Introduction
It has been demonstrated that the increase in the
total number of stromal and epithelial prostatic cells due to the
reduction of cell apoptosis and/or excessive cell proliferation, is
associated with the development of benign prostatic hyperplasia
(BPH) (1–3). BPH, a urinary system disease, is a
common prostate disorder in older males with increasing incidence
as average life span is prolonged in an ageing population (4,5). BPH
has become a major disease worldwide. The overgrown prostate gland
causes increased resistance to urine flow, resulting in lower
urinary tract symptoms (LUTS), including urinary hesitancy,
frequent urination, urgency, thin urine flow and urinary retention
(6). These symptoms have a marked
effect on the physical and mental health of patients and affect
their quality of life.
The pathogenesis of BPH is complex and remains to be
elucidated. It has been widely accepted that the incidence and
development of BPH is closely associated with disordered cell
proliferation, the apoptosis of prostatic cells and dysregulation
of several growth factors (7–12).
Growth factors function via binding to their specific receptors,
which activates the intracellular signal transduction system
involved in the regulation of physiological function (8,9). The
signal transducer and activator of transcription 3 (STAT3) pathway
is one of the main growth factor-mediated signal transduction
pathways and is closely associated with the occurrence of BPH
(13–15). Abnormal activation of the pathway
can lead to a significant increase in the expression levels of the
anti-apoptotic protein B-cell lymphoma 2 (Bcl-2) and the
pro-proliferative protein cyclin D1, which in turn promote cell
growth and the inhibition of apoptosis, which leads to the abnormal
proliferation and malignant transformation of cells (16).
Epidermal growth factor (EGF) is an important factor
in the promotion of mitosis and proliferation. Studies have
demonstrated that EGF has an important effect on prostate growth
and its abnormal expression is a key factor in BPH (16–18).
EGF elicits its biological effects through binding to its receptor
(EGFR). Upon interaction with EGF, the EGFR induces the
phosphorylation/activation of STAT3, a transcription factor
essential for cell survival and proliferation (16,19,20).
The phosphorylation of STAT3 in the cytoplasm induces its
homodimerization, nuclear translocation and DNA binding, resulting
in the expression of various essential genes involved in cell
proliferation and survival (21–24).
Therefore, the suppression of STAT3 activation has been a major
focus in the development of novel treatments for BPH.
Natural products have been considered as alternative
medicines for a number of years. Numerous plants and their
constituents have been observed to possess beneficial therapeutic
effects for various diseases, including BPH (25–28).
Qianliening capsules (QC) are a Traditional Chinese Medicine and
consist of a combination of rhubarb (Radix et Rhizoma Rhei),
leech (Hirudo), Astragalus (Radix Astragali),
Achyranthes (Radix Achyranthis bidentatae) and Dodder (Semen
Cuscutae). These products together confer the QC properties
of heat-clearance, detoxification, promotion of blood circulation,
removal of blood stasis and tonfication of the kidney, the latter
being considered to nourish vitality and referred to as
replenishing the kidney qi in Chinese Medicine (29,30).
It has been reported that QCs have significant therapeutic effects
on BPH by improving a series of LUTS and the dynamic index of urine
flow in patients with BPH (29,31).
In addition, studies involving animal experiments have demonstrated
that QC significantly decreases the prostatic volume and weight and
inhibits prostatic hyperplasia. Previous studies by our group have
shown that this occurs by improving the abnormal ratio of estrogen
to androgen in the serum via regulating the expression of estrogen
receptor (ER) and androgen receptor (AR) (30), inhibiting the expression levels of
pro-proliferative cyclin D1, proliferating cell nuclear antigen and
cyclin-dependent kinase 4 (CDK4) via suppression of the EGF/STAT3
signaling pathway in vivo (16), and inducing prostatic cell
apoptosis through the mitochondrion-dependent apoptotic pathway
in vivo and in vitro (32,33).
However, the precise mechanism of its anti-BPH activity remains to
be fully elucidated. To further investigate the mechanism
underlying the activity of QC in the treatment of BPH, the present
study investigated its effects on the EGF-mediated activities in
human prostatic WPMY-1 cells, including cell proliferation and
apoptosis, levels of phosphorylation, the transcriptional activity
of STAT3 and the expression levels of several STAT3 signaling
target genes. The present study investigated the effect of QC on
the proliferation and apoptotic rate of WPMY-1 cells,
phosphorylation and transcription of STAT3, as well as expression
of the apoptosis-regulating proteins Bcl-2, cyclin D1, CDK4,
B-cell-associated X protein (Bax) and p21. Western blot and
polymerase chain reaction (PCR) analyses, MTT assay, cell cycle
analysis and a luciferase reporter assay were employed to elucidate
the underlying mechanism of the activity of QC. To the best of our
knowledge, the present study was the first to report that the
effect of QC on the proliferation and apoptosis of WPMY-1 cells
proceeds via interference with the STAT3 pathway, which is likely
to be one of the mechanisms underlying its activity in the
treatment of BPH.
Materials and methods
Materials and reagents
QC (Chinese Food and Drug Administration, approval
no. Z09104065) was provided by the Academy of Pharmacology of
Fujian University of Traditional Chinese Medicine (Fuzhou, China).
QC were dissolved in phosphate-buffered saline (PBS) to a
concentration of 0.5 g/ml and stored at 4°C. Dulbecco’s modified
Eagle’s medium (DMEM), fetal bovine serum (FBS),
penicillin-streptomycin, trypsin-EDTA and TRIzol reagent were
purchased from Invitrogen Life Technologies (Carlsbad, CA, USA).
SuperScript II reverse transcriptase was obtained from Promega
Corporation (Madison, WI, USA). All antibodies were purchased from
Cell Signaling Technologies, Inc, (Danvers, MA, USA). The EGF and
all other chemicals used, unless otherwise stated, were obtained
from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture
Human prostate stromal WPMY-1 cells were obtained
from the Cell Bank of the Chinese Academy of Science (Shanghai,
China). The cells were grown in DMEM containing 10% (v/v) FBS, 100
units/ml penicillin and 100 μg/ml streptomycin in a 37°C humidified
incubator with 5% CO2.
Evaluation of cell viability by MTT
assay
The WPMY-1 cells were seeded into 96-well plates at
a density of 1.0×104 cells/well in 0.1 ml medium. After
24 h, with the exception of the control group, the cells were
stimulated with 10 ng/ml EGF and treated with different
concentrations of QC (1, 3 and 5 mg/ml) for 24 h. Subsequently, 10
μl MTT (5 mg/ml in PBS) was added to each well and the samples were
incubated for an additional 4 h at 37°C. The purple-blue MTT
formazan precipitate was dissolved in 0.1 ml dimethyl sulfoxide and
the absorbance was measured at 570 nm using an ELISA reader
(EXL800; BioTek Instruments, Inc., Winooski, VT, USA).
Observation of morphological changes
The WPMY-1 cells were seeded into 12-well plates at
a density of 1.0×105 cells/ml in 1 ml medium. After 24
h, with the exception of the control group, the cells were
stimulated with EGF (10 ng/ml) and treated with various
concentrations of QC (1, 3 and 5 mg/ml) for 24 h. The cell
morphology was observed using a phase-contrast microscope (DM4000B;
Leica Microsystems, Wetzlar, Germany) and images were captured at a
magnification of ×200.
Detection of apoptosis by Annexin
V/propidium iodide (PI) staining
The WPMY-1 cells were seeded into six-well plates at
a density of 1.0×105 cells/ml in 2 ml medium. After 24
h, the cells were stimulated with EGF (10 ng/ml) and treated with
various concentrations of QC for 24 h. Following incubation with
various concentrations of QC (1,3 and 5 mg/ml), the apoptotic rate
of WPMY-1 cells was determined by flow cytometric analysis using
FACSCalibur flow cytometer (Becton-Dickinson, San Jose, CA, USA)
and an Annexin V-fluorescein isothiocyanate (FITC)/PI kit
(Becton-Dickinson). Staining was performed according to the
manufacturer’s instructions. The percentage of cells in early
apoptosis was determined via quantification of Annexin V-positive
and PI-negative cells, while Annexin V-positive and PI-positive
cells were regarded to be in late apoptosis.
Detection of cell cycle distribution by
PI staining
The WPMY-1 cells were seeded into a six-well plate
at a density of 1.0×105 cells/ml in 2 ml medium. After
24 h, with the exception of the control group, the cells were
stimulated with EGF and treated with various concentrations of QC
for 24 h. After 24 h, the cells were harvested, adjusted to a
concentration of 1×106 cells/ml and fixed in 70% ethanol
at 4°C overnight. The fixed cells were washed twice with cold PBS
and incubated with RNase (8 μg/ml) and PI (10 μg/ml) for 30 min in
the dark. The cell cycle distribution was detected by flow
cytometric analysis and the proportion of DNA in different phases
was analyzed using Modfit LT 3.0 software (Verity Software House,
Topsham, ME, USA).
Analysis of caspase-9 and caspase-3
activation
The activation of caspase-3 and -9 was determined
using a colorimetric assay with caspase-9 and -3 activation kits
(Invitrogen Life Technologies) according to the manufacturer’s
instructions. Briefly, following treatment with various
concentrations of QC for 24 h, the EGF-stimulated WPMY-1 cells were
lysed with mammalian cell lysis buffer, containing 20 mM Tris-HCl
(pH 7.4), 150 mM NaCl, 2 mM ethylenediaminetetraacetic acid, 1%
Nonidet P-40, 50 mM NaF and 1X protease inhibitor, for 30 min on
ice. The lysed cells were centrifuged at 16,000 × g for 10 min and
100 μg protein was incubated with 50 μl of the colorimetric
tetrapeptides, Asp-Glu-Val-Asp (DEVD)-p-nitroaniline (pNA),
a specific substrate of caspase-3 or Leu-Glu-His-Asp (LEHD)-pNA, a
specific substrate of caspase-9, at 37°C in the dark for 2 h. The
samples were read at 405 nm using an ELISA reader (EXL800; BioTek
Instruments, Inc.). The data were normalized to the activity of the
caspases in the control cells, which were treated with the PBS
vehicle, and expressed as the fold-change compared with the
control.
Luciferase gene reporter assay
The WPMY-1 cells were seeded into 96-well plates at
a density of 1×104 cells/well in 0.1 ml complete DMEM
until they reached ~60% confluency and then continuously cultured
in FBS- and antibiotic-free medium overnight. The cells were
transfected with a mixture of inducible STAT3-responsive firefly
luciferase and constitutively expressing Renilla luciferase using
Lipofectamine™ LTX with PLUS™ Reagent. Subsequently, 6 h after
transfection, the medium was changed back to complete DMEM with
FBS, penicillin and streptomycin. After 24 h of transfection, the
cells were treated with various contractions of QC (1, 3 and 5
mg/ml) for 1 h followed by EGF for another 24 h. The cell extracts
were prepared and analyzed using the Promega Dural Luciferase
Reporter Assay system according to the manufacturer’s instructions.
The firefly luciferase activity measured was normalized to that of
Renilla luciferase in the same well.
RNA extraction and RT-qPCR analysis
A total of 1×105 WPMY-1 cells were seeded
into 12-well plates. After 24 h, the cells were stimulated with EGF
and treated with various concentrations of QC (1, 3 and 5 mg/ml)
for 24 h. The total RNA from the WPMY-1 cells was isolated using
TRIzol reagent (Invitrogen Life Technologies). Oligo(dT)-primed RNA
(1 μg) was reverse-transcribed with SuperScript II reverse
transcriptase (Promega Corporation) according to the manufacturer’s
instructions. The cDNA obtained was used to determine the mRNA
expression levels of Bcl-2, Bax, cyclin D1, CDK4 and p21 by PCR
with Taq DNA polymerase (Fermentas, Pittsburgh, PA, USA). GAPDH was
used as an internal control. The primers used for amplification of
Bcl-2, Bax, Cyclin D1, CDK4, p21 and GAPDH transcripts are as
described previously (33,34). The samples were analyzed by gel
electrophoresis (1.5% agarose) and the DNA bands were examined
using a gel documentation system (Gel Doc XR+; Bio-Rad, Hercules,
CA, USA).
Western blot analysis
The WPMY-1 cells were seeded into a 25
cm2-culture bottle at a density of 1.0×105
cells/ml in 5 ml medium. After 24 h, the cells were stimulated with
EGF and treated with various concentrations of QC for 24 h. The
treated cells were lysed in mammalian cell lysis buffer (M-PER;
Thermo Fisher Scientific, Rockford, IL, USA) containing protease
(EMD Biosciences, La Jolla, CA, USA) and phosphatase inhibitor
(Sigma-Aldrich) cocktails and centrifuged at 14,000 × g for 15 min.
The protein concentrations in the cell lysate supernatants were
determined using a Bicinchoninic Acid Protein Assay kit (Tiangen
Biotech Co., Ltd, Beijing, China). Equal quantities of protein (20
μg) from each tumor or cell lysate were resolved on 12%
Tris-glycine gels and transferred onto polyvinylidene difluoride
membranes. The membranes were inhibited for 2 h with 5% non-fat dry
milk and incubated with the desired primary monoclonal antibodies
directed against STAT3, phosphorylated (p)-STAT3, Bcl-2, Bax,
cyclin D1, CDK4, p21 and β-actin (all 1:1,000) overnight at 4°C.
Appropriate horseradish peroxidase-conjugated secondary antibodies
with chemiluminescence detection were used to image the
antibody-detected proteins. The membranes were analyzed using
Enhanced eyeECL Plus reagents and scanned using a Storm
PhosphorImager (Chemi Doc XRS+; Bio-Rad).
Statistical analysis
Data are expressed as the mean ± standard deviation
for the indicated number of independently performed experiments.
The data were analyzed using SPSS 17.0 software for Windows (SPSS,
Inc., Chicago, IL, USA). Statistical analyses were performed using
Student’s t-test and analysis of variance. P<0.05 was considered
to indicate a statistically significant difference.
Results
QC inhibits the growth of WPMY-1
cells
Since the development of BPH is associated with a
reduction in prostatic cell apoptosis and/or excessive cell
proliferation and EGF is one of the essential growth factors in
promoting the proliferation and inhibiting the apoptosis of
prostatic cells, the present study used EGF to stimulate the growth
of the WPMY-1 human prostate stromal cell line. As expected, upon
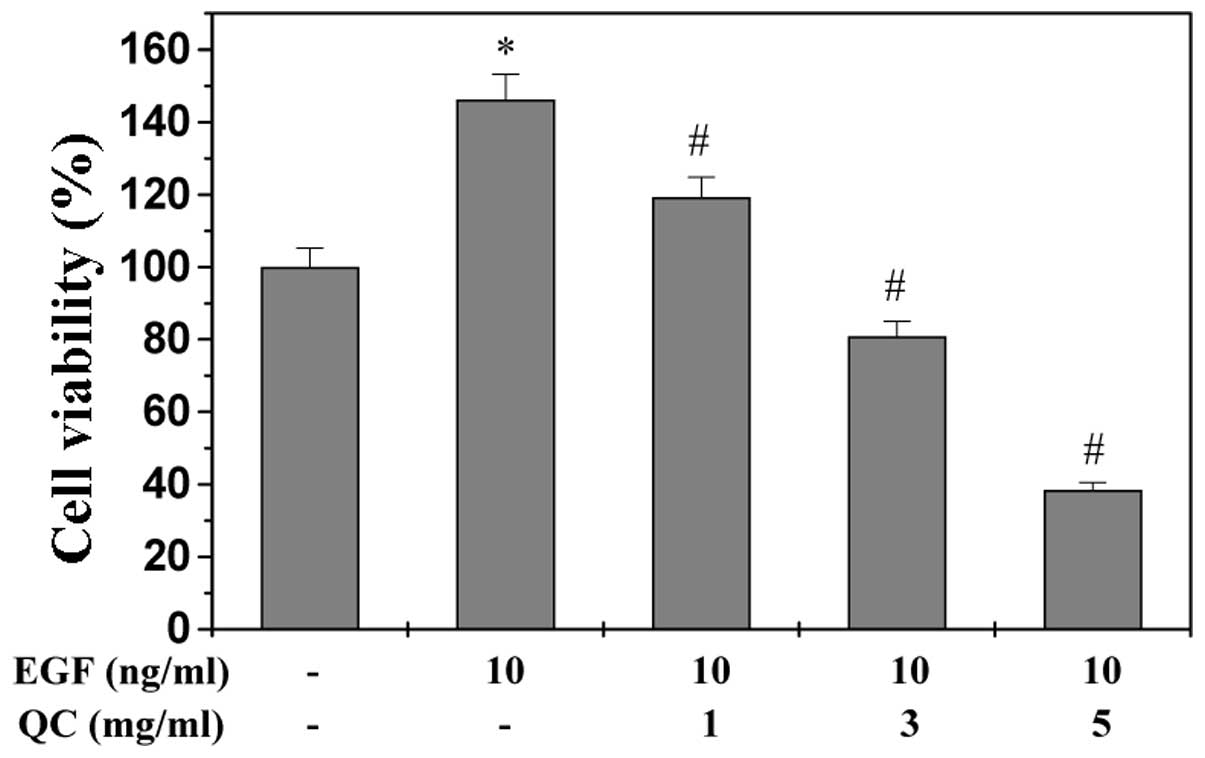
stimulation with 10 ng/ml EGF, the viability of the WPMY-1 cells
increased to 145% of that of the control cells (P<0.01; Fig. 1). However, treatment with 1–5 mg/ml
QC for 24 h led to a dose-dependent reduction in the viability of
EGF-stimulated cells (P<0.01). To further confirm these results,
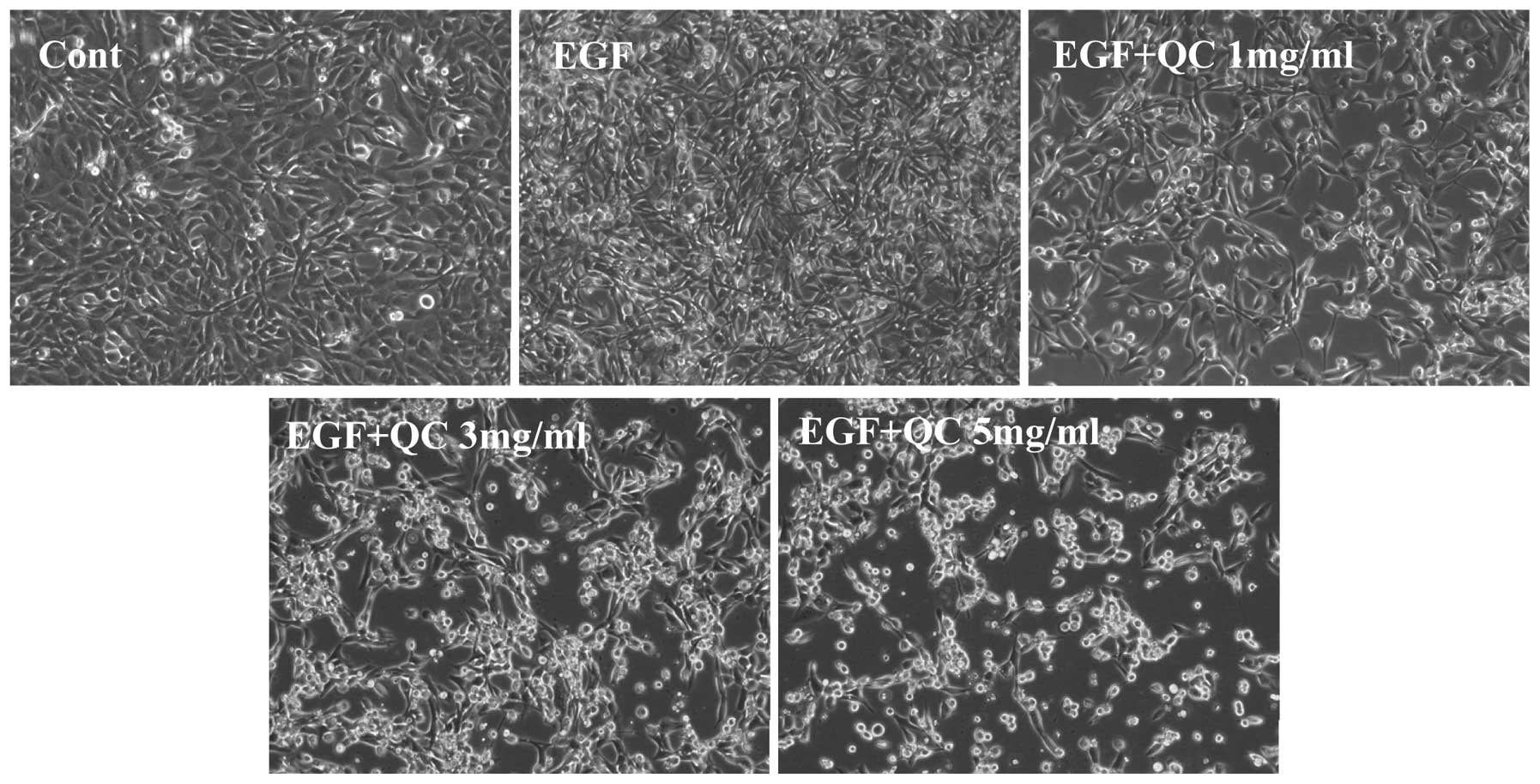
the effect of QC on WPMY-1 cell morphology was assessed via
phase-contrast microscopy, since the morphology of cells in culture
is indicative of the health status of the cells. As shown in
Fig. 2, QC treatment led to a
dose-dependent reduction in WPMY-1 cell density. These data
demonstrated that QC inhibited the growth of EGF-stimulated WPMY-1
cells.
QC induces apoptosis of WPMY-1 cells
The QC-induced apoptosis of the WPMY-1 cells was
examined in vitro using Annexin-V/PI staining followed by
flow cytometric analysis. As shown in Fig. 3A and B, no significant decrease was
observed in the apoptotic rate of WPMY-1 cells stimulated with 10
ng/ml EGF compared with that of the control cells (P>0.05).
However, QC treatment dose-dependently increased the percentage of
cells undergoing apoptosis, demonstrated as early apoptosis in the
lower right and late apoptosis in the upper right quadrants in the
dot plots (P<0.05, vs. EGF-stimulated cells without QC
treatment) (Fig. 3A).
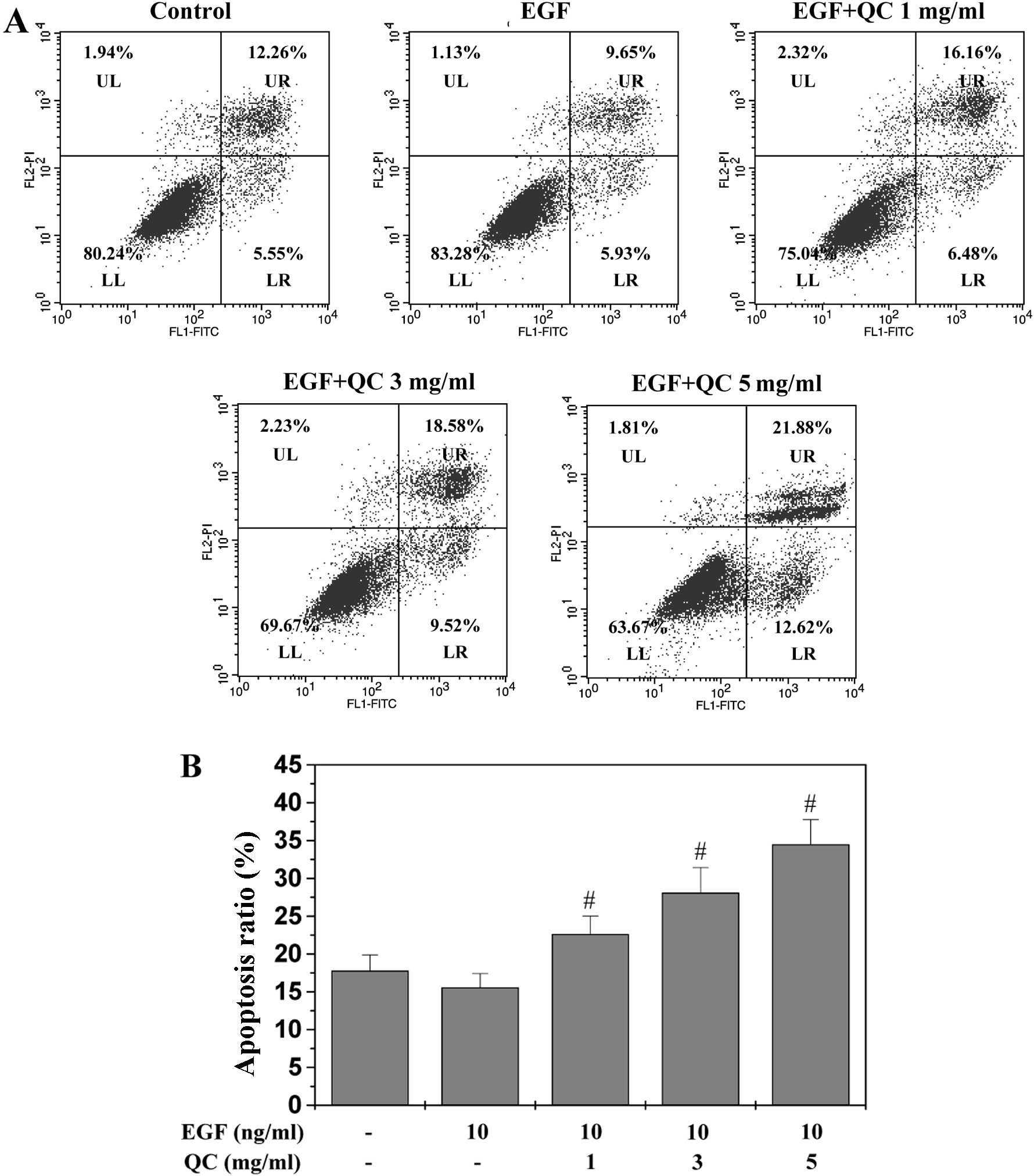 | Figure 3Effect of QC on the apoptosis of
WPMY-1 cells stimulated with EGF. (A) WPMY-1 cells stimulated with
EGF were treated with the indicated concentrations of QC (1, 3 or 5
ml/ml) for 24 h, collected and stained with Annexin V/PI followed
by FACS analysis. Representative FACS analysis scatter-grams of
Annexin V/PI staining reveals four cell populations:
Double-negative stained cells (LL) indicating the live cell
population; Annexin V-positive/PI-negative stained cells (LR);
Annexin V/PI double-positive stained cells (UR) representing early
and late apoptosis, respectively, and Annexin V-negative and
PI-positive stained cells (UL) indicating dead cells. Dot plots are
representative of three independent experiments. (B) Quantification
of FACS analysis. Data are expressed as the mean ± standard
deviation (error bars) from three independent experiments.
#P<0.05, vs. cells treated with EGF but without QC.
QC, Qianliening capsules; EGF, epidermal growth factor; FACS,
fluorescence-activated cell sorting; LL, lower left; LR, lower
right; UR, upper right; UL, upper left; FITC, fluorescein
isothiocyanate; PI, propidium iodide. |
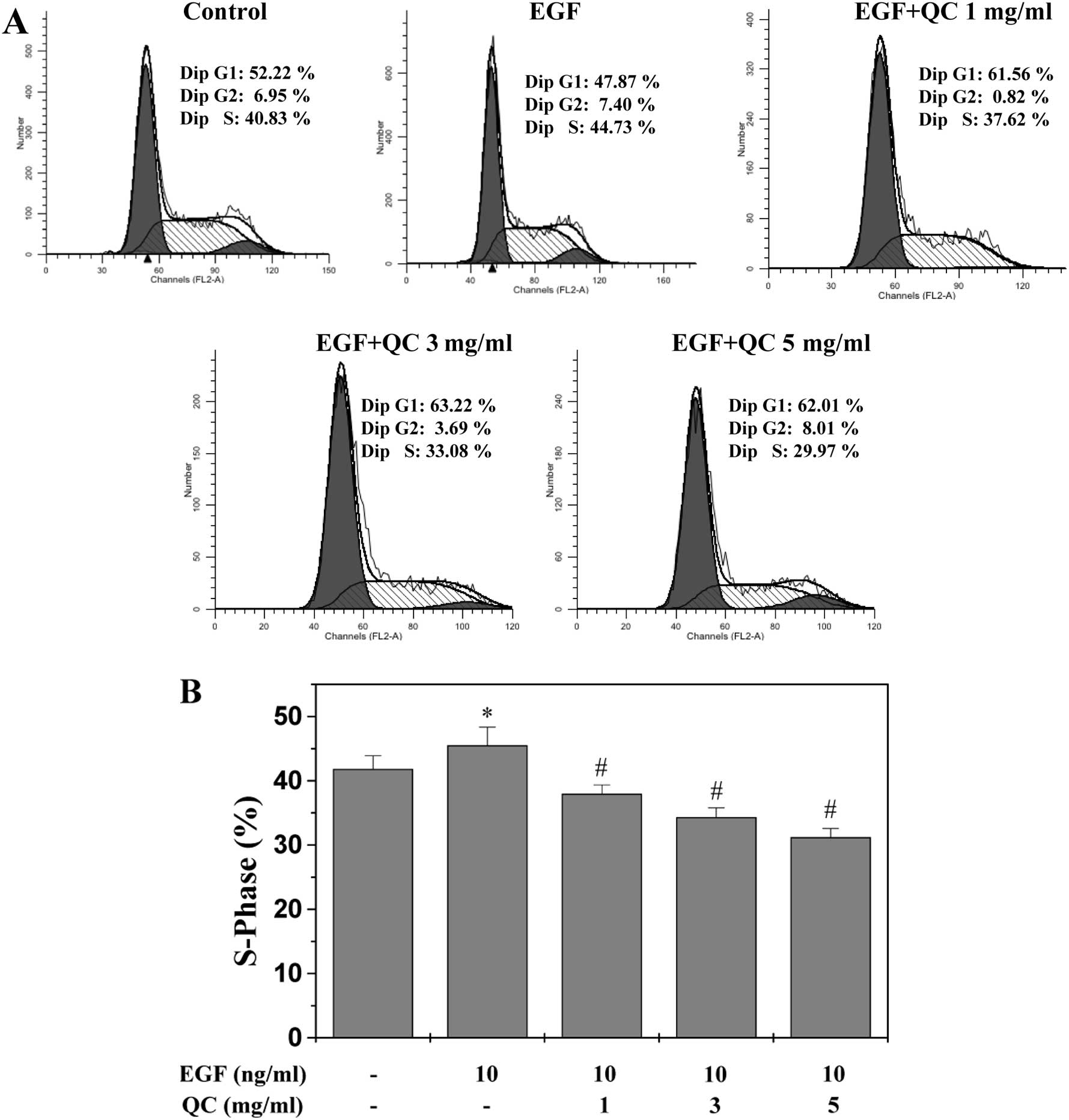
QC inhibits G1/S phase transition of
WPMY-1 cells
The G1/S transition is one of the two main
checkpoints used by cells to regulate the cell cycle progression
and, thus, cell proliferation (35). The present study investigated the
effect of QC on the G1- to S-phase transition in the WPMY-1 cells
via PI staining followed by flow cytometric analysis. As shown in
Fig. 4, the proportion of WPMY-1
cells in S-phase following stimulation with EGF (45.52±2.83%) was
markedly increased compared with that of the control cells
(41.83±2.07%; P<0.05; Fig. 4).
However, treatment with 1, 3 and 5 mg/ml QC for 24 h led to a
dose-dependent reduction in the proportion of the EGF-stimulated
cells in S-phase (37.98±1.38, 34.34±1.44 and 31.22±1.34%,
respectively), compared with that of the EGF-stimulated cells
without QC treatment (45.52±2.83; P<0.01). These results
indicated that QC inhibited the WPMY-1 cell proliferation by
inhibiting cell cycle progression by blocking the G1- to S-phase
transition.
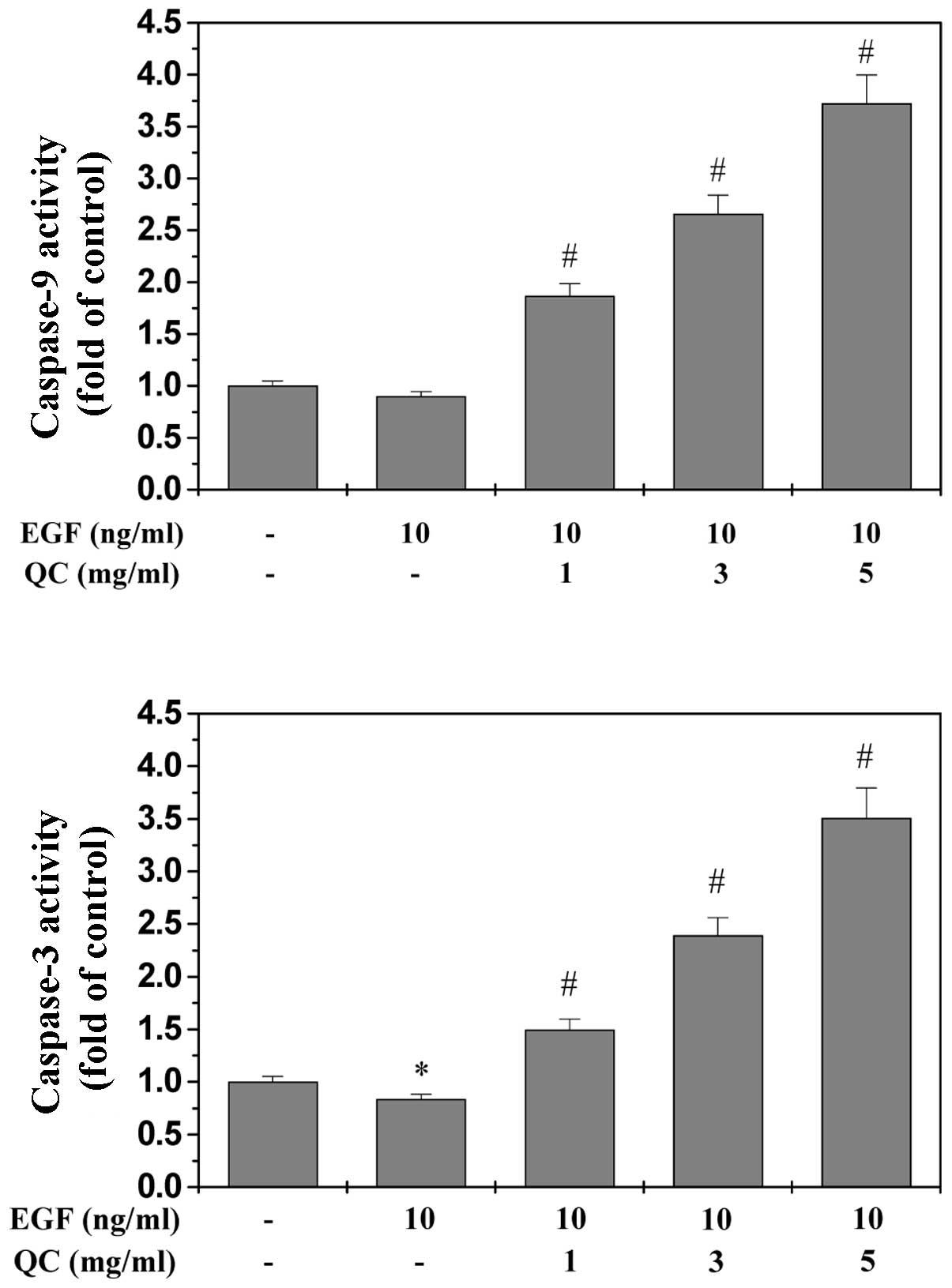
QC induces the activation of caspase-9
and -3 in WPMY-1 cells
The activation of caspase-9 and -3 was examined
using a colorimetric assay with the chromophores LEHD-pNA, a
specific substrate of caspase-9 and DEVD-pNA, a specific substrate
of caspase-3. Caspases, cytoplasmic aspartate-specific cysteine
proteases, are key proteins in the apoptotic response. Caspase-9
can activate caspase-3, which triggers targeting and degradation of
specific and vital cellular proteins. Subsequently, the degradation
of nuclear DNA and apoptotic cell death occurs. Therefore, the
activation of caspases is important in the process of apoptosis. As
shown in Fig. 5A and B, QC
treatment significantly and dose-dependently induced activation of
caspase-9 and -3 in WPMY-1 cells (P<0.01, vs EGF-stimulated
cells without QC treatment). In addition, EGF significantly
inhibited the activation of caspase-3 (Fig. 5B).
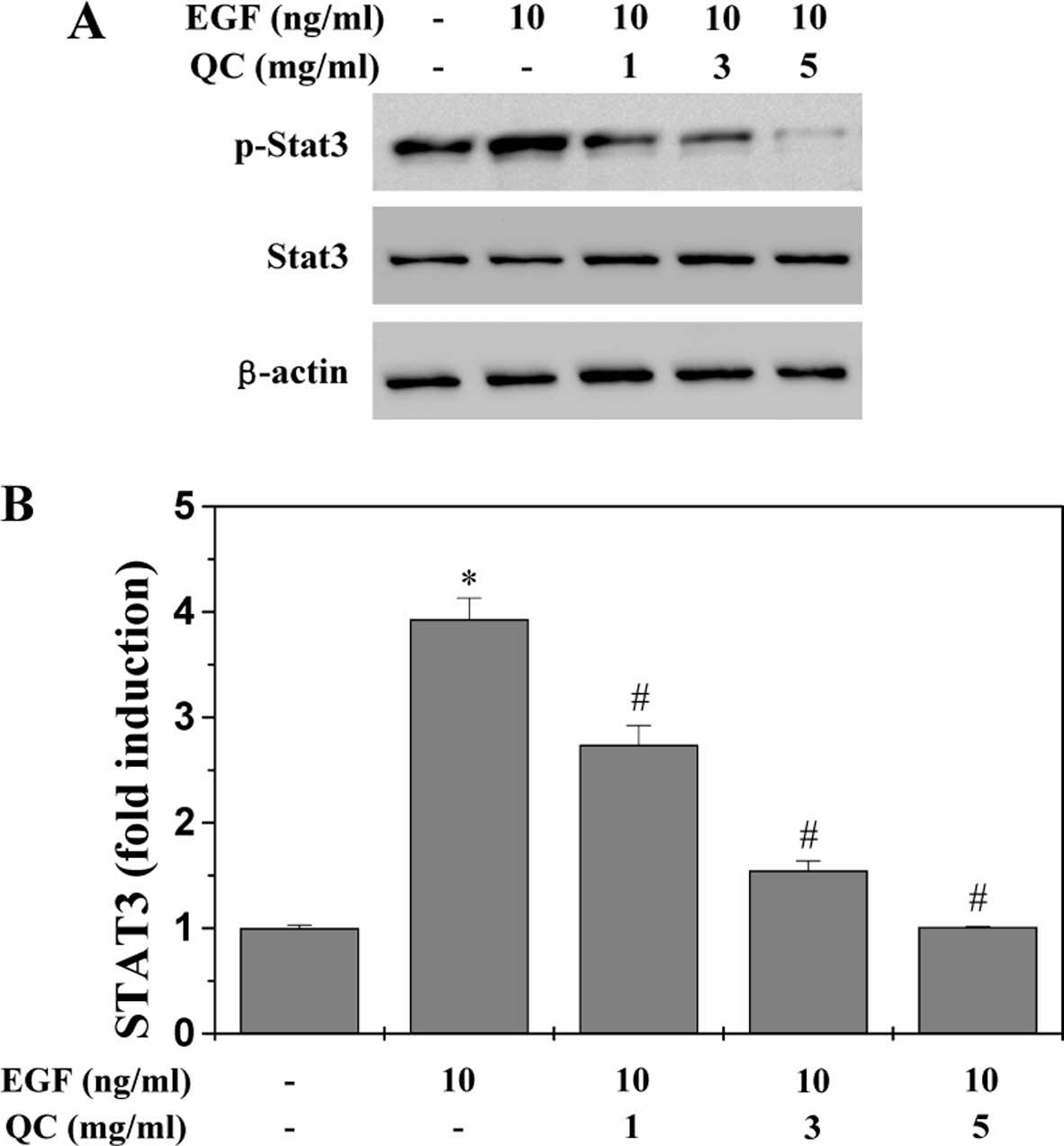
QC inhibits the activation of the STAT3
signaling pathway in WPMY-1 cells
Previous studies have demonstrated that EGF
stimulates STAT3 activation, which upregulates the levels of
phosphorylated STAT3 in BPH prostatic cells (16–20).
Therefore, in the present study, STAT3 activation in WPMY-1 cells
was stimulated with EGF. The activation of STAT3 was determined by
western blot analysis using an antibody which recognizes STAT3
phosphorylated at Tyr705 (pSTAT3). As shown in Fig. 6A, stimulation with 10 ng/ml EGF
significantly increased the levels of pSTAT3 in the WPMY-1 cells,
which, however, was profoundly inhibited by QC in a dose-dependent
manner. The levels of non-phosphorylated STAT3 remained unchanged
following treatment with EGF and/or QC. To further confirm the
inhibitory effect of QC on the activation of STAT3, a dual
luciferase reporter assay was used to examine the transcriptional
activity of STAT3. As shown in Fig.
6B, QC significantly and dose-dependently inhibited the
EGF-stimulated increase in STAT3 transcriptional activity. These
data suggested that QC was potent in inhibiting EGF-mediated STAT3
activation in human prostatic cells.
QC regulates the expression of Bcl-2,
Bax, cyclin D1, CDK4 and p21 in WPMY-1 cells
To further investigate the mechanisms underlying the
activities of QC, RT-qPCR and western blot analyses were performed
to examine the effect of QC on the expression of anti-apoptotic
Bcl-2, pro-apoptotic Bax, pro-proliferative cyclin D1 and CDK4, as
well as anti-proliferative p21, which are important target genes of
the STAT3 signaling pathway. As Fig.
7 shows, the mRNA and protein expression levels of Bcl-2,
cyclin D1 and CDK4 were markedly increased by EGF stimulation and
the expression levels of Bax and p21 were markedly reduced by EGF
stimulation. However, QC treatment profoundly inhibited the
EGF-induced upregulation of Bcl-2, cyclin D1 and CDK4 and increased
the EGF-induced downregulation of Bax and p21 at the
transcriptional and translational levels.
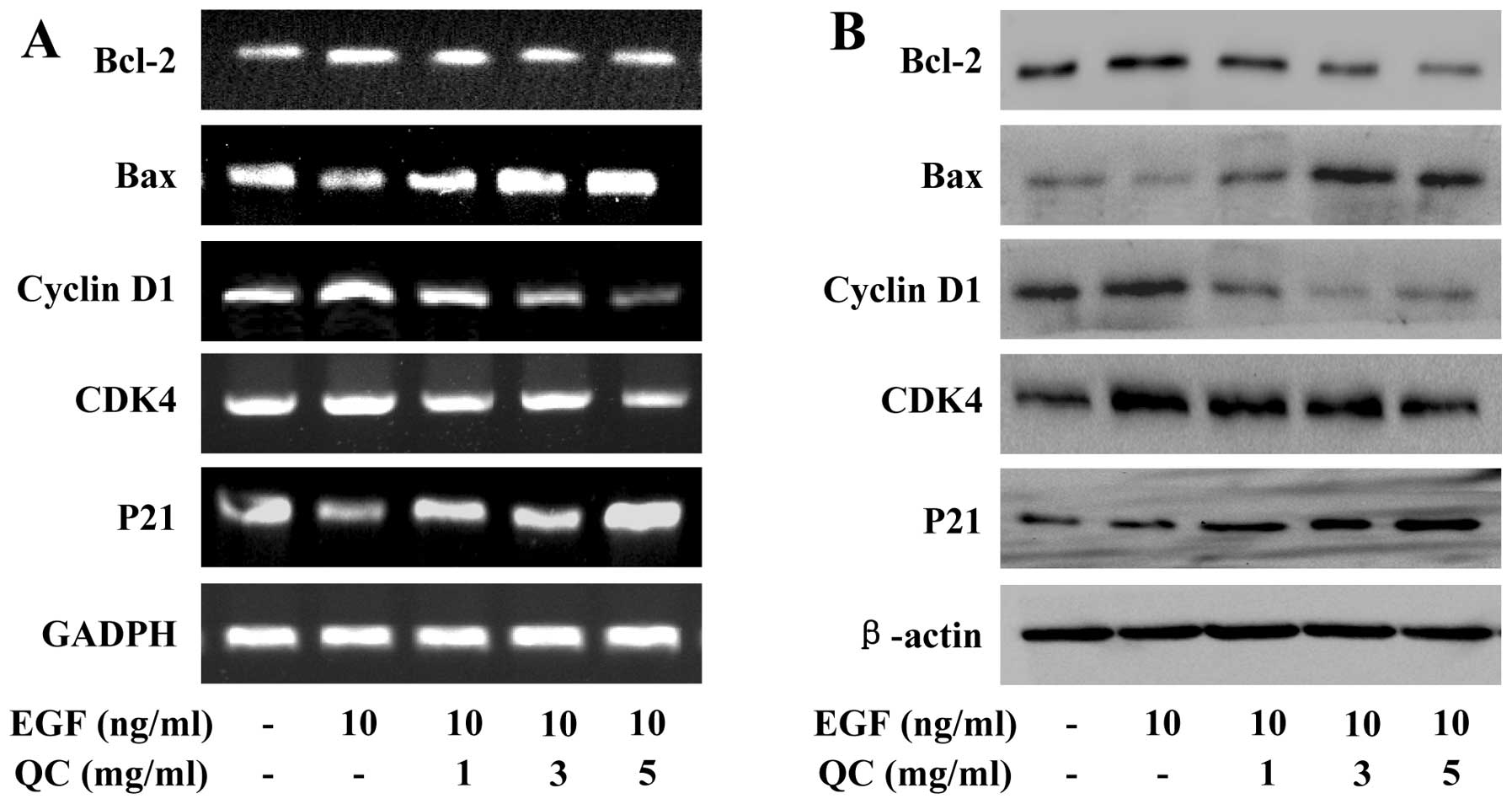 | Figure 7Effect of QC on the expression of
Bcl-2, Bax, Cyclin D1, CDK4 and p21 in WPMY-1 cells stimulated with
EGF. The WPMY-1 cells stimulated with EGF were treated with
indicated concentrations of QC (1, 3 or 5 ml/ml) for 24 h. (A) mRNA
levels of Bcl-2, Bax, Cyclin D1, CDK4 and p21 were determined by
RT-qPCR. (B) Protein expression levels of Bcl-2, Bax, cyclin D1,
CDK4 and p21 were analyzed by western blot analysis. GAPDH and
β-actin were used as the internal controls for the RT-qPCR and
western blot assays, respectively. Data are representative of three
independent experiments. QC, Qianliening capsules; EGF, epidermal
growth factor; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X
protein; CDK4, cyclin-dependent kinase 4; RT-qPCR, reverse
transcription quantitative polymerase chain reaction. |
Discussion
BPH is a non-cancerous enlargement of the prostate
and is characterized by excessive and uncontrolled growth of the
epithelial and stromal cells of the prostate gland. It is an
age-associated disease and is present in 50% of men by the age of
50 and 80% by the age of 70 (4).
To date, BPH treatment includes surgery and medication, although
there is no completely effective treatment for BPH. Surgical
therapy may have improved efficacy, but pharmacotherapy remains the
most common option for BPH treatment. Pharmacotherapies consist of
α-blockers, including Terazosin, Doxazosin and Tamsulosin, which
inhibit α-adrenergic receptors and 5α-reductase inhibitors,
including Finasteride and Dutasteride, which inhibit the production
of dihydrotestosterone by suppressing 5α-reductase (36,37).
Although all may reduce the tissue enlargement and decrease the
blockage of urine flow, they also have their own side effects,
including orthostatic hypotension, reduced libido and ejaculation
or erectile dysfunction (38,39).
Therefore, there is an urgent requirement for the development of
novel agents without or with fewer side effects and toxicity for
BPH treatment. Natural products, including Traditional Chinese
Medicines, usually generate fewer adverse effects and exhibit
therapeutic efficacy (25–28,40).
QC are a Traditional Chinese Medicinal formulation,
which has been used clinically in China for numerous years,
exhibiting significant efficacy in BPH treatment (16,30–33).
However, the mechanism underlying its anti-BPH activity remains to
be fully elucidated. In the present study, the WPMY-1 cell line,
derived from stromal cells of the normal adult prostate, was
treated with different concentrations of QC following stimulation
with EGF in order to clarify the potential molecular mechanisms of
the activity of QC in BPH treatment. The present study, for the
first time, to the best of our knowledge, demonstrated that QC
reduced cell viability, arrested cell cycle through inhibition of
G1-phase progression, induced the activation of caspase-9 and -3,
inhibited the proliferation and induced the apoptosis of the
EGF-stimulated WPMY-1 cells in a dose-dependent manner.
Furthermore, the mechanism underlying the effects of QC on the
WPMY-1 cells proceeded via the STAT3 pathway.
BPH is the pathological, upregulated proliferation
of prostatic cells and a variety of growth factors, including EGF,
are inculpated in its pathogenesis. EGF stimulates the activation
of STAT3 via binding to the EGF receptor (16,19).
STAT3 is one of the main cellular signaling transduction pathways
known to be closely associated with the development of BPH
(13–15). The activation of STAT3 is mediated
by phosphorylation at Tyr 705, leading to its homodimerization,
nuclear translocation and DNA binding, which in turn regulates the
expression of various genes involved in cell proliferation and
survival, including up-regulation of the expression of
pro-proliferative cyclin D1, CDK4 and anti-apoptotic Bcl-2 or
down-regulation of the expression of anti-proliferative p21 and
pro-apoptotic Bax (19–24). The aberrant activation of STAT3
leads to increased cell proliferation and reduced cell apoptosis,
resulting in BPH development. Therefore, modulation of STAT3
signaling has been a promising target for the development of
anti-BPH therapies (16,41).
In the present study, human prostatic WPMY-1 cells
were stimulated with EGF and the results revealed that the
activation of STAT3 was clearly increased upon EGF stimulation,
leading to a significant increase in its phosphorylation levels and
transcriptional activity. However, the EGF-mediated STAT3
activation was profoundly inhibited by QC treatment in a
dose-dependent manner. Consequently, QC treatment significantly
inhibited the EGF-induced up-regulation of cyclin D1, CDK4 and
Bcl-2, and increased the EGF-induced downregulation of p21 and Bax,
which are key target genes of the STAT3 pathway.
In conclusion, the present study reported for the
first time, to the best of our knowledge, that QC inhibited the
growth of WPMY-1 cells and induced their apoptosis via inhibition
of the STAT3 pathway in vitro, which may be one of the
mechanisms underlying the activity of QC in the treatment of
BPH.
Acknowledgements
This study was supported by the Nature Science
Foundation of China (nos. 81173433, 81273928 and 81373817).
Abbreviations:
|
QC
|
Qianliening capsule
|
|
BPH
|
benign prostatic hyperplasia
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
|
EGF
|
epidermal growth factor
|
References
|
1
|
Sirab N, Robert G, Fasolo V, et al:
Lipidosterolic extract of Serenoa repens modulates the expression
of inflammation related-genes inbenign prostatic hyperplasia
epithelial and stromal cells. Int J Mol Sci. 14:14301–14320. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Atawia RT, Tadros MG, Khalifa AE, Mosli HA
and Abdel-Naim AB: Role of the phytoestrogenic, pro-apoptotic and
anti-oxidative properties of silymarin in inhibiting experimental
benign prostatic hyperplasia in rats. Toxicol Lett. 219:160–169.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pollan MC, Benghuzzi HA and Tucci M:
Growth factor expression in early stages of benign prostatic
hyperplasia upon exposure to sustained delivery of androgens.
Biomed Sci Instrum. 39:329–334. 2003.PubMed/NCBI
|
|
4
|
Paolone DR: Benign prostatic hyperplasia.
Clin Geriatr Med. 26:223–239. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roehrborn CG: Male lower urinary tract
symptoms (LUTS) and benign prostatic hyperplasia (BPH). Med Clin
North Am. 95:87–100. 2011. View Article : Google Scholar
|
|
6
|
Gat Y, Gornish M, Heiblum M and Joshua S:
Reversal of benign prostate hyperplasia by selective occlusion of
impaired venous drainage in the male reproductive system: novel
mechanism, new treatment. Andrologia. 40:273–281. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Steiner MS: Review of peptide growth
factors in benign prostatic hyperplasia and urological malignancy.
J Urol. 153:1085–1096. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Z and Olumi AF: Diabetes, growth
hormone-insulin-like growth factor pathways and association to
benign prostatic hyperplasia. Differentiation. 82:261–271. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Descazeaud A, Weinbreck N, Robert G, et
al: Transforming growth factor β-receptor II protein expression in
benign prostatic hyperplasia is associated with prostate volume and
inflammation. BJU Int. 108:E23–E28. 2011. View Article : Google Scholar
|
|
10
|
Voss M, Trojan L, Steidler A, et al: Serum
vascular endothelial growth factor C level in patients with
prostate cancer and benign prostatic hyperplasia. Anal Quant Cytol
Histol. 30:199–202. 2008.PubMed/NCBI
|
|
11
|
Ma Z, Tsuchiya N, Yuasa T, et al:
Polymorphisms of fibroblast growth factor receptor 4 have
association with the development of prostate cancer and benign
prostatic hyperplasia and the progression of prostate cancer in a
Japanese population. Int J Cancer. 123:2574–2579. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang W, Zhang X, Mize GJ and Takayama TK:
Protease-activated receptor-1 upregulates fibroblast growth factor
7 in stroma of benign prostatic hyperplasia. Prostate.
68:1064–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Haura EB, Turkson J and Jove R: Mechanisms
of disease: Insights into the emerging role of signal transducers
and activators of transcription in cancer. Nat Clin Pract Oncol.
2:315–324. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Siejka A, Schally AV, Block NL and
Barabutis N: Antagonists of growth hormone-releasing hormone
inhibit the proliferation of human benign prostatic hyperplasia
cells. Prostate. 70:1087–1093. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Siejka A, Schally AV, Block NL and
Barabutis N: Mechanisms of inhibition of human benign prostatic
hyperplasia in vitro by the luteinizing hormone-releasing hormone
antagonist cetrorelix. BJU Int. 106:1382–1388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin JM, Zhou JH, Xu W, et al: Qianliening
capsule treats benign prostatic hyperplasia via suppression of the
EGF/STAT3 signaling pathway. Exp Ther Med. 5:1293–1300.
2013.PubMed/NCBI
|
|
17
|
Lin J, Hong Z, Zhou H, et al: Expression
of the growth factor related to angiogenesis on the prostatic
hyperplasia in rats. J Fujian Univ Tradit Chin Med. 18:63–65.
2008.(In Chinese).
|
|
18
|
bin Jia, Hong Tang, Weimin Li and Wenqing
Cai: The effects of epidermal growth factor on the expression of
Bcl-2, Bax and c-myc in mice prostate cells. Chin J Gerontol.
27:251–252. 2007.(In Chinese).
|
|
19
|
Park OK, Schaefer TS and Nathans D: In
vitro activation of STAT3 by epidermal growth factor receptor
kinase. Proc Natl Acad Sci USA. 93:13704–13708. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhong Z, Wen Z and Darnell JE Jr: Stat3: a
STAT family member activated by tyrosine phosphorylation in
response to epidermal growth factor and interleukin-6. Science.
264:95–98. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Catlett-Falcone R, Landowski TH, Oshiro
MM, et al: Constitutive activation of Stat3 signaling confers
resistance to apoptosis in human U266 myeloma cells. Immunity.
10:105–115. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Karni R, Jove R and Levitzki A: Inhibition
of pp60c-Src reduces Bcl-XL expression and reverses the transformed
phenotype of cells overexpressing EGF and HER-2 receptors.
Oncogene. 18:4654–4662. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Coqueret O and Gascan H: Functional
interaction of STAT3 transcription factor with the cell cycle
inhibitor p21WAF1/CIP1/SDI1. J Biol Chem. 275:18794–18800. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bienvenu F, Gascan H and Coqueret O:
Cyclin D1 represses STAT3 activation through a Cdk4-independent
mechanism. J Biol Chem. 276:16840–16847. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang YP, Du J, Hong ZF, et al: Effects of
Kangquan recipe on sex steroids and cell proliferation in rats with
benign prostatic hyperplasia. Chin J Integr Med. 15:289–292. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Boyle P, Robertson C, Lowe F and Roehrborn
C: Meta-analysis of clinical trials of Permixon in the treatment of
symptomatic benign prostatic hyperplasia. Urology. 55:533–539.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vacherot F, Azzouz M, Gil-Diez-De-Medina
S, et al: Induction of apoptosis and inhibition of cell
proliferation by the lipido-sterolic extract of Serenoa repens
(LSESr, Permixon) in benign prostatic hyperplasia. Prostate.
45:259–266. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Quiles MT, Arbós MA, Fraga A, et al:
Antiproliferative and apoptotic effects of the herbal agent Pygeum
africanum on cultured prostate stromal cells from patients with
benign prostatic hyperplasia (BPH). Prostate. 70:1044–1053. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin JM, Huang YP, Zhou Jh and Hong ZF:
Therapeutic efficacy of Qianliening capsules in the treatment of
benign prostatic hyperplasia. Asia-Pacific Traditional Medicine.
9:140–143. 2013.(In Chinese).
|
|
30
|
Zhou J, Lin J, Xu W, et al: Qianliening
capsule treats benign prostatic hyperplasia through regulating the
expression of sex hormones, estrogen receptor and androgen
receptor. Afr J Pharm and Pharmacol. 6:173–180. 2012. View Article : Google Scholar
|
|
31
|
Zhong X, Lin J, Zhou J, et al: Qianliening
capsule treats benign prostatic hyperplasia (BPH) by
down-regulating the expression of PCNA, CyclinD1 and CDK4. Afr J
Biotechnol. 11:7731–7737. 2012.
|
|
32
|
Zheng HY, Xu W, Lin JM, Peng J and Hong
ZF: Qianliening capsule treats benign prostatic hyperplasia via
induction of prostatic cell apoptosis. Mol Med Rep. 7:848–854.
2013.PubMed/NCBI
|
|
33
|
Hong ZF, Lin JM, Zhong XY, et al:
Qianliening capsule inhibits human prostate cell growth via
induction of the mitochondrion-dependent cell apoptosis. Chin J
Integr Med. 18:824–830. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lin JM, Chen YQ, Cai QY, et al:
Scutellaria barbata D Don inhibits colorectal cancer growth via
suppression of multiple signaling pathways. Integr Cancer Ther.
13:240–248. 2014. View Article : Google Scholar
|
|
35
|
Lin JM, Chen YQ, Wei LH, et al: Ursolic
acid promotes colorectal cancer cell apoptosis and inhibits cell
proliferation via modulation of multiple signaling pathways. Inter
J Oncol. 43:1235–1243. 2013.
|
|
36
|
Roehrborn CG, Nuckolls JG, Wei JT, et al:
The benign prostatic hyperplasia registry and patient survey: study
design, methods and patient baseline characteristics. BJU Int.
100:813–819. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Black L, Naslund MJ, Gilbert TD Jr, Davis
EA and Ollendorf DA: An examination of treatment patterns and costs
of care among patients with benign prostatic hyperplasia. Am J
Manag Care. 12:S99–S110. 2006.PubMed/NCBI
|
|
38
|
Roehrborn C, Boyle P, Nickel JC, Hoefner K
and Andriole G: ARIA3001 ARIA3002 and ARIA3003 Study Investigators:
Efficacy and safety of a dual inhibitor of 5-alpha-reductase types
1 and 2 (dutasteride) in men with benign prostatic hyperplasia.
Urology. 60:434–441. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Roderick MacDonald and Wilt Timothy J:
Alfuzosin for treatment of lower urinary tract symptoms compatible
with benign prostatic hyperplasia: A systematic review of efficacy
and adverse effects. Urology. 66:780–788. 2005. View Article : Google Scholar
|
|
40
|
Youle RJ and Strasser A: The BCL-2 protein
family: opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View Article : Google Scholar
|
|
41
|
Verma V, Sharma V, Singh V, et al:
Labda-8(17),12,14-trien-19-oic acid contained in fruits of
Cupressus sempervirens suppresses benign prostatic hyperplasia in
rat and in vitro human models through inhibition of androgen and
STAT-3 signaling. Phytother Res. 28:1196–203. 2014. View Article : Google Scholar : PubMed/NCBI
|