Introduction
Naturally occurring sulfated polysaccharides (SPs)
are commonly found in three major groups of marine algae (1). SPs are historically known to exhibit
a number of therapeutic and biological effects, including
antioxidant (2),
anti-proliferative, anti-viral (3), anti-coagulant (4) and anti-tumor/cancer therapy (5–7)
abilities. Previous studies have shown that numerous algal
bioactive molecules, SPs included, may be implicated in the low
incidences of cancer in countries that traditionally consume high
levels of marine food (8,9).
In 2011, breast cancer was the most frequently
diagnosed type of cancer and the leading cause of cancer mortality
among females in the United States, accounting for 23% of the total
number of cancer cases and 14% of cancer-related mortalities
(10). Several epidemiological
studies have provided evidence that marine algae consumption
correlates with lower breast cancer rates in East Asia (11–16).
Furthermore, intake of seaweed in the diet has been associated with
a low risk of developing breast cancer (11). The potential anti-cancer effects of
marine algae are partially attributed to polysaccharide compounds,
particularly those which are sulfated, including carrageenans
(17,18). Carrageenans are a family of linear
SPs which are divided into three categories (κ, I or λ) depending
on their degree of sulfonation, solubility and gelling properties
(19). A number of studies
concerning novel anti-cancer drugs have determined that the
modulation of signal-transduction pathways, inhibition of cell
proliferation, induction of apoptosis, inhibition of tumor
metastasis and inhibition of angiogenesis are all mechanisms which
are involved in the control of carcinogenesis (17,18).
Therefore, increasing the levels of apoptosis in cancer cells may
be an effective method of chemopreventative and chemotherapeutic
intervention in numerous types of cancer (17,18).
In the present study, the therapeutic effects of
Laurencia papillosa against human breast cancer cells were
investigated. The identified extracted sulfated carrageenan (ESC)
was evaluated for its effects on the viability and proliferation of
MDA-MB-231 human breast cancer cells.
Materials and methods
Plant material and extract
preparation
The red alga L. papillosa was collected from
Syrian coastal waters and processed at the Marine Biology
Laboratory of Tishreen University (Lattakia, Syria). The collected
algal biomass was washed and air-dried at 60°C to a constant
weight, followed by heating in water (1.5% w/v) for 12 h with
mechanical stirring. The carrageenan extract was dissolved in
Milli-Q water (Millipore, Billerica, MA, USA), filtered and
immediately mixed with three volumes of ethanol (95%)
(Sigma-Aldrich, St. Louis, MO, USA) which caused precipitation of
the carrageenan. The extract was collected and oven dried at
50–60°C to a constant weight.
Fourier transform infrared (FT-IR)
analysis
The IR spectra of the extracted polysaccharide were
determined using a Nicolet 6700 FT-IR spectrometer (Thermo
Scientific, Waltham, MA, USA). The polysaccharide was ground with
spectroscopic-grade potassium bromide (KBr) powder, dispersed in a
KBr disk and pressed into 1-mm pellets for FT-IR measurement in the
wave-number range of 600–4,000 cm−1 using 16 scans.
Cell culture
MDA-MB-231 cells [provided by Professor P. Bécuwe
from the Cancer Research Unit (EA SIGRETO), Nancy, France] were
cultured in RPMI-1640 medium containing 10% fetal bovine serum
(FBS), 50 U/ml penicillin/streptomycin and 2 mM L-glutamine. The
cells were cultured at 37°C in 5% CO2. All materials
used in the cell culture were supplied by Gibco-BRL (Carlsbad, CA,
USA).
XTT assay
Cells were seeded at a density of 2×103
in a 96-well plate. Following a 24-h culture the cells were treated
with ESC (1, 10, 50 or 100 μM) and incubated for 24, 48 or 72 h.
Cell viability was measured using an XTT assay kit (Roche,
Mannheim, Germany) following the manufacturer’s instructions. The
number of living cells was quantified by measuring absorbance at a
wavelength of 490 nm using a microplate reader (Multiskan EX
Microplate Readers; Thermo Scientific) and the absorption of the
controls was set to 100%. A graph of cell viability percentage
against ESC concentration was produced from the mean absorbance
values, by calculating the percentage growth of the ESC-treated
cells compared with the growth of the untreated cells. Treatment
with each ESC concentration was conducted in triplicate.
Cell bioimaging
Cells (2×103) were seeded into a slide
chamber (Nalge Nunc International, Penfield, NY, USA) and cultured
for 24 h, followed by treatment with ESCs at the desired
concentration. Formaldehyde-fixed cells (4% formaldehyde) were
inspected using a ×10 objective lens of an Olympus inverted
microscope (Olympus CK2; Olympus Corporation, Tokyo, Japan). Images
were then captured using a microscope-branched Olympus DP70 camera
(Olympus Corporation).
DAPI staining
Cells (2×105) were first cultured in a
slide chamber. Following treatment with 50 μM ESC, the cells were
fixed using 4% formaldehyde. The cells were washed in
phosphate-buffered saline and incubated in 1 μg/ml DAPI
(Sigma-Aldrich) (dissolved in methanol) for 5 min in the dark.
Slides were mounted and observed using a Nikon ECLIPSE 80i
fluorescence microscope (Nikon, Tokyo, Japan). Images were captured
using a microscope-branched Nikon DS-Ri1 camera (Nikon).
DNA fragmentation assay
Cells were treated with 50 μM ESC for 12, 16 and 24
h prior to harvesting. Genomic DNA was extracted using previously
described methods (20). The DNA
samples were separated using a 1.5% agarose gel and DNA fragments
were visualized by UV transillumination following ethidium bromdie
staining. Fluorescence intensity was quantified using a Gel Doc
2000 system (Bio-Rad, Hercules, CA, USA) in order to determine the
amount of DNA that was degraded upon treatment with 50 μM ESC.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Cells were seeded at a density of 1×106
cells per 75 cm2 flask and cultured for 24 h. The cells
were treated with 50 μM ESC for 6, 18, 24 and 48 h prior to being
harvested. Total RNA was extracted using a RNeasy kit (Qiagen,
Valencia, CA, USA). The cDNA was directly prepared from total RNA
using thr M-MLV RT First-Strand Synthesis System (Invitrogen Life
Technologies, Carlsbad, CA, USA) and an oligo (dT) 12–18 primer
(Invitrogen Life Technologies) according to the manufacturer’s
instructions. Transcript levels of caspase-8, caspase-3, caspase-9,
p53, Bcl-2, Bax and a GAPDH reference gene were determined using
transcript-specific primers (16).
qPCR was performed with a StepOne/Plus Real-Time PCR system
(Applied Biosystems, Foster City, CA, USA) according to the
manufacturer’s instructions for relative expression (ΔΔCt method).
Thus, the expression levels were expressed as relative fold
differences compared with the expression levels of the reference
gene.
Statistical analysis
The results were expressed as the mean value ± the
standard error of the mean of individual experiments. Comparisons
of the means were conducted using a one-way analysis of variance
followed by Bonferroni’s post hoc test (Prism software, version 6.0
for windows; GraphPad Software, La Jolla, CA, USA).
Results
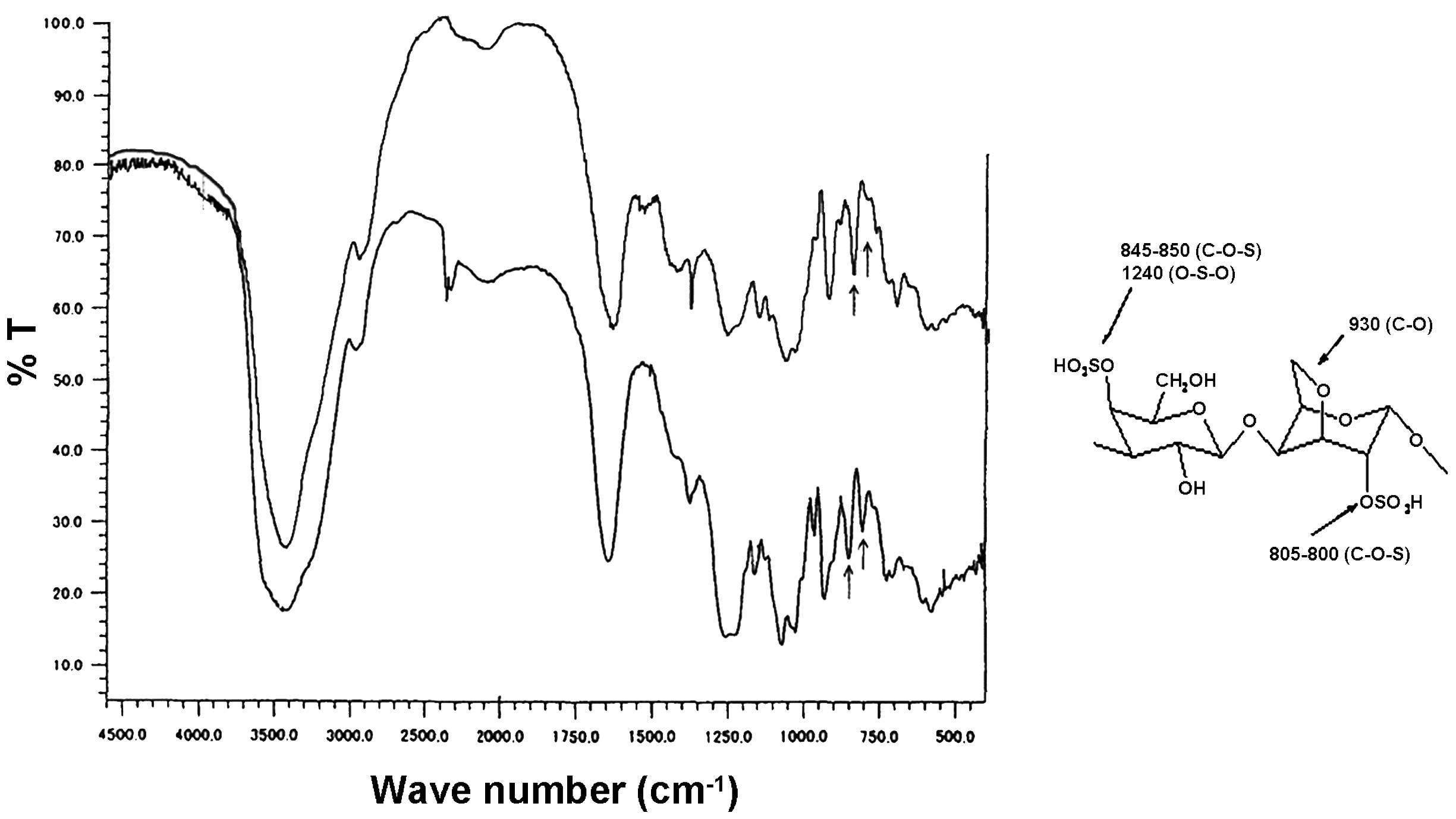
FT-IR spectroscopic analysis of L.
papillosa extract
The simple yet efficient extraction method yielded
considerable levels of SPs, which are originally found in
appreciable levels in the red alga L. papillosa. Comparing
the FT-IR spectrum of the ESC with the standard FT-IR spectra of
the most common carrageenans (κ-, I- and λ-carrageenans) revealed
that the obtained ESC FT-IR spectrum is similar to the standard
FT-IR spectrum of I-Carrageenan (Fig.
1) (22,23). Notably, the spectrum obtained in
the current study possessed two characteristic absorption bands at
847 and 802 cm−1 which are associated with
I-carrageenan. The distinctive band at 802 cm−1 is
associated with the sulfate group linked to the anhydrogalactose
ring, whereas the clear 847 cm−1 band corresponds to the
sulfated group bonded to the galactose ring. The broad band at
~1250 cm−1 is readily assigned to the S=O stretching
vibration of sulfate groups (24,25).
Furthermore, the spectrum showed no marked peaks around 823 or 835
cm−1 which are usually observed in the other SPs,
confirming the lack of other sulfate ester substitutions.
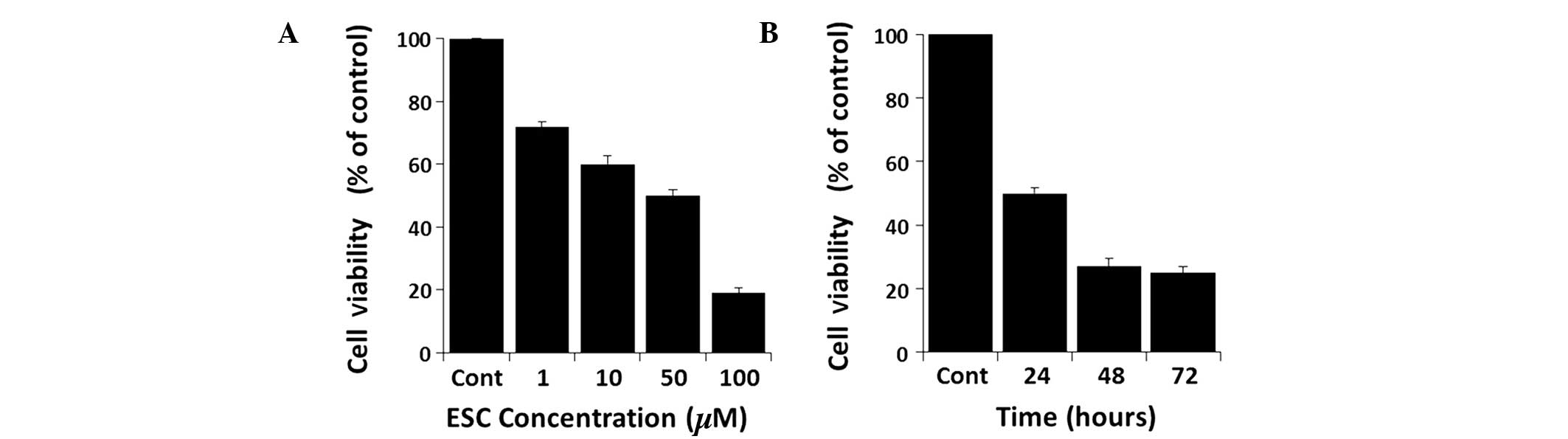
ESC inhibits the proliferation of
MDA-MB-231 cells in a time- and concentration-dependent manner
Cells treated with series of increasing
concentrations of ESC showed a reduction in cell viability in a
time- and concentration-dependent manner (Fig. 2). Therefore, to elucidate the
pathway that cells were following when exposed to ESC, it was
important to determine the half maximal inhibitory concentration
(IC50). ESC inhibited the proliferation of MDA-MB-231
cells in a concentration-dependent manner, with an IC50
value of ~50 μM (Fig. 2A). A
time-course study at a concentration of 50 μM revealed that ESC
markedly reduced the cell viability in a time-dependent manner, as
shown in Fig. 2B.
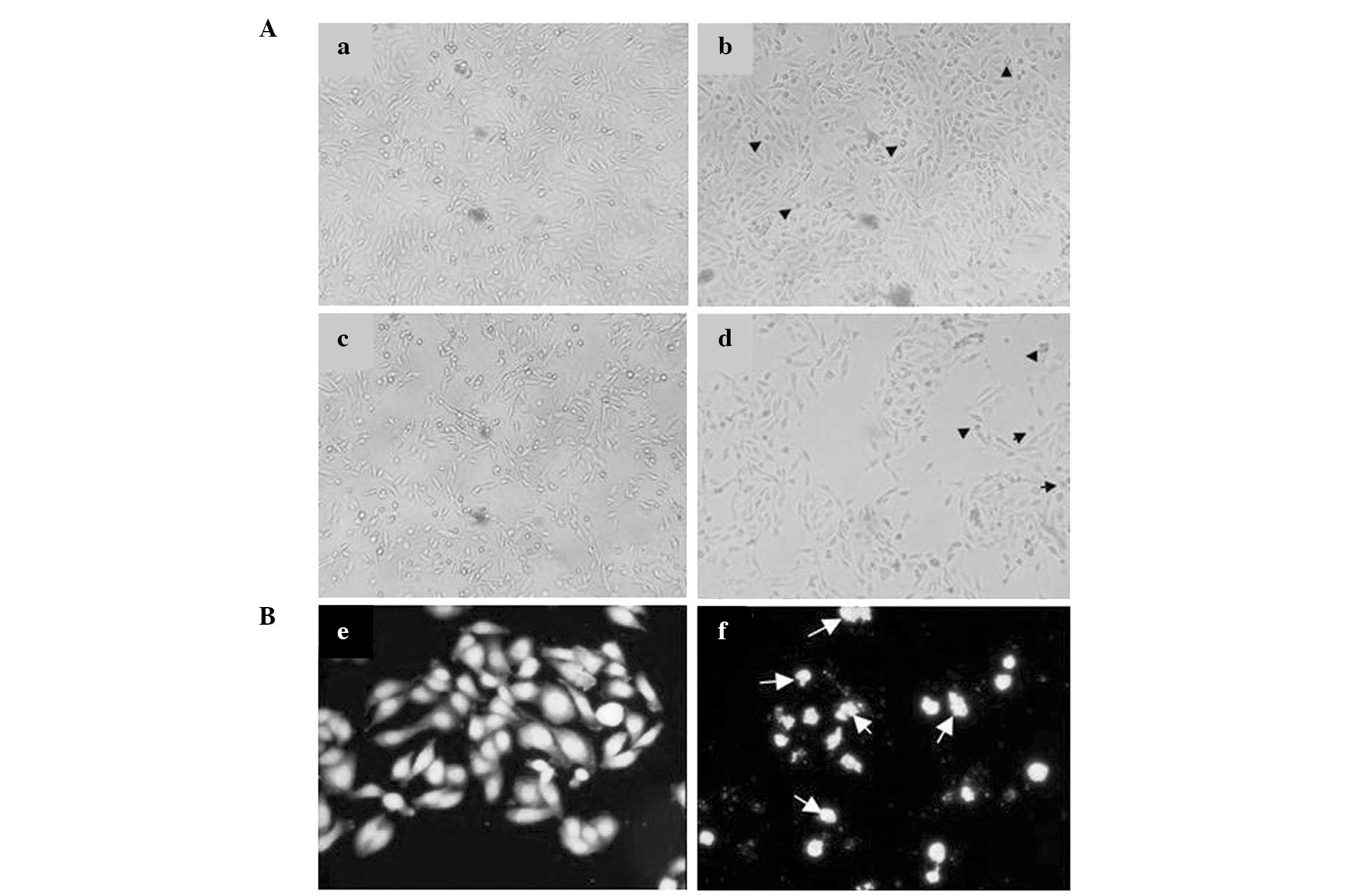
ESC induces morphological changes in
MDA-MB-231 cells
To visually confirm the aforementioned biochemical
results, bioimaging of ESC-treated cells was performed. Fig. 3A shows that cell proliferation was
notably reduced by increasing concentrations of ESC. Control cells
showed healthy growing patterns compared with those of the cells
treated with 5 μM of ESC, which had a rounded, shrunken appearance
reflecting the classical signs of programmed cell death. The signs
of biological injury in the compound-treated cells were clearer at
higher concentrations (20 and 50 μM). Higher concentrations of ESC
led to a reduction in cell proliferation, evident by a greater
number of shrunken rounded cells compared with those observed at
lower concentrations. In addition, the morphological changes of
ESC-treated cells were observed via DAPI staining. Apoptotic
bodies, one of the morphological signs of apoptosis, were present
in the 50 μM ESC-treated cells stained with DAPI (Fig. 3B). Furthermore, nuclear
condensation, which leads to the breakdown of nuclear DNA strands
into multiple oligonucleosomal-sized fragments, was observed.
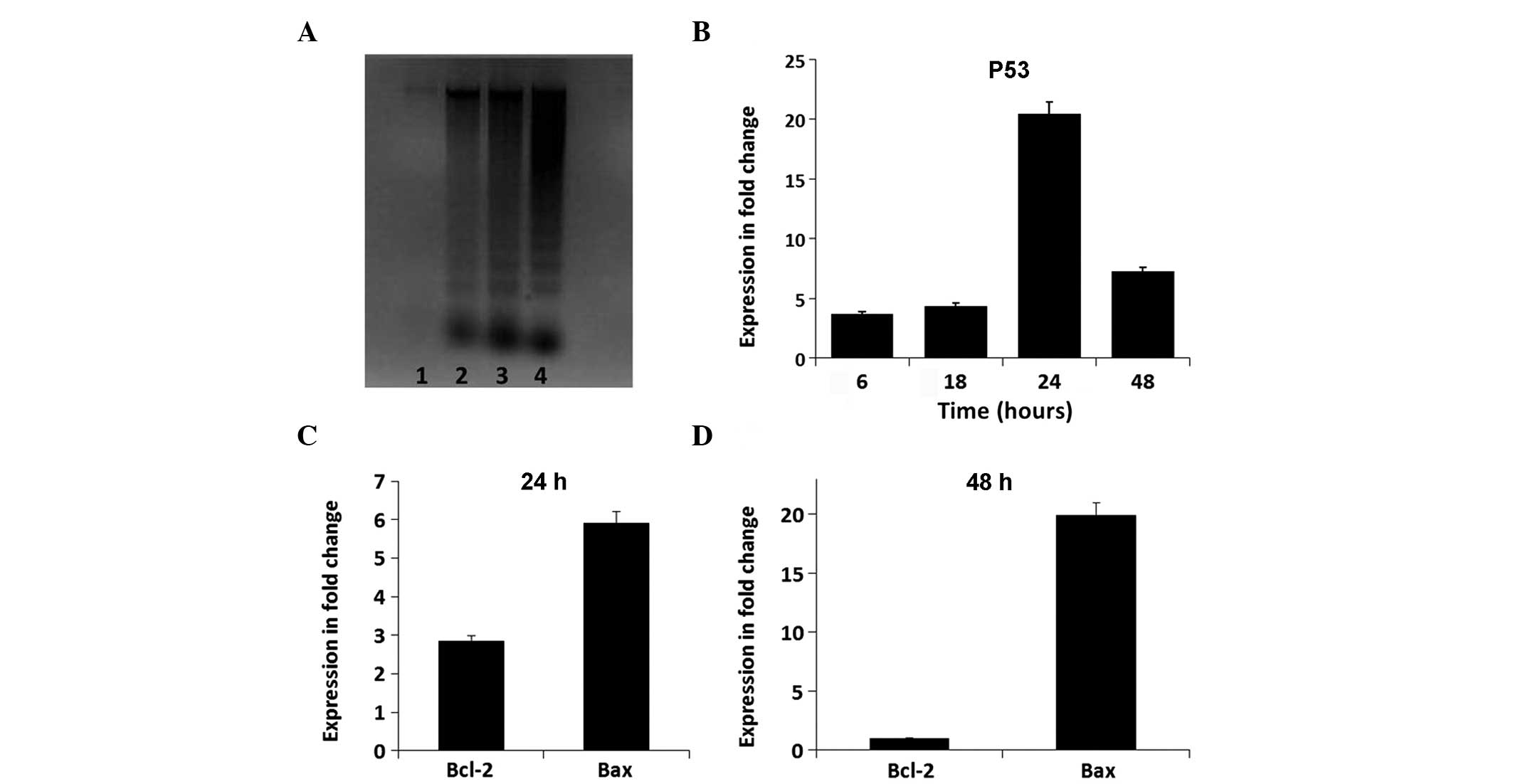
ESC-treated cells showed an increase in the levels of DNA
fragmentation following 12, 16 and 24 h of ESC treatment,
confirming activation of apoptosis (Fig. 4A).
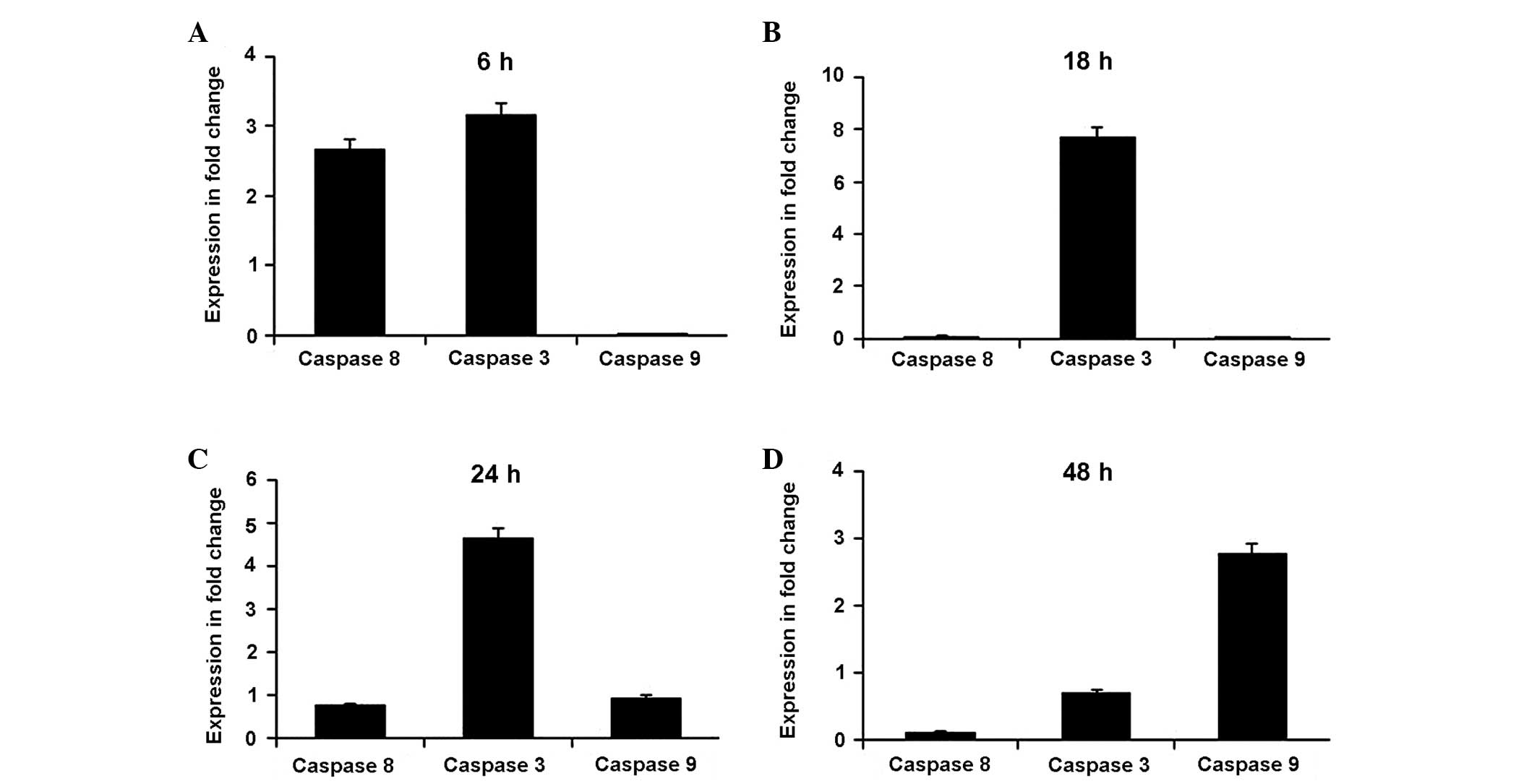
ESC alters apoptotic gene activity and
caspase activation in MDA-MB-231 cells
The relative expression levels of genes involved in
apoptosis were analyzed, including those of caspase-8, caspase-3,
caspase-9, p53, Bcl-2 and Bax. Figs.
4 and 5 summarize the gene
expression changes. In the initial 6 h, ESC increased the
expression levels of caspase-8 and caspase-3 by several fold
compared with those of the untreated cells (Fig. 5A). Subsequently, ESC maintained
high levels (a several fold increase) of caspase-3 expression for
18 and 24 h as a result of the early expression of caspase-8,
compared with those of the untreated cells (Fig. 5B and C). The expression levels of
caspase-9 only began to increase relative to the control cells
after 24 h, indicating the possibility of indirect activation by
ESC. The expression levels were markedly increased by several fold
after 48 h (Fig. 5D). In parallel,
the expression levels of the oncogenic p53 gene were increased by
3.72-fold at 6 h and increased to 20.45-fold relative to the
controls at 24 h (Fig. 4B). In the
same time period, the apoptotic Bax:Bcl-2 expression ratio in 50 μM
ESC-treated cells was increased in a time-dependent manner.
Comparably, the expression levels of Bcl-2 were reduced
from2.85-fold at 24 h to 0.97-fold at 48 h. Conversely, Bax
expression levels were significantly increased in ESC-treated cells
to5.8-fold at 24 h and to 19.95-fold at 48 h compared with those in
the controls, indicating that the ESC-treatment induced apoptosis
by increasing the Bax:Bcl-2 ratio (Fig. 4C and D).
Discussion
Marine alga metabolites have been recognized as a
source of diverse and novel pharmacological molecules and
compounds. Carrageenans, complex SPs, are considered to be major
constituent compounds in a large group of edible red algae
(8,26). In the present study, FT-IR analysis
revealed that the SPs extracted from L. papillosa have the
characteristic spectra of I-carrageenan carbohydrate. The IR
spectroscopic analysis of an algal extract from Eucheuma
serra highlighted similar unique characteristics of
I-carrageenan (23). In the last
decade, several studies have indicated that a number of red algal
SPs demonstrate anti-proliferative, pro-apoptotic, DNA-damaging,
anti-angiogenic, growth-inhibiting, cell cycle-arresting and
anti-metastatic functions (27,28).
Therefore, algal polysaccharides have become compounds of great
interest due to their anti-cancerous activity (6,7). The
anti-cancer mechanisms of SPs have been hypothesized to involve
inhibition of the proliferation of tumor cells via the induction of
apoptosis, which has been demonstrated in a number of tumor models,
including those of melanoma, nasopharyngeal and gastric carcinomas
and breast cancer (11,29). The current study revealed that ESC
exerts a cytotoxic inhibitory effect on MDA-MB-231 cells in a time-
and concentration-dependent manner. Subsequently, the inhibition of
the ESC-treated cells led to apoptosis. Similar studies have shown
that the sulfated oligosaccharide PI-88 demonstrates effective
anti-tumor activity in a pancreatic islet mouse melanoma via
apoptosis (30). These results,
along with the results of the present study, demonstrate that ESC
has anti-proliferative properties that lead to apoptosis.
Induction of apoptosis via cytotoxic drug treatment
has been shown to be a significant method of triggering cell death
in a number of types of cancer (31). Therefore, an understanding of the
events of apoptosis and its signaling pathway may allow for the
development of novel agents for cancer treatment (32). Notably, the results of the current
study demonstrated that ESC effectively induces the extrinsic
pathway of apoptosis via regulation of the key molecule caspase-8
in MDA-MB-231 cells. In addition, ESC-treated cells exhibit
features that characterize apoptosis induced by the main executors
caspase-3 and caspase-9 (33),
including nuclear condensation, DNA fragmentation and cell
shrinkage (34–36). The Bax and Bcl-2 proteins are also
known to regulate apoptosis promoted by different stimuli (37,38).
p53 is a direct transcriptional activator of the Bax gene (39), which interacts with Bcl-2 to
enhance outer mitochondrial membrane permeabilization. The
increased expression levels of p53 induce an increase in the
Bax:Bcl-2 ratio, resulting in the release of cytochrome c,
the activation of caspase and ultimately apoptosis (40,41).
The biological activity of ESC algal extracts observed in the
present study indicates a potential mechanism for the induction of
cell apoptosis. These results concur with those of several previous
studies on apoptosis induction via SP treatment (27,28).
In conclusion, the results of the current study
demonstrate that ESCs from the red alga L. papillosa inhibit
cell growth and induce apoptosis in MDA-MB-231 cells via the
recruitment of caspase-3, caspase-8 and caspase-9, the
re-modulation of the Bax:Bcl-2 ratio, and DNA damage. ESC may serve
as a potential therapeutic agent and could be a promising target
molecule in cancer prevention. Further studies are required to
evaluate the potential anti-proliferative and anti-cancer
activities of this extract in vivo.
Acknowledgements
This study was supported and funded by the Atomic
Energy Commission of Syria (AECS). The authors thank the Director
General of the AECS and the Head of the AECS Molecular Biology and
Biotechnology Department for their support.
References
|
1
|
Pomin VH: Fucanomics and galactanomics:
marine distribution, medicinal impact, conceptions, and challenges.
Mar Drugs. 10:793–811. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cofrades S, López-López I, Bravo L, et al:
Nutritional and antioxidant properties of different brown and red
Spanish edible seaweeds. Food Sci Technol Int. 16:361–370. 2010.
View Article : Google Scholar
|
|
3
|
Pujol CA, Scolaro LA, Ciancia M,
Matulewicz MC, Cerezo AS and Damonte EB: Antiviral activity of a
carrageenan from Gigartina skottsbergii against intraperitoneal
murine herpes simplex virus infection. Planta Med. 72:121–125.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Magalhaes KD, Costa LS, Fidelis GP, et al:
Anticoagulant, antioxidant and antitumor activities of heterofucans
from the seaweed Dictyopteris delicatula. Int J Mol Sci.
12:3352–3365. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fu BD, Bi WY, He CL, et al: Sulfated
derivatives of 20(S)-ginsenoside Rh2 and their inhibitory effects
on LPS-induced inflammatory cytokines and mediators. Fitoterapia.
84:303–307. 2013. View Article : Google Scholar
|
|
6
|
Chen LL, Chen X, Choi H, et al: Exploiting
antitumor immunity to overcome relapse and improve remission
duration. Cancer Immunol Immunother. 61:1113–1124. 2012. View Article : Google Scholar :
|
|
7
|
Namvara F, Mohameda S, et al:
Polyphenol-rich seaweed (Eucheuma cottonii) extract suppresses
breast tumour via hormone modulation and apoptosis induction. Food
Chem. 130:376–382. 2012. View Article : Google Scholar
|
|
8
|
Yang C, Chung D, et al: Effects of
molecular weight and hydrolysis conditions on anticancer activity
of fucoidans from sporophyll of Undaria pinnatifida. Int J Biol
Macromol. 43:433–437. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
DeSantis C, Howlader N, Cronin KA and
Jemal A: Breast cancer incidence rates in U.S. women are no longer
declining. Cancer Epidemiol Biomarkers Prev. 20:733–739. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yuan YV and Walsh NA: Antioxidant and
antiproliferative activities of extracts from a variety of edible
seaweeds. Food Chem Toxicol. 44:1144–1150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reddy BS, Cohen LA, McCoy GD, Hill P,
Weisburger JH and Wynder EL: Nutrition and its relationship to
cancer. Adv Cancer Res. 32:237–345. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Teas J: The consumption of seaweed as a
protective factor in the etiology of breast cancer. Med Hypotheses.
7:601–613. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hebert JR and Rosen A: Nutritional,
socioeconomic, and reproductive factors in relation to female
breast cancer mortality: findings from a cross-national study.
Cancer Detect Prevent. 20:234–244. 1996.PubMed/NCBI
|
|
15
|
Kodama M, Kodama T, Miura S and Yoshida M:
Nutrition and breast cancer risk in Japan. Anticancer Res.
11:745–754. 1991.PubMed/NCBI
|
|
16
|
Teas J, Vena S, Cone DL and Irhimeh M: The
consumption of seaweed as a protective factor in the etiology of
breast cancer: proof of principle. J Appl Phycol. 25:771–779. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim SK and Karagozlu MZ: Marine algae:
natural product source for gastrointestinal cancer treatment. Adv
Food Nutr Res. 64:225–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Khanavi M, Nabavi M, Sadati N, et al:
Cytotoxic activity of some marine brown algae against cancer cell
lines. Biol Res. 43:31–37. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Patel S: Therapeutic importance of
sulfated polysaccharides from seaweeds: updating the recent
findings. 3. Biotech. 2:171–185. 2012.
|
|
20
|
Miller SA, Dykes DD and Polesky HF: A
simple salting out procedure for extracting DNA from human
nucleated cells. Nucleic Acids Res. 16:12151988. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lo YL and Liu Y: Reversing multidrug
resistance in Caco-2 by silencing MDR1, MRP1, MRP2, and
BCL-2/BCL-xL using liposomal antisense oligonucleotides. PLoS One.
9:e901802014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fenoradosoa TA, Delattre C, Laroche C, et
al: Highly sulphated galactan from Halymenia durvillei
(Halymeniales, Rhodophyta), a red seaweed of Madagascar marine
coasts. Int J Biol Macromol. 45:140–145. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin LH, Tako M and Hongo F: Isolation and
characterization of iota-carrageenan from Eucheuma serra
(Togekirinsai). J Appl Glycosci. 47:303–310. 2000. View Article : Google Scholar
|
|
24
|
Chiovitti A, Bacic A, Craik DJ, Kraft GT
and Liao ML: A nearly idealized 6′-O-methylated iota-carrageenan
from the Australian red alga Claviclonium ovatum (Acrotylaceae,
Gigartinales). Carbohydr Res. 339:1459–1466. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liao ML, Chiovitti A, Munro SL, Craik DJ,
Kraft GT and Bacic A: Sulfated galactans from Australian specimens
of the red alga Phacelocarpus peperocarpos (Gigartinales,
Rhodophyta). Carbohydr Res. 296:237–247. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karnjanapratum S and You S: Molecular
characteristics of sulfated polysaccharides from Monostroma nitidum
and their in vitro anticancer and immunomodulatory activities. Int
J Biol Macromol. 48:311–318. 2011. View Article : Google Scholar
|
|
27
|
Xue M, Ge Y, Zhang J, et al: Anticancer
properties and mechanisms of fucoidan on mouse breast cancer in
vitro and in vivo. PLoS One. 7:e434832012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
El Gamal AA: Biological importance of
marine algae. Saudi Pharm J. 18:1–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu Y, Tang W, Li CL, et al: Cytotoxicity
of a newly synthesized nitroxide derivative of
4-ferrocenecarboxyl-2,2,6,6-tetramethylpiperidine-1-oxyl in high
metastatic lung tumor cells. Pharmazie. 61:1028–1033. 2006.
|
|
30
|
Johnstone KD, Karoli T, Liu L, et al:
Synthesis and biological evaluation of polysulfated oligosaccharide
glycosides as inhibitors of angiogenesis and tumor growth. J Med
Chem. 53:1686–1699. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Green DR and Kroemer G: The
pathophysiology of mitochondrial cell death. Science. 305:626–629.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guessous I, Cornuz J and Paccaud F: Lung
cancer screening: current situation and perspective. Swiss Med
Wkly. 137:304–311. 2007.PubMed/NCBI
|
|
34
|
Shah S, Gapor A and Sylvester PW: Role of
caspase-8 activation in mediating vitamin E-induced apoptosis in
murine mammary cancer cells. Nutr Cancer. 45:236–246. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Riedl SJ and Shi Y: Molecular mechanisms
of caspase regulation during apoptosis. Nat Rev Mol Cell Biol.
5:897–907. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cheah YH, Nordin FJ, Tee TT, Azimahtol HL,
Abdullah NR and Ismail Z: Antiproliferative property and apoptotic
effect of xanthorrhizol on MDA-MB-231 breast cancer cells.
Anticancer Res. 28:3677–3689. 2008.
|
|
37
|
Gao Z, Shao Y and Jiang X: Essential roles
of the Bcl-2 family of proteins in caspase-2-induced apoptosis. J
Biol Chem. 280:38271–38275. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cheah YH, Azimahtol HL and Abdullah NR:
Xanthorrhizol exhibits antiproliferative activity on MCF-7 breast
cancer cells via apoptosis induction. Anticancer Res. 26:4527–4534.
2006.
|
|
39
|
Miyashita T and Reed JC: Tumor suppressor
p53 is a direct transcriptional activator of the human bax gene.
Cell. 80:293–299. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rassouli FB, Matin MM, Iranshahi M and
Bahrami AR: Investigating the cytotoxic and apoptosis inducing
effects of monoterpenoid stylosin in vitro. Fitoterapia.
82:742–749. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Luo G, Guan X and Zhou L: Apoptotic effect
of citrus fruit extract nobiletin on lung cancer cell line A549 in
vitro and in vivo. Cancer Biol Ther. 7:966–973. 2008. View Article : Google Scholar : PubMed/NCBI
|