Introduction
Hirschsprung’s disease (HSCR), one of the most
common congenital disorders, is characterized by the absence of
intestinal ganglion cells in the myenteric and submucosal plexuses
(1). The disease often presents
with intestinal obstruction and massive distension of the bowel
following birth (2). The incidence
is ~1 in 5,000 live births, and this figure is slightly higher in
Asian people (~28 per 100,000 live births). Although the causes of
HSCR remain unclear, it is known to be a complex disease, which is
influenced by numerous genetic and environmental factors. To date,
mutations in the ret proto-oncogene (3), endothelin receptor B (4), endothelin 3 (5), glial derived neurotrophic factor
(6) and sex determining region
Y-box 10 (7), which are all known
to be involved in the formation of the enteric nervous system, have
been identified in patients with HSCR. However, changes in these
genes do not account for all cases of HSCR. At present,
unidentified genes, or combinations of genes, underlie as many as
50–70% of cases.
The Wnt signaling pathway is involved in a number of
biological process, including cell proliferation, tumorigenesis and
embryonic development (8,9). The present study investigated the
association between HSCR disease and the Wnt signaling pathway. It
has previously been demonstrated that the WNT8A gene is involved in
susceptibility to HSCR and is important in the development of this
disease (10). The dishevelled
(DVL) protein, which has three homologous subtypes, DVL-1, DVL-2
and DVL-3, is a cytoplasmic protein that is important in embryonic
development, cell differentiation and tumor formation (11–13).
It is also known to be a key part of the Wnt signaling pathway
(14). A previous study showed
that in patients with HSCR, the expression of DVL-1 and DVL-3 in
aganglionic colon segments was higher than that in ganglionic
segments (15). However, the
correlation between expression of the DVL-2 protein and HSCR has
not been confirmed. Therefore in the current study, differential
expression of DVL-2 was detected using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR),
western blotting and immunohistochemistry staining.
Materials and methods
Patients and specimens
This study was approved by the Ethics Committee of
China Medical University (Ethical no. 2013PS07K; Liaoning, China).
Colonic tissues were obtained from 50 patients (41 males and 9
females) with pathologically confirmed HSCR pre- or post-operative
at Shengjing Hospital of China Medical University. HSCR was
diagnosed by a barium enema X-ray and suction rectal biopsy. All
patients with HSCR exhibited aganglionosis restricted to the
sigmoid colon. Their ages ranged from 0.5 to 4.5 years old, with an
average of 1.5 years. Based on the results of the barium enema and
the intestinal morphology observed during and after the operation,
the surgical specimens were divided into aganglionic colon segments
and ganglionic colon segments. These classifications were further
confirmed by histological examination of a frozen section of each
segment. Aganglionic and ganglionic colon segment tissues were
collected and identified by histopathological examination of
hematoxylin and eosin (H&E)-stained slides. Each tissue
specimen was divided into two; one part was frozen at −80°C for
molecular analysis and the other was fixed in 10% neutral-formalin
and embedded with paraffin.
Reagents and instruments
A biotin streptavidin detection system for
immunohistochemical staining, and a DAB-0031/1031 kit were obtained
from Fuzhou Maixin Biotech Co., Ltd. (Fuzhou, China). The
PrimeScript RT reagent kit was obtained from Takara Biotechnology
Co., Ltd. (Dalian, China). The electrophoresis apparatus was
obtained from Bio-Rad Laboratories (Hercules, CA, USA). The mixed
SYBR green kit from Takara Biotechnology was made for RT-qPCR
conducted using a LightCycler® 480 instrument (Roche
Diagnostics, Basel, Switzerland).
Design and synthesis of primers
Human β-actin was used as an internal control and
the length of the amplified fragment was 317 base pairs (bp). The
other primers were constructed using the cDNA χ sequence of DVL-2,
for which the length was 129 bp. These two pairs of primers were
synthesized and purified by the Shanghai Biological Engineering
Company (Shanghai, China). The primer sequences were as follows:
Forward: 5′-AGA GCT ACG AGC TGC CTG AC-3′ and reverse: 5′-AGC ACT
GTG TTG GCG TAC AG-3′ for human β-actin; and forward: 5′-CCA CCT
TTA CCT CCT TTG-3′ and reverse: 5′-CAC TAC TGA CTC GGT TTC TG-3′
for human DVL-2.
RNA extraction, RT-qPCR)
Tissues (~100 mg) from the aganglionic and
ganglionic intestines were used for total RNA extraction using the
RNA extraction reagent, TRIzol® (Invitrogen Life
Technologies, Carlsbad, CA, USA), according to the manufacturer’s
instructions. The harvested RNA was diluted to a concentration of 1
μg/μl, aliquoted and stored at −80°C. For cDNA synthesis, two
reagent kits were used (One Step PrimeScript® mRNA cDNA
Synthesis kit and PrimeScript® RT reagent kit, Takara
Biotechnology Co., Ltd.).
RT-qPCR was performed in triplicate for each
specimen using SYBR® Green PCR Master Mix in a
LightCycler® (Roche Molecular Biochemicals, Co.,
Mannheim, Germany). The reference gene, β-actin (Takara
Biotechnology, Co., Ltd.; DR3783), was used as an endogenous
control. The following conditions were used: 5 min pre-denaturation
at 95°C, 40 cycles of 5 sec denaturation at 95°C and 30 sec
annealing at 52.5°C. Following the termination of PCR, the
production was analyzed automatically by the Lightcycler system.
The amplification process was followed by a melting curve analysis
and the cycle threshold (Ct) value was recorded. For the genes
investigated here, one cycle change in Ct corresponded to a 2.1±0.2
(standard error of the mean) change in RNA dilution. The relative
expression of the gene was calculated by the 2−ΔΔCt
method (16).
H&E and immunohistochemical
staining
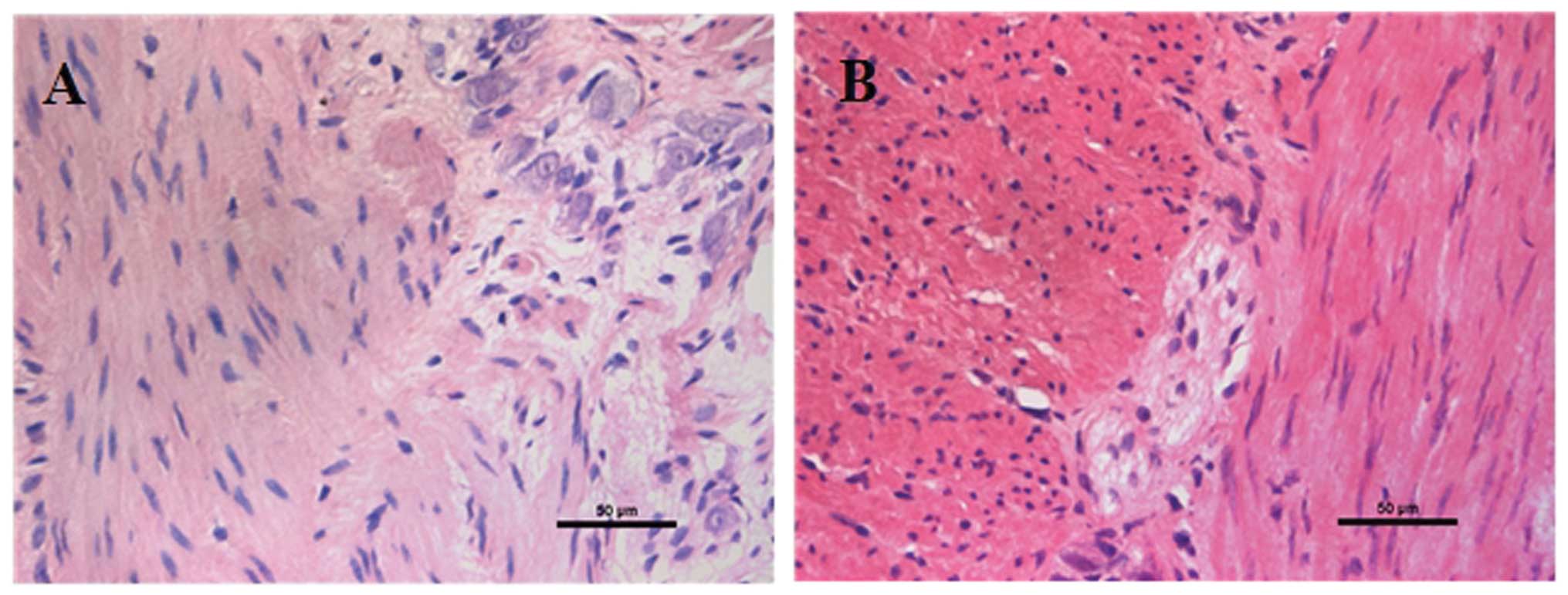
Diagnosis of HSCR was based on H&E staining of
ganglion cells. Review of surgical pathological reports following
resection of the colonic segment confirmed whether the initial
biopsy diagnosis of HSCR had been accurate. Aganglionic and
ganglionic segments were categorized according to the results of
H&E staining (Fig. 1).
Segments were fixed in 10% neutral-formalin and embedded in
paraffin.
Immunohistochemistry (IHC) was performed on 5-μm
sections obtained from formalin-fixed, paraffin embedded blocks,
using a biotin streptavidin complex method. For antigen retrieval,
slides were incubated in a microwave oven for 10 min in 0.01 mol/l
citrate buffer (pH=6; Zhongshan Golden Bridge Biotechnology Co.,
Ltd., Beijing, China), followed by cooling at room temperature.
Following incubation in 10% normal goat serum (Beijing TransGen
Biotech Co. Ltd., Beijing, China) in phosphate-buffered saline
(PBS; Zhongshan Golden Bridge Biotechnology Co., Ltd.) for 30 min
in order to block nonspecific binding sites, samples were incubated
at 4°C overnight with a polyclonal rabbit anti-DVL-2 antibody
(1:2,000; Millipore, Billerica, MA, USA; catalog no. AB5972).
Samples were washed with PBS and then incubated with anti-rabbit
IgG peroxidase-conjugated antibody (1:2,000; Beijing TransGen
Biotech Co. Ltd.; catalog no. I10318) for 20 min at 37°C and washed
by PBS. The final reaction product was stained with
diaminobenzidine (Beijing TransGen Biotech Co. Ltd.). After 10 min
washing with PBS, nuclei were counterstained with hematoxylin. The
negative control was produced using an equivalent quantity of PBS
in place of the rabbit anti-DVL-2. Brown and yellow deposition
represented a positive reaction. Density and distribution were
observed under a light microscope (Nikon E800, Nikon Corporation,
Kanagawa, Japan). The density of the positively stained area was
calculated, at ×400 magnification, as the sum of the areas occupied
by the positively stained area of the tissues. Images were captured
and the area of staining in each image was calculated using a NISE
Elements Basic Research (version 2.30, Kawasaki, Kanagawa, Japan)
analysis system. Negative controls were performed using an
equivalent quantity of PBS instead of rabbit anti-DVL-2. Two
pathologists independently reviewed the IHC-stained slides and
agreed on a diagnoses by consensus.
Western blot analysis
Colon tissue specimens (~50 mg) were cut into small
pieces using surgical scissors, sonicated in protein lysis buffer
(Beijing TransGen Biotech Co. Ltd.) and centrifuged at 13,600 × g
for 15 min at 4°C. The protein concentration was measured using the
Bradford method (17), specimens
were adjusted to obtain the same protein concentration, and then
aliquoted and stored at −80°C. Samples containing equal quantities
of protein (50 μg) were separated by sodium-dodecyl sulfate
polyacrylamide gel electrophoresis on a 10% gel, and then
electrotransferred onto polyvinylidene fluoride membranes (EMD
Millipore). Blots were blocked with 5% non-fat for 1 h at 37°C,
followed by incubation with anti-DVL2 antibodies (1:1,000)
overnight at 4°C. The following day, the blots were washed in TBST,
and incubated with goat anti-rabbit IgG (1:2,000 dilution; Beijing
ZhongShan Jinqiao Biological Technology Co., Ltd., Beijing, China;
catalog no. ZB-2301) for 90 min at 37°C prior to detection by
enhanced chemiluminescence (Pierce Biotechnology, Inc., Rockford,
IL, USA). The grayscale values of the DVL-2 band were normalized to
the values of the corresponding β-actin band to determine the
relative expression levels of the DVL-2 protein. Experiments were
repeated three times, independently.
Statistical analysis
The Statistical Program for Social Sciences, version
13.0 (SPSS, Inc., Chicago, IL, USA), was used for statistical
analysis. A t-test was used to compare the expression level of
DVL-2 in aganglionic colon segments with that of the normal
segments. Data are expressed as the mean ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
RT-qPCR analysis
The optical density value of RNA calculated by
A260/A280 ranged from 1.8 to 2.0. In the analysis of RT-qPCR data,
the mRNA expression levels of DVL-2 were normalized to the mRNA
expression levels of β-actin in each specimen. The results showed
that the mRNA expression levels of DVL-2 in the aganglionic
segments were 72% lower than those in the ganglionic segments
(P<0.03; Table I).
 | Table IRelative quantity of DVL-2 mRNA in
aganglionic and ganglionic segments of colon tissue. |
Table I
Relative quantity of DVL-2 mRNA in
aganglionic and ganglionic segments of colon tissue.
| Segment | DVL-2 average Ct
value | β-actin average Ct
value | ΔCt | ΔΔCt | Relative
expression |
|---|
| Ganglionic | 26.73±1.59 | 23.94±1.95 | 2.79 | 0.00 | 1.00 |
| Aganglionic | 28.57±1.03 | 24.06±1.80 | 4.51 | 1.82 | 0.28 |
IHC staining results
The aganglionic and ganglionic segments were first
defined by H&E staining, which demonstrated the absence of
focal ganglion cells (Fig. 1).
Immunohistochemical staining was performed and positive reaction
was predominantly detected in the mucosal and submucosal layers of
the ganglionic colonic segments. The brown-yellow depositions
observed in the ganglionic segments tissues were richer and more
widespread in the submucosa. The distribution was reticulodromous
in the circular muscular layer, while that of the aganglionic
segment tissues was punctiform. DVL-2 immunoreactivity was greater
in the ganglionic segments than the aganglionic segments (Fig. 2).
Western blot analysis
Expression of the DVL-2 protein was evaluated by
western blotting with specific antibodies in samples from the same
group of patients with HSCR. In accordance with the results from
the RT-qPCR experiments, a significant decrease in the DVL-2
protein was detected in aganglionic segments compared with the
matched ganglionic segments (Fig.
3). The protein level of DVL-2 was 11.31±2.23 in the
aganglionic colon segment, compared with 35.21±2.66 in the
ganglionic segments P<0.05).
Discussion
HSCR is a congenital disorder, characterized by an
absence of enteric neurons in the myenteric and submucosal plexuses
of the terminal gastrointestinal tract. It usually presents with
intestinal obstruction and massive distension of the proximal bowel
(megacolon) following birth (2).
To date, numerous genes have been reported to be involved in the
etiology of HSCR, however, the mechanisms underlying the motility
dysfunction observed in HSCR remain unclear. Furthermore, for
patients with HSCR, a significant problem is that of persistent
postoperative disturbances in bowel motility, even following
surgery. The reasons for this phenomenon also remain to be
elucidated. In the present study, RT-qPCR, IHC staining and western
blotting were used to investigate the differential expression in
mRNA and protein levels of DVL-2 between aganglionic and normal
ganglionic colonic tissues, in order to gain information on bowel
motility disturbance. Aganglionic and normal colonic segment
tissues, derived from 50 patients with sporadic HSCR were analyzed.
The results showed that the expression of DVL-2 mRNA in the
aganglionic segments was lower than that in the ganglionic segments
(Table I) and that these
differences were statistically significant (P<0.05). Results of
IHC experiments to assess the DVL-2 protein levels confirmed a
significant reduction in DVL-2 expression in aganglionic colonic
segments compared with the ganglionic segments.
As a member of the Wnt signaling pathway, DVL exerts
its biological function predominantly through the canonical and
non-canonical Wnt signaling pathways (18,19).
Within the central nervous system, DVL is widely expressed in early
embryonic and postnatal nervous tissues, and is crucial for the
formation of connections between neurons (11). When DVL-2 expression is inhibited,
neuronal precursor cell proliferation is reduced. A previous study
showed that in patients with HSCR, the expression of DVL-1 and
DVL-3 in aganglionic colonic segments was greater than that in
ganglionic segments (15). The
different expressions between DVL-1, DVL-3 and DVL-2 in aganglionic
colon segments may indicate that different subtypes of DVL gene
have different roles in the development of the enteric nervous
system. When the Wnt signaling pathway becomes abnormal, resulting
in a reduced quantity of DVL-2, synapse formation is reduced. In
order to restore this function, expression of DVL-1 and DVL-3
increases, stimulating synapse formation by increasing synaptic
assembly, in order to promote normal development of ganglions.
There are a number of mechanisms regarding the role
of DVL-2 in HSCR that require further investigation, including the
mechanism by which a reduced expression of DVL-2 results in the
development of HSCR. The present study demonstrated a differential
expression of DVL-2 mRNA and protein between the aganglionic and
the ganglionic colon segments, suggesting that DVL-2 may be an
important factor in the pathogenesis of HSCR. The difference in
expression of DVL-2 mRNA and protein between the aganglionic
segments and the ganglionic segments provides a starting point for
further investigation into the molecular basis of HSCR.
Acknowledgements
This study was supported by the Shenyang Science and
Technology Plan Project (grant no. F13-316-1-01).
References
|
1
|
Amiel J, Sproat-Emison E, Garcia-Barcelo
M, et al: Hirschsprung disease, associated syndromes and genetics:
a review. J Med Genet. 45:1–14. 2008. View Article : Google Scholar
|
|
2
|
Whitehouse FR and Kernohan JW: Myenteric
plexus in congenital megacolon; study of 11 cases. Arch Int Med
(Chic). 82:75–111. 1948. View Article : Google Scholar
|
|
3
|
Lantieri F, Griseri P and Ceccherini I:
Molecular mechanisms of RET-induced Hirschsprung pathogenesis. Ann
Med. 38:11–19. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kusafuka T, Wang Y and Puri P: Novel
mutations of the endothelin-B receptor gene in isolated patients
with Hirschsprung’s disease. Hum Mol Genet. 5:347–349. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bidaud C, Salomon R, Van Camp G, et al:
Endothelin-3 gene mutations in isolated and syndromic Hirschsprung
disease. Eur J Hum Genet. 5:247–251. 1997.PubMed/NCBI
|
|
6
|
Salomon R, Attié T, Pelet A, et al:
Germline mutations of the RET ligand GDNF are not sufficient to
cause Hirschsprung disease. Nat Genet. 14:345–347. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pan ZW, Lou J, Luo C, Yu L and Li JC:
Association analysis of the SOX10 polymorphism with Hirschsprung
disease in the Han Chinese population. J Pediatr Surg.
46:1930–1934. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Martin BL and Kimelman D: Canonical Wnt
signaling dynamically controls multiple stem cell fate decisions
during vertebrate body formation. Dev Cell. 22:223–232. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gurney A, Axelrod F, Bond CJ, et al: Wnt
pathway inhibition via the targeting of Frizzled receptors results
in decreased growth and tumorigenicity of human tumors. Proc Natl
Acad Sci USA. 109:11717–11722. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao H, Chen D, Liu X, et al: Polymorphisms
and expression of the WNT8A gene in Hirschsprung’s disease. Int J
Mol Med. 32:647–652. 2013.PubMed/NCBI
|
|
11
|
Almuedo-Castillo M, Saló E and Adell T:
Dishevelled is essential for neural connectivity and planar cell
polarity in planarians. Proc Natl Sci Acad USA. 108:2813–2818.
2011. View Article : Google Scholar
|
|
12
|
Zhang X, Zhu J, Yang GY, et al:
Dishevelled promotes axon differentiation by regulating atypical
protein kinase C. Nat Cell Biol. 9:743–754. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pulvirenti T, Van Der Heijden M, Droms LA,
et al: Dishevelled 2 signaling promotes self-renewal and
tumorigenicity in human gliomas. Cancer Res. 71:7280–7290. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao C and Chen YG: Dishevelled: The hub of
Wnt signaling. Cell Signal. 22:717–727. 2010. View Article : Google Scholar
|
|
15
|
Chen D, Mi J, Wu M, Wang W and Gao H: The
expression of dishevelled gene in Hirschsprung’s disease. Int J
Clin Exp Pathol. 6:1791–1798. 2013.
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔct method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
17
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–54.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu C, Li Y, Semenov M, et al: Control of
beta-catenin phosphorylation/degradation by a dual-kinase
mechanism. Cell. 108:837–847. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Habas R, Dawid IB and He X: Coactivation
of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate
gastrulation. Genes Dev. 17:295–309. 2003. View Article : Google Scholar : PubMed/NCBI
|