubated in 0.05%
trypsin (Beyotime Institute of Biotechnology) at 37°C for 30 min
followed by incubation in 3% H2O2 (Beyotime
Institute of Biotechnology) for 10 min. Primary labeling was
conducted using mouse anti-human CD34 monoclonal antibody
(Sigma-Aldrich) at a dilution of l:100 at 4°C overnight. Following
rewarming of the sections, the secondary antibody was added to the
slides followed by 3,3′-diaminobenzidine for a color reaction. The
sections were subsequently stained with hematoxylin. PBS was used
in place of the primary antibody as a negative control. To quantify
the vascular area, three areas of high vascular density were
selected at low magnification (×40) and the microvessels were
quantified in each region at high magnification (×200). The average
for each section was considered to indicate the microvascular
density.
Statistical analysis
Values are presented as the mean of ≥three
independent experiments ± standard deviation unless otherwise
stated. An unpaired Student’s t-test or analysis of variance was
used to determine the significance of differences between
experimental groups. The statistical analyses were performed using
SPSS version 13.0 software (SPSS Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant difference
between values.
Results
Successful construction of pAd-EGFP-T42
and pAd-EGFP-4xT42
The pAd-EGFP-T42 and pAd-EGFP-4xT42 plasmid DNA was
extracted and digested using Pac I. Following Pac I digestion, the
fragment sizes were determined as 3,000 bp and >15 kb, which
confirmed that the plasmid backbone was pAd-BL-dest and that the
pAd-EGFP-T42 and pAd-EGFP-4xT42 inserts were correct (20).
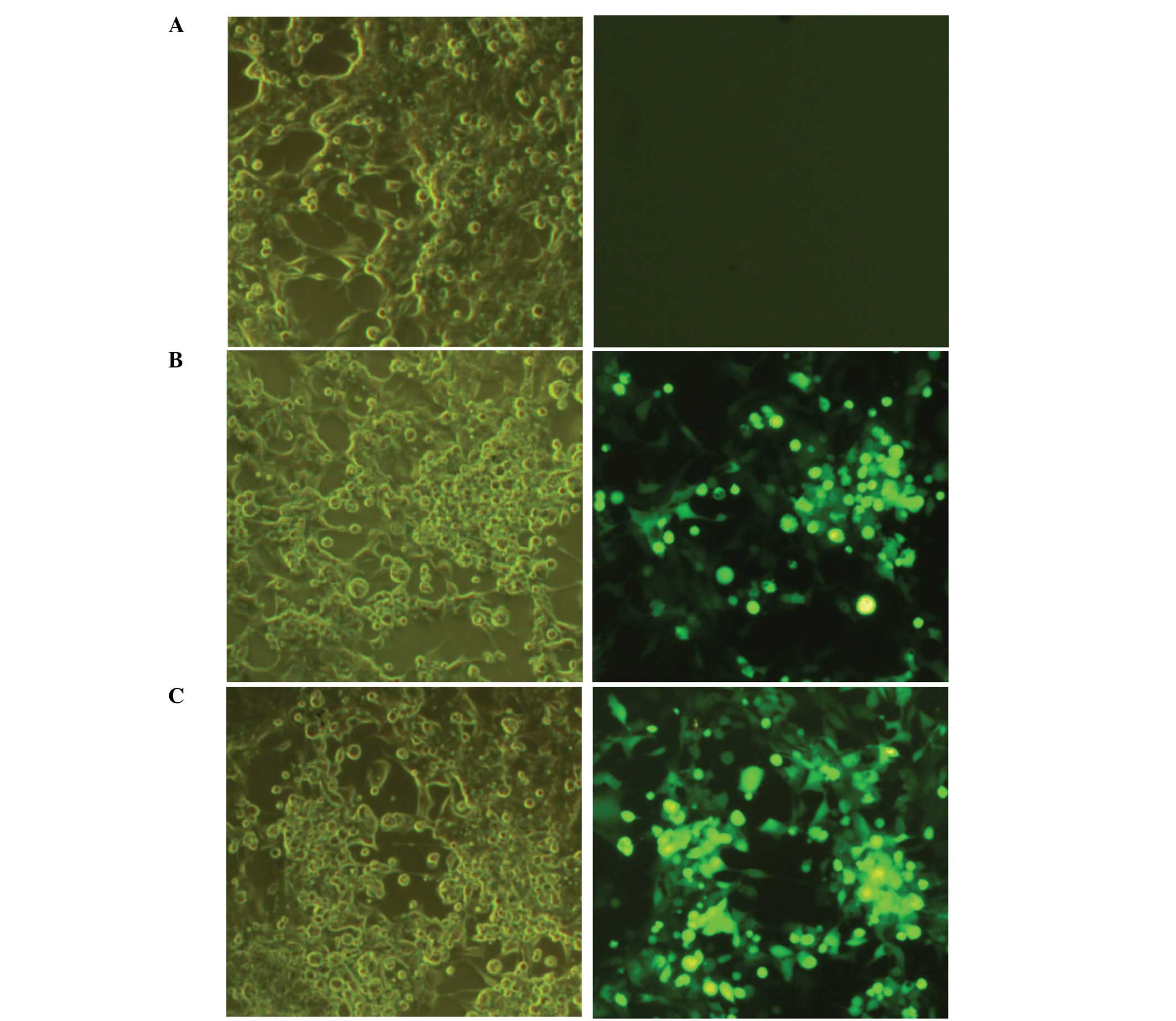
Transfection of 293 cells with
pAd-EGFP-4xT42
Following 24 h of transfection with pAd (empty
vector), pAd-EGFP-T42 or pAd-EGFP-4xT42, the virus demonstrated a
cytopathic effect on the transfected 293 cells and EGFP was
successfully identified by fluorescent microscopy (Fig. 1). Consistent with these results,
RT-PCR of the pAd-EGFP-T42 and pAd-EGFP-4xT42 adenoviruses
indicated that T42/4xT42 was expressed following transfection. The
virus titer of the 293 cells was also measured at 5×109
Pfu/ml.
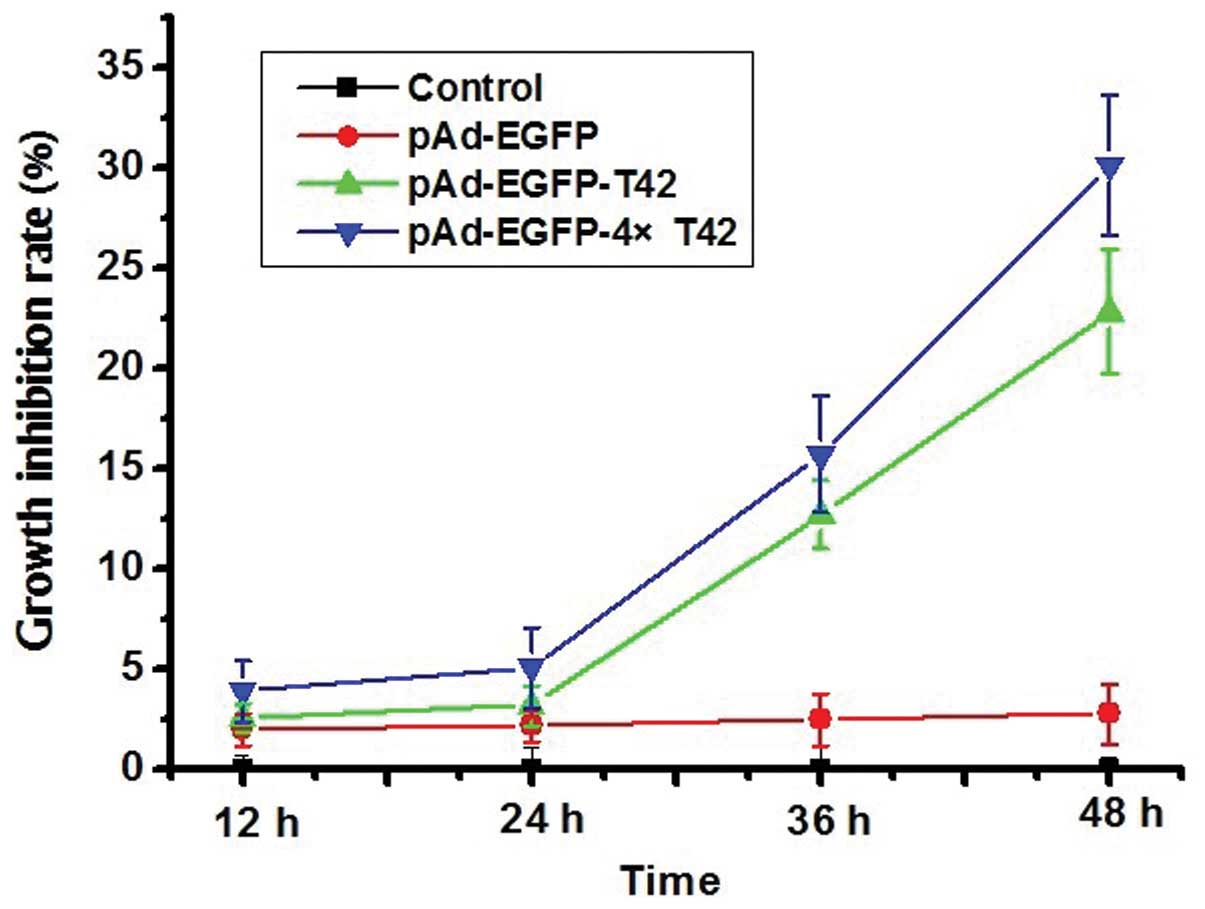
pAd-EGFP-4xT42 and pAd-EGFP-T42 enhance
tumor cell growth inhibition rate
The cell growth inhibition rate was significantly
enhanced in the pAd-EGFP-T42 and pAd-EGFP-4xT42 transfection groups
compared with that of the control group at 36 and 48 h (P<0.05).
However, there was no significant difference in the cell growth
inhibition rate between the pAd-EGFP plasmid transfection group and
that of the control group at any time-point (Fig. 2).
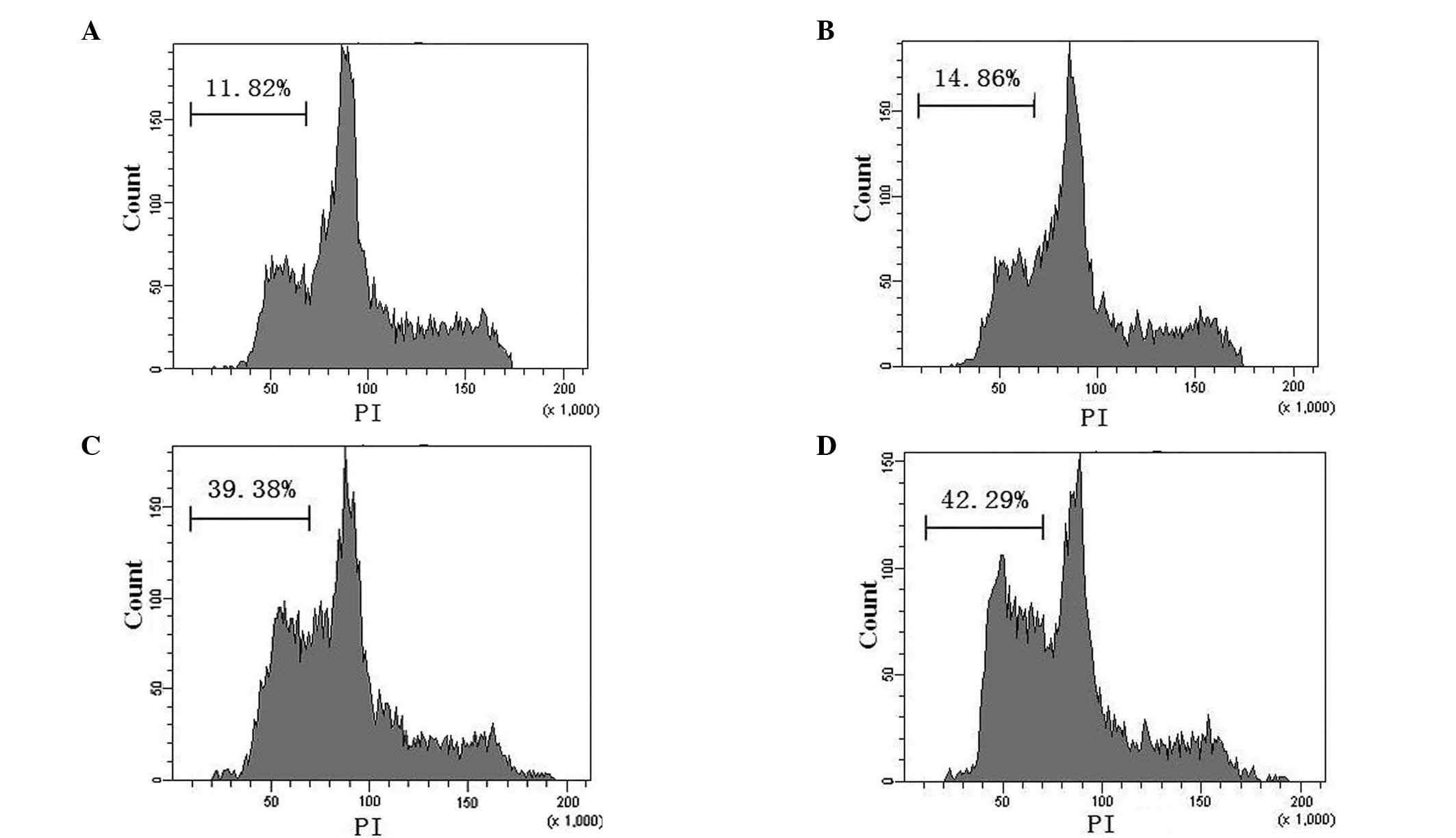
pAd-EGFP-4xT42 and pAd-EGFP-T42 enhance
MCF-7 cell apoptosis
The results of the flow cytometric analysis of
apoptosis are exhibited in Fig. 3.
The apoptotic rates of the blank control, pAd-EGFP, pAd-EGFP-T42
and pAd-EGFP-4xT42 groups were 11.82±2.31, 14.86±1.02, 39.38±2.17
and 42.29±3.26%, respectively. The apoptotic rates of the MCF-7
cells infected with pAd-EGFP-T42 or pAd-EGFP-4xT42 were
significantly higher than those of the cells infected with the
control adenovirus and the non-transfected cells in the blank
control group (P<0.05). There was no significant difference in
the apoptotic rate of the control group and that of the pAd-EGFP
group (P>0.05) or between that of the pAd-EGFP-T42 group and
that of the pAd-EGFP-4xT42 group.
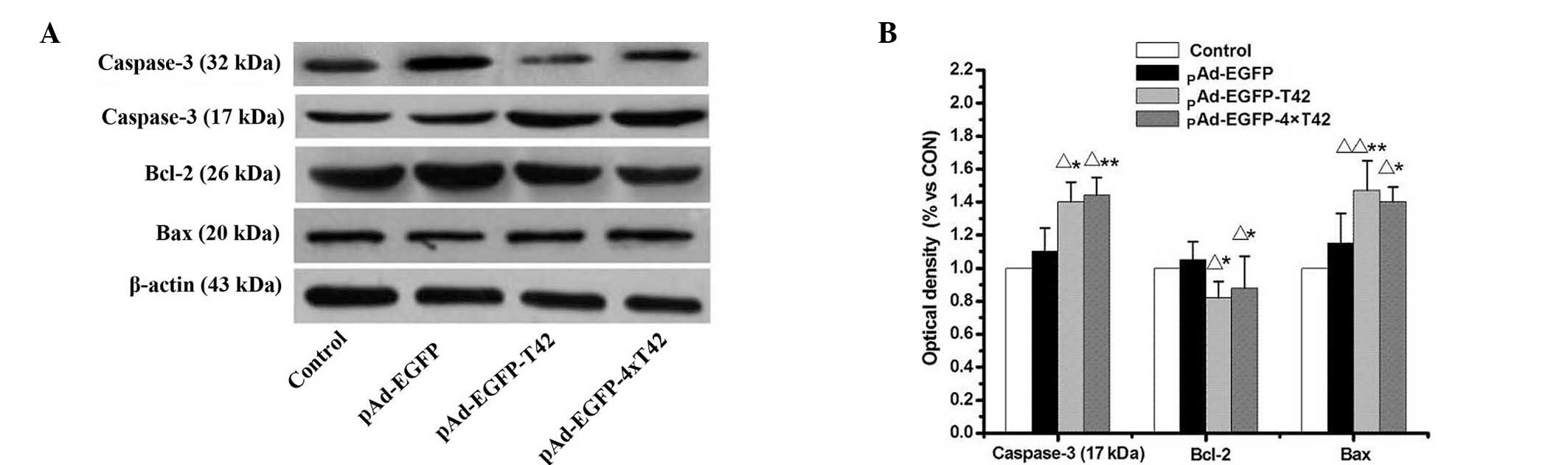
pAd-EGFP-T42 and pAd-EGFP-4xT42 increases
pro-apoptotic protein expression in MCF-7 cells
Western blot analysis was used to evaluate the
expression levels of apoptosis-associated proteins in MCF-7 cells
treated with pAd-EGFP-T42 and pAd-EGFP-4xT42. As indicated in
Fig. 4, expression levels of Bcl-2
were significantly decreased, while expression levels of Bax were
significantly enhanced in MCF-7 cells treated with pAd-EGFP-T42 and
pAd-EGFP-4xT42 in comparison to those of the control and pAd-EGFP
groups. The levels of the active 17 kDa cleaved caspase-3 fragment
were significantly increased in MCF-7 cells transfected with
pAd-EGFP-T42 and pAd-EGFP-4xT42, compared with those of the control
and pAd-EGFP groups.
Protective effects of pAd-EGFP-T42 and
pAd-EGFP-4xT42 on a xenograft mouse model
Mice were sacrificed by cervical dislocation 28 days
after injection of adenovirus, and the tumors were excised for
measurement. The average tumor sizes in each of the four groups
were 122.46±8.08, 113.28±6.37, 63.32±13.10 and 54.86±13.59
mm3, respectively. The inhibition rates of tumor growth
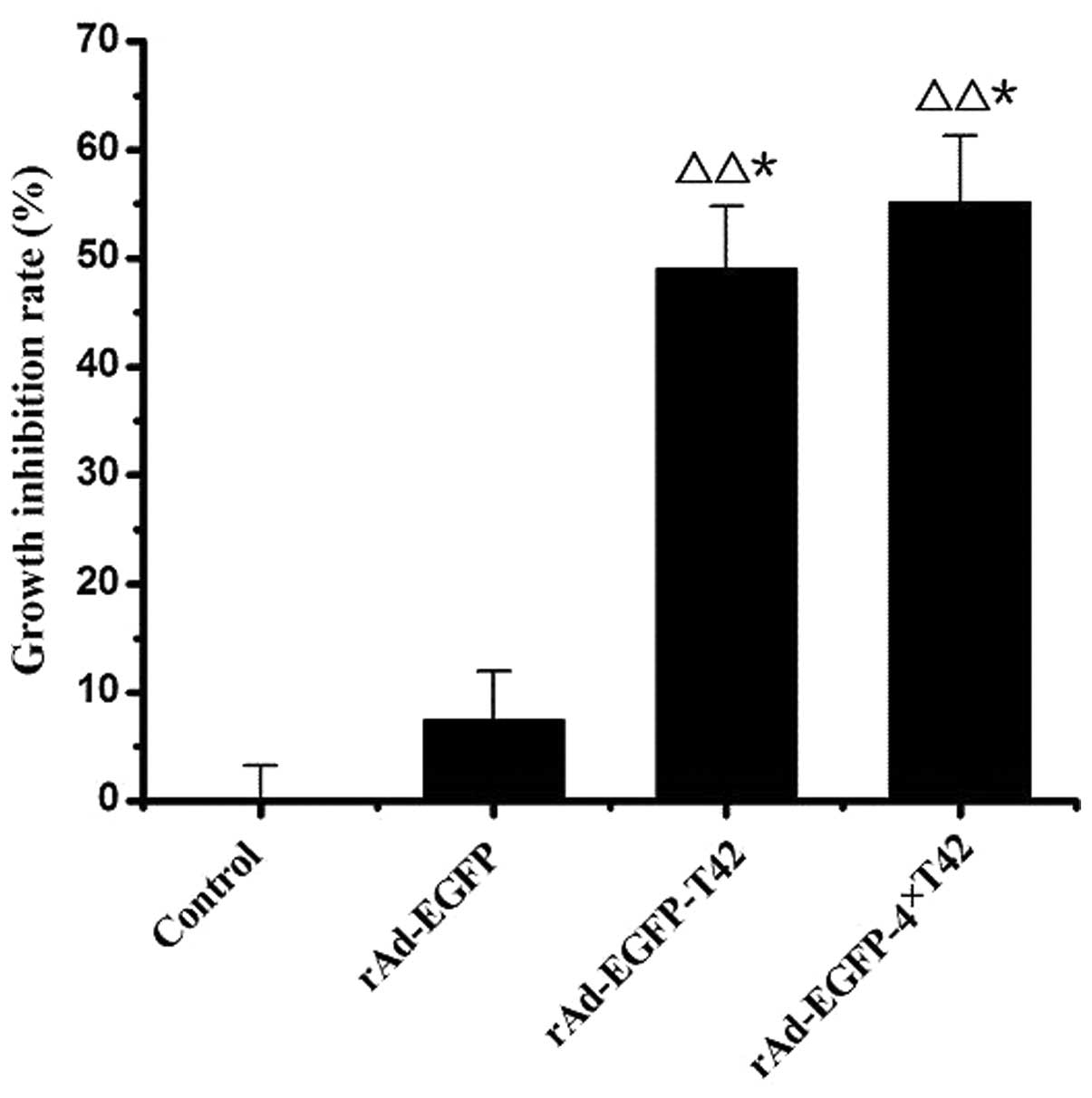
are presented in Fig. 5. There was
a significant increase in the tumor growth inhibition rate of the
pAd-EGFP-T42- and pAd-EGFP-4xT42-transfected groups and that of the
control group (P<0.05). There was also a significant increase in
the tumor growth inhibition rate of the pAd-EGFP-4xT42 group
compared to that of the pAd-EGFP group (P<0.05).
pAd-EGFP-T42 and pAd-EGFP-4xT42 decrease
tumor microvessel density
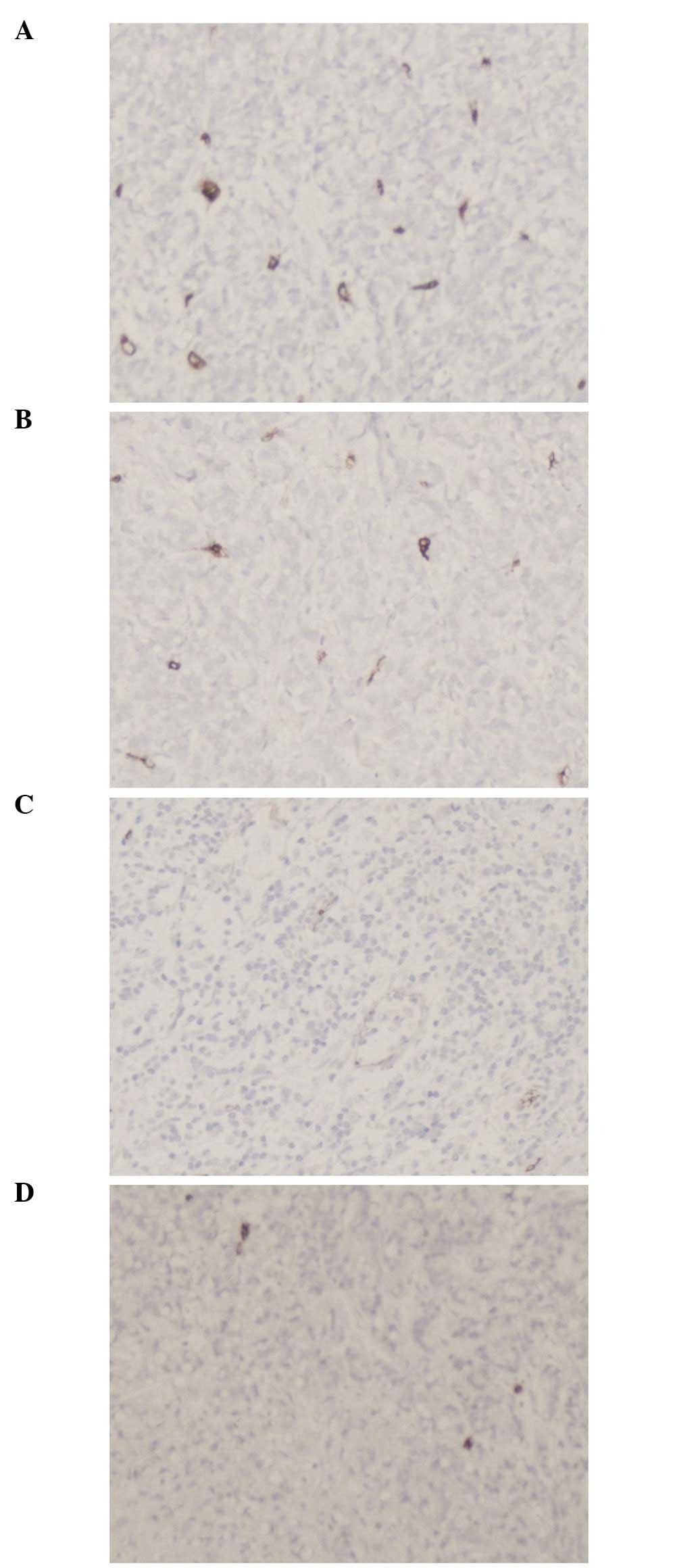
Irregular neovascularization was detected in the
tumor tissue, in particular at the edge of the tumor, by
immunohistochemical staining. In the tumors of the mice infected
with pAd-EGFP-T42 and pAd-EGFP-4xT42, there was a decreased
microvessel density compared to that of the tumors of the mice
transfected with pAd-EGFP. The results of the microvessel count are
presented in Table I and Fig. 6.
 | Table INeo-angiogenesis density in tumors of
various groups. |
Table I
Neo-angiogenesis density in tumors of
various groups.
| Group | Blank control | pAd-EGFP | pAd-EGFP-T42 | pAd-EGFP-4xT42 |
|---|
| Microvessel
density | - | 18.88±2.53 | 12.71±3.48 | 11.97±3.31 |
Discussion
In 2000, Maeshima et al (10) reported that tumstatin was able to
selectively inhibit the angiogenesis of tumor tissue, without
influencing normal angiogenesis. Tumstatin has been described as an
endogenous antitumor agent (11,21).
Amino acids 54–132 at the N-terminus of the protein have
demonstrated anti-angiogenic effects, while amino acids 185–203 at
the C-terminus have displayed antitumor activity (21).
Through binding with αVβ3 integrin, tumstatin
inhibits activation of the transduction pathway involving focal
adhesion kinase, phosphatidylinositol-4,5-bisphosphate 3-kinase and
Akt and the mammalian target of rapamycin pathway (22). As a result, tumstatin prevents the
dissociation of eukaryotic initiation factor 4E protein from
4E-binding protein 1, which leads to the inhibition of
cap-dependent protein synthesis in endothelial cells (23). Twenty-one peptides function to
preserve the anti-angiogenic activity of tumstatin (24). Studies have also demonstrated that
19 peptides are involved in the inhibition of adhesion, chemotaxis
and proliferation of various cells by binding to the
CD47/integrin-associated protein and αvβ3 proteins on the surface
of tumor cells and forming a complex (25). In the present study, the 19-residue
tumstatin peptide, a fragment with antitumor activity, and the
21-residue peptide were ligated. The RGD structure was also added,
as well as amino acids -GG-, to form a 21 peptide-GG-19 peptide
construct, which consisted of 42 peptides in total. RGD has been
shown to inhibit the proliferation and migration of tumor cells and
was therefore predicted to increase the antitumor capability of the
42-residue peptide (26). These
small peptides from tumstatin promoted cell apoptosis via
extracellular effects. However, to the best of our knowledge, it
had not previously been elucidated whether the tumstatin T42
peptide was able to influence the intracellular activity of tumor
cells. Therefore, in the present study, eukaryotic cells were
infected with an adenovirus carrying the target gene and the
expression levels of target proteins in these eukaryotic cells were
subsequently evaluated. In addition, the antitumor effects of the
T42 peptide were further examined by infecting breast cancer cells
with an adenovirus carrying the T42 target gene.
The adenovirus Ad5, selected for use in the present
study, has been improved by advancements in biotechnology. The Ad5
genome cannot produce the packaging protein E1 unless it is
provided by the host 293 cells; therefore, its genome is able to be
amplified in 293 cells (27).
However, normal human tissue cells do not express the E1 protein;
therefore, the packaging protein E1 of Ad5 will not be present and
the virus will be unable to proliferate in human cells (28). The virus is capable of being
engineered to produce a specific target protein, so it is safe for
use in gene therapy research (29). Following the construction of the
adenoviral vector containing the T42 or 4xT42 gene coupled with
EGFP, GFP was observed using fluorescence microscopy, which
confirmed that the EGFP fragment had been successfully incorporated
into the 293 cells. RT-PCR verified that the T42 and 4xT42 plasmid
were expressed by 293 cells. Furthermore, the fluorescence observed
following transfection indicated that the adenoviruses were
successfully packaged and transfected effectively. At seven days
post-transfection, a large proportion of the 293 cells shed and
appeared disintegrated under microscopic examination, which was
consistent with previous studies (30). When transfections are performed
without the use of a fluorescent indicator, the amount of time in
which viruses are produced is difficult to accurately determine. By
contrast, in the present study, it was ensured that the maximum
viral titer and conditions for viral amplification were
optimized.
The successful construction and packaging of the
pAd-EGFP-T42 and pAd-EGFP-4xT42 adenoviruses and the preliminary
results regarding their function indicated that the adenovirus
promoted apoptosis and inhibited the growth of cancer cells, which
provided a foundation for further investigation into gene therapies
for cancer. The growth inhibition ratios of the transfected groups
compared to those of the control group at four different
time-points were calculated using the MTT assay. The results of
these experiments demonstrated that no significant inhibitory
effect of the pAd-EGFP-T42 and pAd-EGFP-4xT42s plasmid on the
growth inhibition rate was evident at 12 or 24 h in comparison to
that of the control group. However, the inhibitory effect increased
over time, and at 36 and 48 h there was a significant difference
between the growth inhibition rate of pAd-EGFP-T42 and
pAd-EGFP-4xT42 plasmid groups and that of the control group
(P<0.05), while there was no significant difference between that
of the pAd-EGFP plasmid group and that of the control group.
Flow cytometry was used to determine the apoptotic
rates of the cells in the recombinant adenovirus pAd-EGFP-T42 and
pAd-EGFP-4xT42 groups, the pAd-EGFP adenovirus control group and
the blank control group. The apoptotic rates of the cells infected
with pAd-EGFP-T42 and pAd-EGFP-4xT42 were significantly higher than
those of the cells infected with the control adenovirus or the
non-transfected cells in the blank control group (P<0.05). These
results demonstrated that transfection of pAd-EGFP-T42 and
pAd-EGFP-4xT42 were able to significantly enhance the apoptotic
rate of tumor cells at 48 h post-transfection, which was consistent
with the results of the MTT assay.
The results of the present study revealed that
pAd-EGFP-T42 and pAd-EGFP-4xT42 suppressed the growth of MCF-7
cells in vitro and the growth of tumor xenografts in
vivo. In MCF-7 cells treated with pAd-EGFP-T42 or
pAd-EGFP-4xT42, there was significant downregulation of Bcl-2
expression and upregulation of Bax and caspase-3 expression, which
are key executors during apoptosis. These results indicated that
the mitochondrial pathway may be involved in regulating the
induction of apoptosis (31).
There are distinct mechanisms that mediate the anti-angiogenic and
antiproliferative activities of these tumstatin peptides. Mice
treated with pAd-EGFP-T42 or pAd-EGFP-4xT42 had fewer CD34-positive
vessels. The results observed were similar to those of previous
reports indicating that the 19 peptide fragments were able to
inhibit tumor proliferation and the 21 peptide fragments were able
to inhibit endothelial cell proliferation. Therefore, the
recombinant adenovirus packaged in the present study had both
abilities.
Recombinant adenoviruses carrying the T42 or 4xT42
genes (pAd-EGFP-T42/pAd-EGFP-4xT42) were successfully constructed.
The results of the present study revealed that the adenoviruses
constructed were able to inhibit the proliferation of breast cancer
cells in vivo and in vitro. Of note, no significant
difference was observed between the pAd-EGFP-T42- and the
pAd-EGFP-4xT42-transfected groups. pAd-EGFP-T42 and pAd-EGFP-4xT42
may function via the mitochondrial pathway to inhibit transplant
tumor angiogenesis. The adenoviral constructs exhibited antitumor
effects when the peptide was expressed in the cells in
vitro, and also upon the expression of the peptide gene by the
recombinant virus in vivo. However, the target of the T42
peptide remains to be elucidated. In conclusion, the results of the
present study indicated the relevance of the T42 peptide in the
development of novel cancer treatment strategies, which requires
further study in order to elucidate the mechanism of the antitumor
effects of this adenovirus.
Acknowledgements
The authors would like to thank the Science and
Technology Funds of the Hei Longjiang Education Department of China
for the grant they provided to support the present study (no.
12511188).
Abbreviations:
|
CPE
|
cytopathic effect
|
|
DMEM
|
Dulbecco’s modified Eagle’s medium
|
|
DMSO
|
dimethyl sulfoxide
|
|
EGFP
|
enhanced green fluorescent protein
|
|
FBS
|
fetal bovine serum
|
|
MOI
|
multiplicity of infection
|
|
MVD
|
microvascular density
|
|
PBS
|
phosphate-buffered saline
|
|
Pfu
|
plaque-forming unit
|
|
RT-PCR
|
reverse-transcription polymerase chain
reaction
|
References
|
1
|
Tazawa H, Kagawa S and Fujiwara T:
Advances in adenovirus-mediated p53 cancer gene therapy. Expert
Opin Biol Ther. 13:1569–1583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu SX, Xia ZS and Zhong YQ: Gene therapy
in pancreatic cancer. World J Gastroenterol. 20:13343–13368. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Folkman J: Tumour angiogenesis:
therapeutic implications. N Engl J Med. 285:1182–1186. 1971.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hlatky L, Hahnfeldt P and Folkman J:
Clinical application of antiangiogenic therapy: microvessel
density, what it does and doesn’t tell us. J Natl Cancer Inst.
94:883–893. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Albig AR and Schiemann WP: Fibulin-5
antagonizes vascular endothelial growth factor (VEGF) signaling and
angiogenic sprouting by endothelial cells. DNA Cell Biol.
23:367–379. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sim BK: Angiostatin and endostatin:
endothelial cell-specific endogenous inhibitors of angiogenesis and
tumor growth. Angiogenesis. 2:37–48. 1998. View Article : Google Scholar
|
|
7
|
Ribatti D: History of research on
angiogenesis. Chem Immunol Allergy. 99:1–14. 2014. View Article : Google Scholar
|
|
8
|
Hamano Y, Zeisberg M, Sugimoto H, et al:
Physiological levels of tumstatin, a fragment of collagen IV alpha3
chain, are generated by MMP-9 proteolysis and suppress angiogenesis
via alphaVbeta3 integrin. Cancer Cell. 3:589–601. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Su XJ, Wu FJ, Yuan LJ and Lin XS:
Determination of anti-Hepg2 cells activity and purification of
tumor chalone T42 peptide. J Jilin Univ, Med Ed. 36:86–89.
2010.
|
|
10
|
Maeshima Y, Colorado PC, Torre A, et al:
Distinct antitumor properties of a type IV collagen domain derived
from basement membrane. J Biol Chem. 275:21340–21348. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maeshima Y, Manfredi M, Reimer C, et al:
Identification of the anti-angiogenic site within vascular basement
membrane-derived tumstatin. J Biol Chem. 276:15240–15248. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saus J, Wieslander J, Langeveld JP,
Quinones S and Hudson BG: Identification of the Goodpasture antigen
as the alpha 3(IV) chain of collagen IV. J Biol Chem.
263:13374–13380. 1988.PubMed/NCBI
|
|
13
|
Wang SJ, Liu XH, Ji YB and Chen N: Study
on Biological Activity of Two Modified Anti-tumor Peptide of
Tumstatin. Biochemistry and Biophysics. 34:1152–1161. 2007.(In
Chinese).
|
|
14
|
Gao Y, Yu Y, Lu S, Sun BC and Wang XH:
Cloning and expression of human tumstatin gene in E. Coli. Chinese
Journal of Biochemical Pharmaceutics. 26:324–327. 2005.(In
Chinese).
|
|
15
|
Li Y, Liu XH, Lin XS, et al: Inhibitory
effect of tumstatin related peptide T42 on human umbilical vein
endothelial cells and human gastric adenocarcinoma. J Clin Rehab
Tissue Engineer Res. 11:1837–1840. 2007.(In Chinese).
|
|
16
|
Su XJ, Lin XS, Wu FJ and Yuan LJ:
Antitumor activity of tumor chalone 42 peptide combined with
cyclophosphamide. China Cancer. 5:334–337. 2010.
|
|
17
|
Jones MS II, Harrach B, Ganac RD, et al:
New adenovirus species found in a patient presenting with
gastroenteritis. J Virol. 81:5978–5984. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bernt KM, Ni S, Tieu AT and Lieber A:
Assessment of a combined, adenovirus-mediated oncolytic and
immunostimulatory tumor therapy. Cancer Res. 65:4343–4352. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takehashi M, Kanatsu-Shinohara M, Inoue K,
et al: Adenovirus-mediated gene delivery into mouse spermatogonial
stem cells. Proc Natl Acad Sci USA. 104:2596–2601. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qi DD, Chen YL, Guo JZ, Zhu GM, Zhang T,
Jiang S and Wang YZ: Constructiong of recombinant adenovirus vector
with 4T42 peptide gene, and study of the effect of anti- SKBR3 cell
in vitro. Chinese Journal of Gerontology. 32:2781–2783. 2012.(In
Chinese).
|
|
21
|
Maeshima Y, Yerramalla UL, Dhanabal M, et
al: Extracellular matrix-derived peptide binds to alpha(v)beta(3)
integrin and inhibits angiogenesis. J Biol Chem. 276:31959–31968.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kawaguchi T, Yamashita Y, Kanamori M, et
al: The PTEN/Akt pathway dictates the direct alphaVbeta3-dependent
growth-inhibitory action of an active fragment of tumstatin in
glioma cells in vitro and in vivo. Cancer Res. 66:11331–11340.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maeshima Y, Sudhakar A, Lively JC, et al:
Tumstatin, an endothelial cell-specific inhibitor of protein
synthesis. Science. 295:140–143. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang GM, Zhang YM, Fu SB, et al: Effects
of cloned tumstatin-related and angiogenesis-inhibitory peptides on
proliferation and apoptosis of endothelial cells. Chin Med J
(Engl). 121:2324–2330. 2008.
|
|
25
|
Shahan TA, Ziaie Z, Pasco S, et al:
Identification of CD47/integrin-associated protein and
alpha(v)beta3 as two receptors for the alpha3(IV) chain of type IV
collagen on tumor cells. Cancer Res. 59:4584–4590. 1999.PubMed/NCBI
|
|
26
|
Moreno-Manzano V, Lucio-Cazana J, Konta T,
Nakayama K and Kitamura M: Enhancement of TNF-alpha-induced
apoptosis by immobilized arginine-glycine-aspartate: involvement of
a tyrosine kinase-dependent, MAP kinase-independent mechanism.
Biochem Biophys Res Commun. 277:293–298. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Spector DJ, Halbert DN and Raskas HJ:
Regulation of integrated adenovirus sequences during adenovirus
infection of transformed cells. J Virol. 36:860–871.
1980.PubMed/NCBI
|
|
28
|
Graham FL, Smiley J, Russell WC and Nairn
R: Characteristics of a human cell line transformed by DNA from
human adenovirus type 5. J Gen Virol. 36:59–74. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Silman NJ and Fooks AR: Biophysical
targeting of adenovirus vectors for gene therapy. Curr Opin Mol
Ther. 2:524–531. 2000.
|
|
30
|
He TC, Zhou S, da Costa LT, Yu J, Kinzler
KW and Vogelstein B: A simplified system for generating recombinant
adenoviruses. Pro Natl Acad Sci USA. 95:2509–2514. 1998. View Article : Google Scholar
|
|
31
|
Li YJ, Sun LC, He Y, et al: The anti-tumor
properties of two tumstatin peptide fragments in human gastric
carcinoma. Acta Pharmacol Sin. 30:1307–1315. 2009. View Article : Google Scholar : PubMed/NCBI
|