Introduction
Obesity may be regarded as a low-level chronic
inflammatory state, and is associated with type 2 diabetes
mellitus, cardiovascular disease and a number of cancer types, such
as breast cancer, colorectal cancer and prostate cancer (1,2).
Adipose tissue is viewed as an endocrine organ,
which produces a wide range of pro-inflammatory cytokines, such as
monocyte chemoattractant protein-1 (MCP-1), interlukin-6 (IL-6) and
tumor necrosis factor-α (TNF-α). These molecules are collectively
termed adipokines (3).
Immunological dysregulation in obese subjects may result from
interactions between white adipose tissue and the immune system
(4). Obesity affects macrophage
accumulation in fat stores, as well as T cell-mediated responses
(5). Evidence has arisen
suggesting that a reduction in levels of circulating T regulatory
cells (Tregs) has an impact on the increased risk of metabolic and
cardiovascular dysfunctions observed in obese patients (6). Myeloid-derived suppressor cells
(MDSCs) are a population of myeloid cells, which act to suppress
the proliferation and function of effector T cells (7) via alteration of the
signal-transducing molecule, TCRζ, on the surface of T cells
(8). Decreased TCRζ chain
expression in circulating T cells has been reported in a number of
types of cancer, including melanoma, and ovarian, breast, renal and
oral squamous cell carcinoma, and is associated with a compromised
antitumor response as a result of reduced T cell proliferation and
cytokine production (9,10). MDSCs have been investigated in
numerous types of cancer. However, there have been few studies into
the phenotype and functions of these cells in obese patients.
The S100A8 and S100A9 proteins have been identified
as members of the S100 Ca2+-binding protein family, and
are known to form a heterodimeric complex, S100A8/A9, which is
involved in mediating the inflammatory response (11). Recently, it has been suggested that
S100A8/A9 proteins may serve as a novel marker for monocytic MDSCs
(12). However, whether S100A8/A9
proteins are also elevated in obese subjects, and thus affect the
frequency of monocytic MDSCs in these individuals, requires further
investigation.
The present study characterized the proportion and
phenotypes of MDSCs in the circulation of obese subjects compared
with those in lean subjects and examined whether MDSCs correlate
with levels of liver enzymes. In addition, the levels of S100A9/9
were measured and the proportion of
CD14+S100A9+ cells in obese and lean subjects
was examined.
Materials and methods
Subject recruitment and blood sample
preparation
A total of 16 adult Chinese men, including eight
overweight/obese subjects (BMI >25 kg/m2) and eight
lean healthy controls (BMI <25 kg/m2) were recruited
between February and June 2013. Subjects with type 2 diabetes
mellitus, hepatitis or other medical comorbidities likely to impact
on the immune system were excluded. The clinical information from
the two groups is summarized in Table
I. The study was approved by the Medical Ethical Committee of
the Second Hospital of Jiaxing, (Jiaxing, China). Blood samples
were collected from all study subjects using vacuette sodium
heparin-containing tubes (BD Biosciences, Franklin Lakes, NJ, USA)
and further processed within 24 h of collection. Plasma was
isolated by centrifugation at 1000 × g for 10 min and stored at
−20°C for further analyses. Liver serum parameters, alanine
transaminase (ALT) and aspartate transaminase (AST), were
determined by an automated chemistry analyzer (D2400/P800; Roche
Diagnostics, Basel, Switzerland).
 | Table IDescription of clinical information
of the two groups. |
Table I
Description of clinical information
of the two groups.
| Lean subjects
(n=8) | Overweight/obese
subjects (n=8) |
|---|
| Age (years) | 30.10±2.714 | 32.55±2.413 |
| BMI
(kg/m2) | 21.01±0.80 | 28.81±0.99a |
| Monocyte
number | 0.418±0.063 | 0.407±0.0256 |
Ultrasonography
Ultrasonography was performing using a B-mode of
Ultrasound (iU22 xMATRIX, Philips Healthcare, Andover, MA, USA).
Screening for fatty liver was based on a B ultrasonic diagnosis
according to the standard guidelines of the Chinese Medical
Association (13).
Flow cytometric analysis
To determine the proportion and phenotypes of MDSCs
in total plasma leukocytes, multicolor fluorescence-activated cell
sorting (FACS) analysis was conducted using freshly-collected whole
blood and fluorochrome-conjugated mouse anti-human monoclonal
antibodies against CD11b, CD14, CD16, CD15, CD33 and HLADR, as well
as isotype control antibodies (BD Pharmingen, San Diego, CA, USA).
S100A9 was obtained from BioLegend Inc. (San Diego, CA, USA) and
staining was performed according to the manufacturer’s
instructions. Intracellular staining of TCRζ (BD Pharmingen) was
conducted using the methods described previously (14). In this experiment, peripheral blood
mononulcear cells (PBMCs) were isolated from 4 ml whole blood.
Blood in a vacuette tube was transferred to a 15 ml conical tube
(BD Falcon, Franklin Lakes, NJ), diluted to a volume of 8 ml with 1
× PBS (Solarbio, Beijing, P.R. China), and underlayed with 4 ml of
Ficoll Paque (Sigma-Aldrich, St. Louis, MO, USA). The PBMCs were
collected at the interface layer following centrifugation of the
conical tube at 400 × g for 30 min. Data were collected using a BD
FACSCanto II flow cytometer, and analyzed with DIVA software
(version V6.1.3, BD Pharmingen).
ELISA assay
Plasma was obtained from obese and lean subjects,
and stored at −20°C. The plasma concentration of S100A8/9 was
measured using a commercially available ELISA kit, according to the
manufacturer’s instructions (Cuisabio Biotech Co., Ltd., Wuhan,
China).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Differences in means between groups of data were analyzed
using Student’s unpaired t-test. Correlation analysis was performed
using Pearson’s correlation with the coefficient of correlation
(r2). Statistical analysis was performed, and figures
created, using Prism Version 5.0 software (GraphPad Software Inc.,
La Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
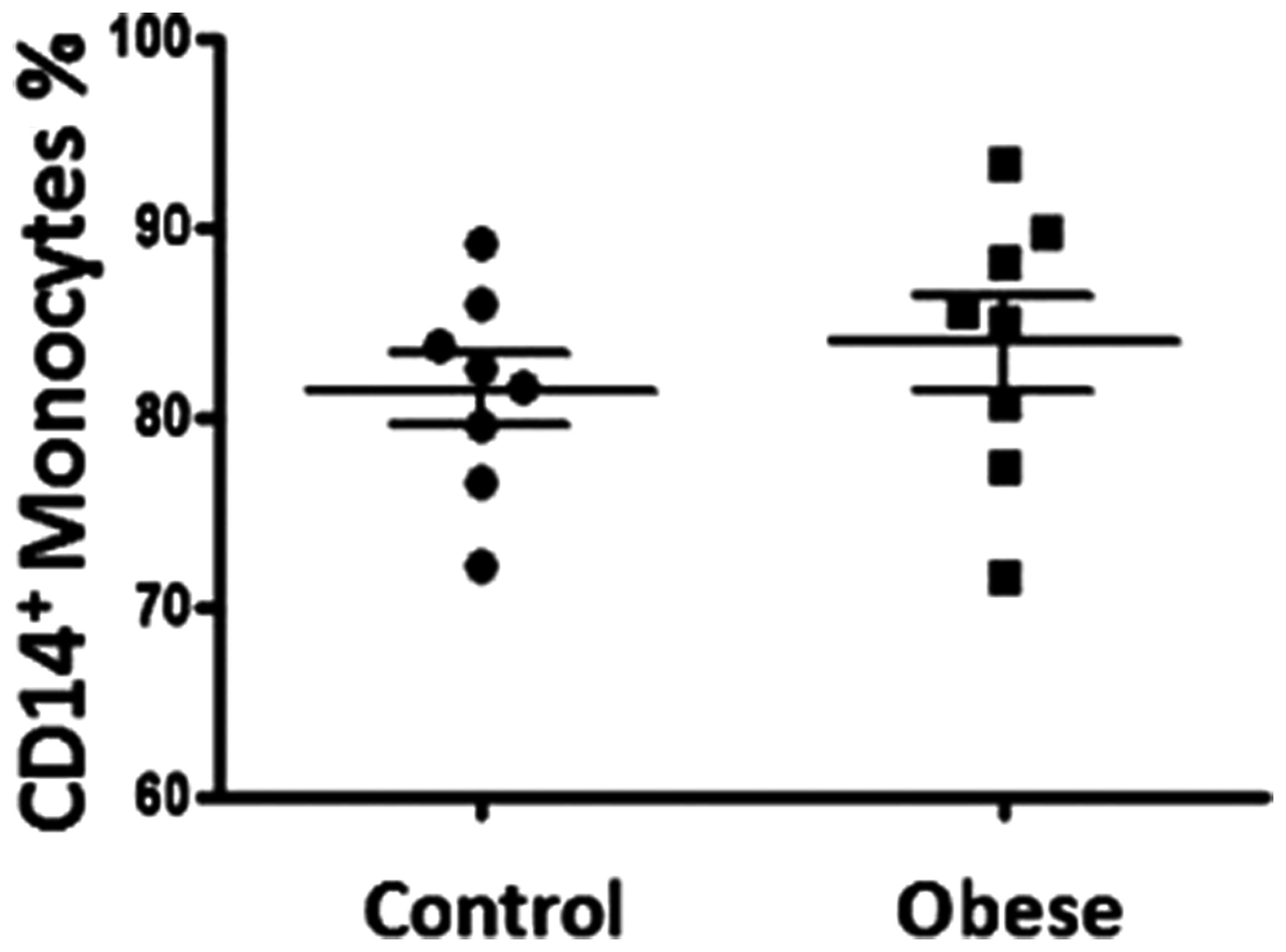
CD14+ monocyte numbers in the
peripheral blood of obese subjects
The total number of monocytes in the peripheral
blood in each group was analyzed. The absolute number of monocytes
in peripheral blood was not significantly different in obese
individuals compared with lean controls (P>0.05; Table I). The percentage of
CD14+ monocytes was further analyzed in whole blood by
flow cytometry. No significant difference was observed between the
obese and lean groups (83.91±2.490 compared with 81.36±1.893%,
respectively; P>0.05; Fig.
1).
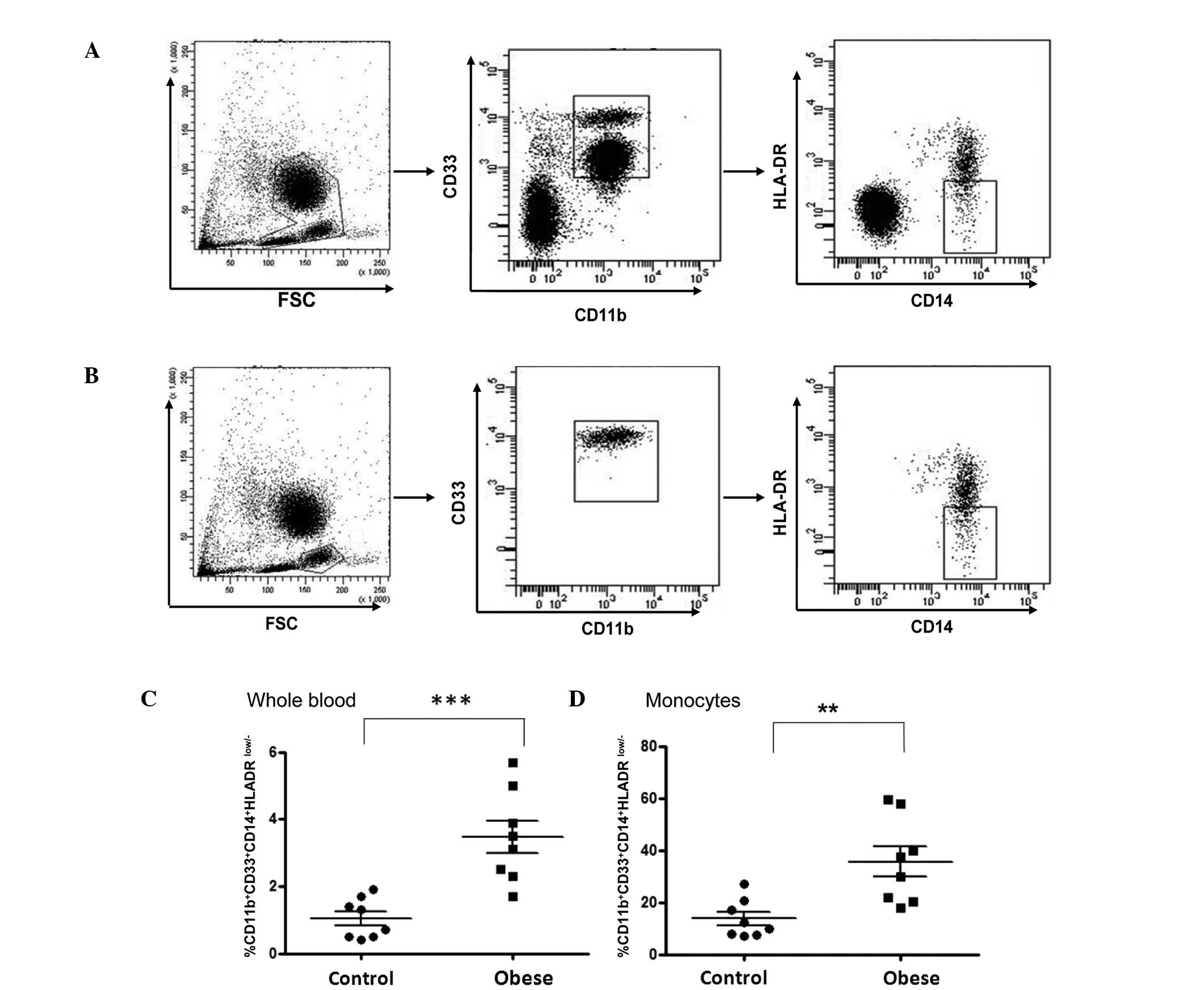
Phenotypical characterization of
myeloid-derived suppressor cells in obese subjects
To determine whether the proportion of granulocytic
and monocytic MDSCs, two previously identified subtypes of MDSCs
(15), was increased in obese
subjects, these two populations were analyzed using flow cytometric
analysis. MDSCs have been defined as a heterogeneous cell
population with a granulocytic phenotype
CD33+CD11b+CD14−HLADR−
in renal cell carcinoma, glioblastoma and gastric cancer (16–18).
In addition, monocytic MDSCs, with a phenotype of
CD14+CD11b+HLADRlow/−, have also
been described in circulating leukocytes of patients with melanoma
(19). In the present study,
granulocytic MDSCs were defined as those expressing CD33 and CD11b,
but that were CD14−, with low/− HLADR. Monocytic MDSCs
have alternatively been described as a population of cells with a
typical
CD33+CD11b+CD14+HLADRlow/−
phenotype. The gating strategy used is shown in Fig. 2. Cells were gated on the whole
blood cell fraction (Fig. 2A) as
well as the monocytic fraction only (Fig. 2B). The percentage of monocytic
MDSCs was significantly increased in the obese group compared with
that in the age-matched lean control group when gated on the whole
cell fraction (3.46±0.48 compared with 1.05±0.21%, respectively;
P<0.001; Fig. 2C) as well as
when gated on the monocytic fraction only (35.56±5.77 compared with
13.69±2.57, respectively; P<0.01; Fig. 2D). However, there was no
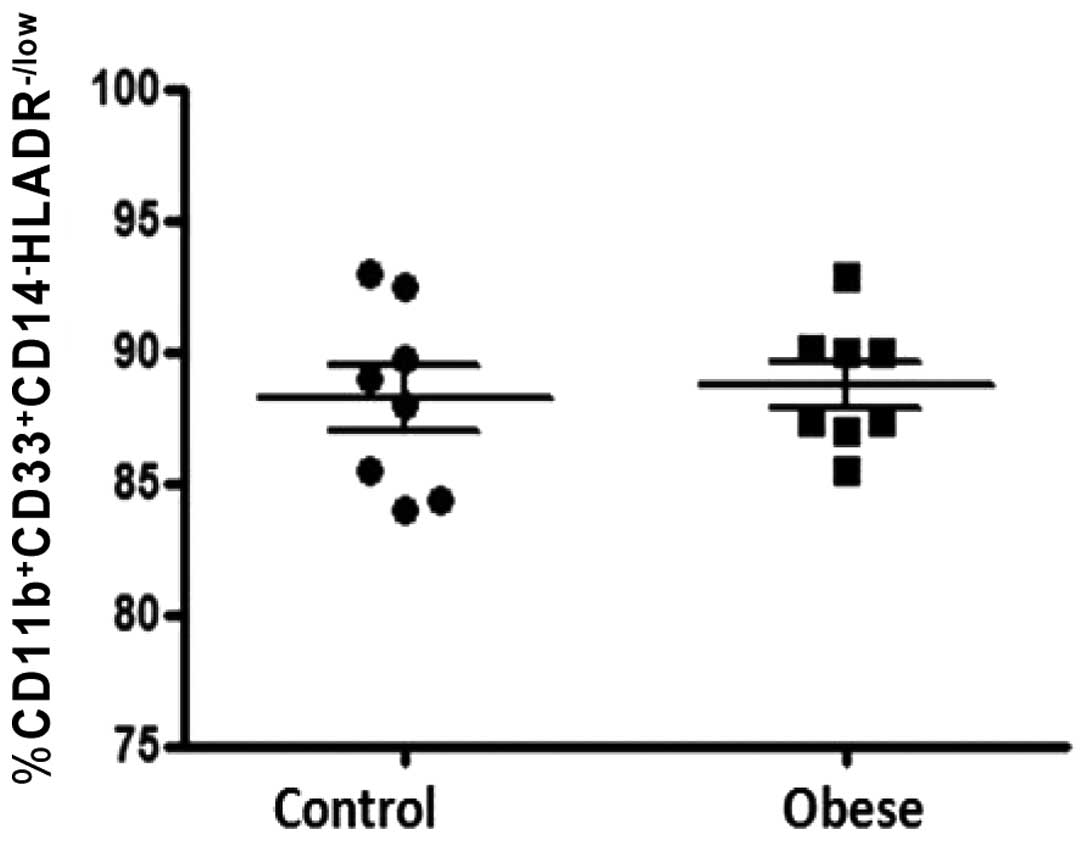
significant difference in the percentage of
CD33+CD11b+CD14−HLADRlow/−
between obese and lean control groups when gated on whole blood
cell fraction (88.71±0.84 compared with 88.19±1.25%, respectively;
P>0.05; Fig. 3).
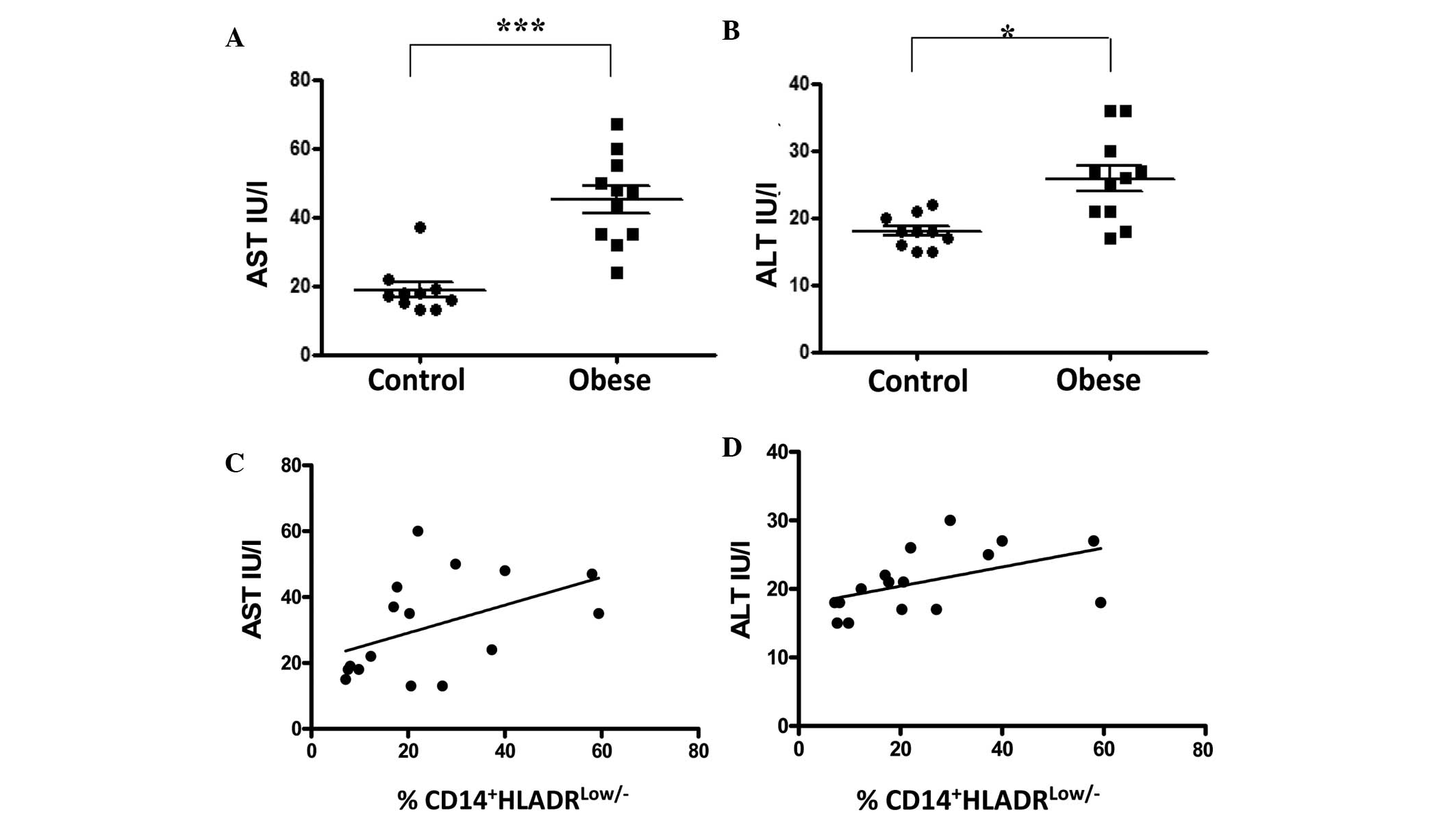
Serum levels of liver enzymes are
correlated with the proportion of
CD14+HLADRlow/− cells in obese subjects
Elevated serum levels of ALT and AST are markers of
liver injury. In the present study, it was observed that the
overweight obese individuals had elevated circulating levels of ALT
compared with lean control subjects (23.88±1.49 vs. 18.25±0.92
IU/l, respectively; P<0.05; Fig.
4A). Levels of AST were also significantly higher in the obese
group than in the lean group (42.75±3.94 compared with 19.38±2.75
IU/l, respectively; P<0.0001; Fig.
4B). The association between serum levels of ALT and AST and
the proportion of CD14+HLADRlow/− monocytes
in peripheral blood was examined. A positive correlation was
observed between AST and CD14+HLADRlow/−
monocytes (r=0.50, 95% CI −0.009 to 0.803; P<0.05; Fig. 4C); as well as between ALT and
CD14+HLADRlow/− monocytes (r=0.58, 95% CI
0.102 to 0.840; P<0.05; Fig.
4D).
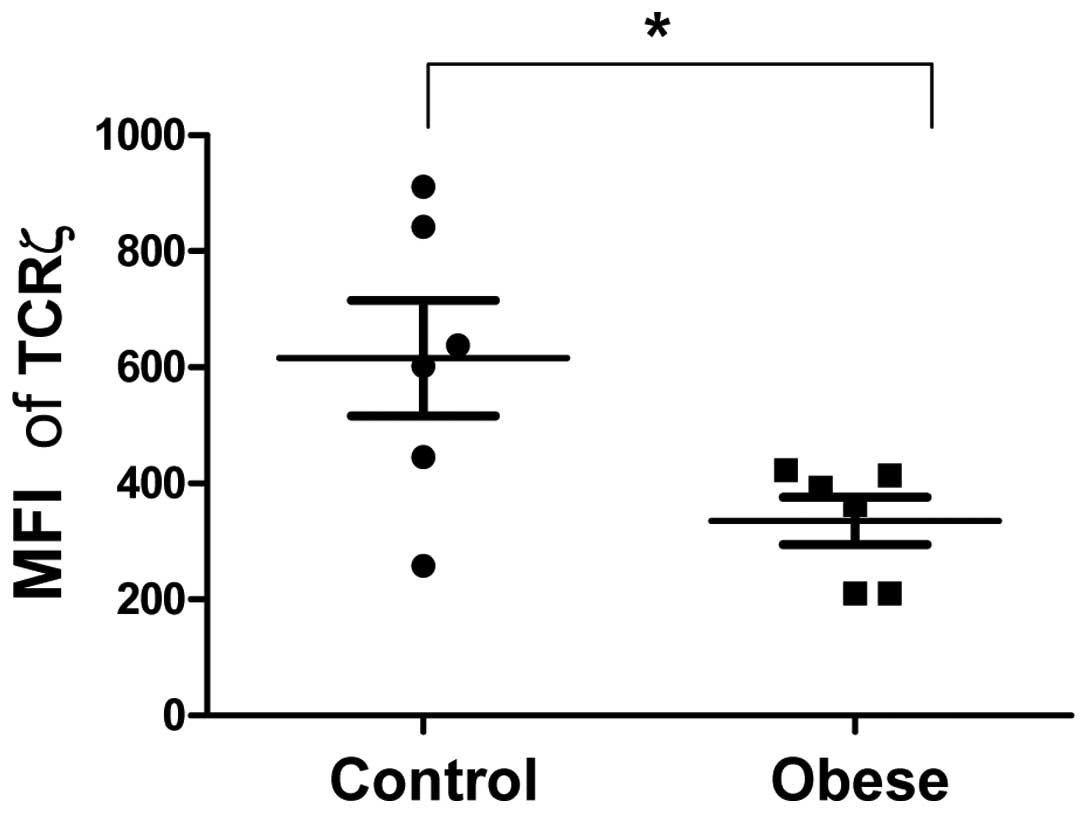
Expression of TCRζ is decreased in the
PBMCs of obese subjects
To evaluate the expression of TCRζ in the peripheral
T cell population, flow cytometric analysis was performed to
measure TCRζ mean fluorescence intensity (MFI) in the
CD3+ resting T cell population. The expression of TCRζ
was reduced in obese subjects compared with lean controls
(335.35±40.57 compared with 616±99.36 MFI, respectively; P=0.0259;
Fig. 5)
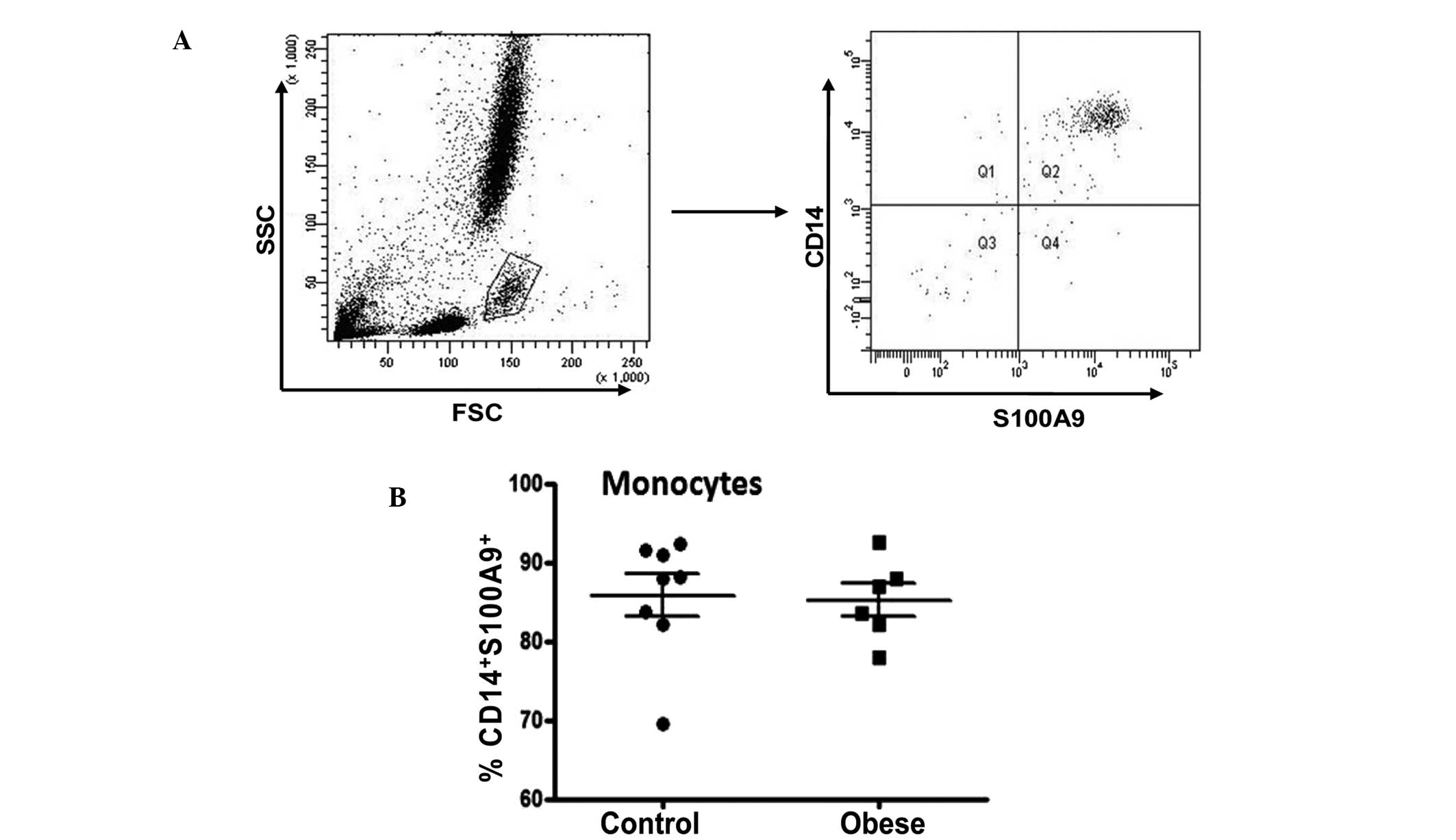
Expression of S100A9 in CD14+
monocytes
The pro-inflammatory heterodimeric S100A8/9 complex
is elevated in a number of chronic inflammatory diseases, including
rheumatoid arthritis, cystic fibrosis and inflammatory bowel
disease (20,21). S100A9 has been proposed as a novel
marker for monocytic human MDSCs in certain types of malignancy
(12). To investigate whether
S100A9 serves as a marker for monocytic MDSCs in obesity,
S100A9-specific antibodies were used to detect this intracellular
molecule. As shown in Fig. 6A,
cells were gated on the monocytic fraction following flow cytometry
using whole peripheral blood. The majority of CD14+
monocytes were S100A9+ (Fig. 6A), and there was no difference in
the proportion of CD14+S100A9+ between obese
subjects and lean controls (85.20±2.07 compared with 85.80±2.67%,
respectively; P>0.05; Fig. 6B).
No statistically significant correlation was identified between
CD14+HLADRlow/− MDSC and
CD14+S100A9+ cells in the monocyte population
(data not shown). In monocytes, a small population with a
CD16+CD14− phenotype has previously been
identified (22). The current
study found that S100A9 was present in CD14+ cells but
not in CD16+ cells (data not shown).
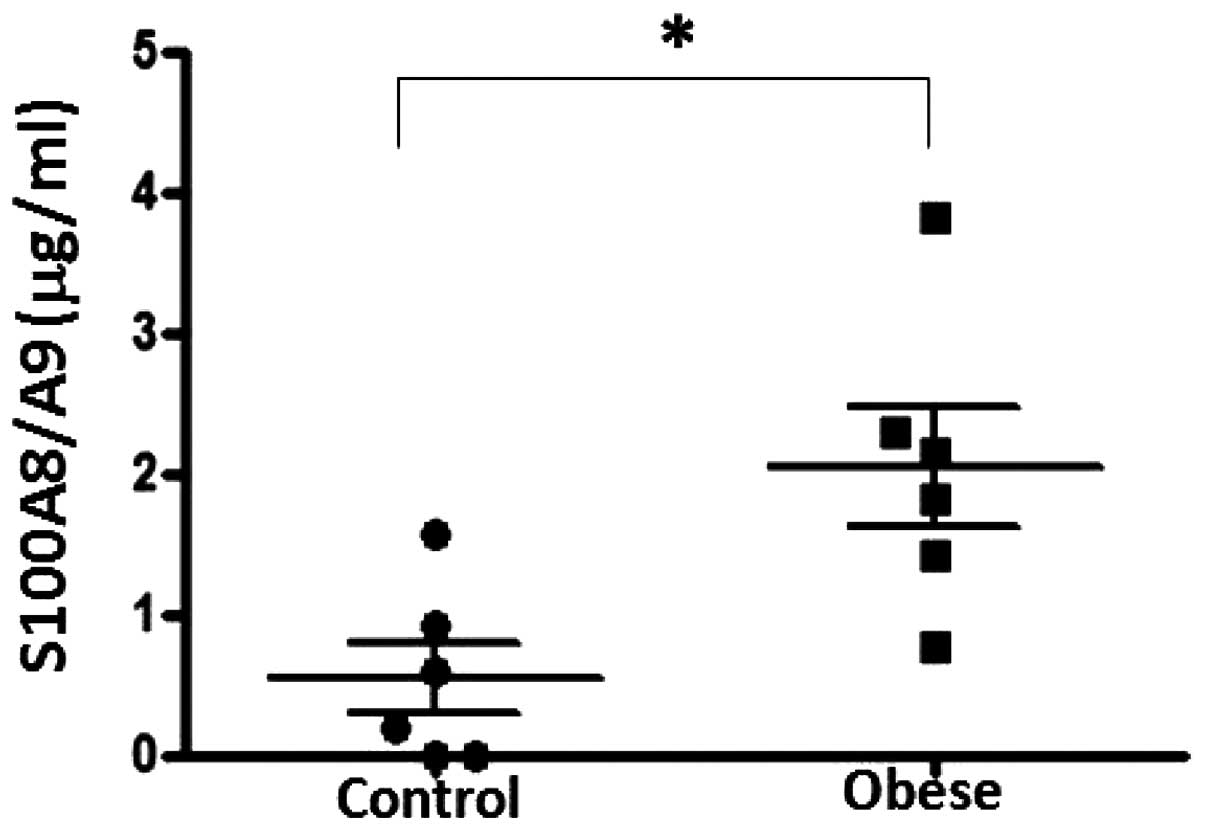
Plasma levels of S100A8/A9 in obese
subjects
The plasma levels of S100A8/A9 were significantly
increased in obese subjects compared with age-matched lean controls
(2.04±0.423 compared with 0.548±0.252 μg/ml, respectively;
P<0.05; Fig. 7).
Discussion
Monocytes respond to immune-stimulating cytokines
(23). These are derived from
pluripotent hematopoietic stem cells in the bone marrow, and can
rapidly and efficiently differentiate into dendritic cells
(23). The impaired immune
response observed in obesity may be associated with the
accumulation of macrophages in adipose tissue as a result of the
attraction of monocytes from the circulation (23). The present study reports that an
increased frequency of monocytic MDSCs appears to be amongst the
immunosuppressive responses promoted by obesity.
The current study reports an increased population of
CD14+HLADRlow/−-monocytes in obese subjects
compared with lean subjects. CD14+HLADRlow/−
monocytic MDSCs have been documented in patients with melanoma
(19), and Non-Hodgkin lymphoma
(NHL) (24). The increased
proportion of CD14+HLADRlow/− monocytes leads
to immunosuppression by impairing T cell functions, including
inhibiting T cell proliferation, reducing their response to stimuli
and reducing production of interferon γ (24).
Decreased monocyte expression of HLADR has been
observed in a range of malignancies, such as hepatocellular
carcinoma, prostate cancer and glioblastoma (25). In addition to tumors, an increased
frequency of CD14+HLADRlow/− cells has also
been reported in non-malignant conditions, such as sepsis (26), pancreatitis (27) and acute liver failure (28). Furthermore, a low percentage of
monocytic HLADR expression serves as a predictor of poor clinical
outcome in acute liver failure (29).
White adipose tissue is now regarded as a key
endocrine organ, secreting a wide range of adipokines including
leptin, adiponectin, TNF-α and IL-6. These molecules act locally or
distantly to regulate energy balance and other physiological
processes, such as insulin sensitivity and inflammatory responses
(30,31). Pro-inflammatory cytokines may
contribute to the loss of HLADR expression in monocytes in obese
subjects. Obesity is associated with a spectrum of liver
abnormalities and is known to cause nonalcoholic fatty liver
disease (NAFLD) (32). In addition
to regulating energy balance, the liver is also involved in
mediating the immune response. Although no liver biopsies were
performed in the current study, ultrasound examination indicated
fatty livers in all obese subjects. In the present study, a
significant positive correlation was observed between the
proportion of CD14+HLADRlow/− monocytes and
levels of the liver enzymes, AST and ALT. This data led to the
hypothesis that the induction of these immunosuppressive monocytes
may contribute to liver dysfunction in obesity. Recently, data has
shown that human activated hepatic stellate cells are able to
convert mature peripheral blood monocytes into MDSCs, and that this
action may prevent ensuing liver injury (33). Therefore, further studies are
required to investigate whether the elevated number
CD14+HLADRlow/− monocytes originates from the
injured liver or from the circulation. Furthermore, the question of
how those immunosuppressive cells interact with hepatocytes in
NAFLD should also be addressed.
MDSCs have been reported to suppress autologous T
cell proliferation and reduce the expression of TCRζ on the
surfaces of CD8+ cells. The ζ chain of TCR is a key
component responsible for the initiation of immune responses
mediated by T cells and natural killer cells (34). Reduced TCRζ expression in
circulating lymphocytes has been reported in patients with ovarian
cancer, breast cancer, oral cancer and melanoma (35). The reduced expression of TCRζ
molecule in obese subjects indicates that
CD14+HLADRlow/− monocytes may lead to
immunosuppression via regulation of TCRζ expression. The
upregulation of CD14+HLADRlow/− and
downregulation of T cell function may be associated with the
increased incidence of cancer in obese patients.
S100A8/9 is produced by myeloid and tumor cells
(34), and elevated S100A8/9 can
in turn induce the production of MDSCs in patients with cancer
(36). Circulating levels of
S100A8/9 have been shown to be upregulated in a number of tumor
types, including gastric cancer (18). Blocking S1008/9 and its receptor,
RAGE, on the surface of MDSCs may restore T cell proliferation
(18). Recently, S100A9 has been
proposed to be a novel marker for monocytic MDSCs in colon cancer
(12). In the present study,
S100A9 was found to be highly expressed in the majority of
CD14+ monocytes and no association was found between
CD14+S100A9+ and
CD14+HLADRlow/− cells. Thus, whether S100A9
should be used as a marker for monocytic MDSCs requires further
clarification. The elevation of S100A8/9 in the plasma of obese
subjects may further induce the production of monocytic MDSCs in
obesity.
In conclusion, to the best of our knowledge, the
current study reports for the first time that the proportion of an
immunosuppressive monocytic population with a
CD14+HLADRlow/− phenotype is significantly
elevated in the circulation of obese subjects compared with that of
lean control subjects. The increased frequency of this cell
population is positively correlated with the concentration of liver
enzymes in the plasma. The expression of the TCRζ molecule was
found to be downregulated in obese patients. In addition
CD14+HLADRlow/− monocytes are not correlated
with CD14+S100A9+ expression. The plasma
concentration of S100A8/9 was found to be increased in obesity.
These findings suggest that the upregulation of
CD14+HLADRlow/− may be responsible for the
impaired T-cell function and liver injury observed in obesity.
Acknowledgements
The authors would like to thank Dr Bing Chen
(University of Liverpool, Liverpool, UK) for her revisions to the
manuscript. This study was supported by grants from the Chinese
National Science Fund for Young Scholars (grant no. 81101707), the
Zhejiang Traditional Chinese Medicine Foundation Project (grant no.
2011ZA104) and the Science and Technology Bureau of Jiaxing (grant
no. 2012AY1071-2).
References
|
1
|
Renehan AG, Tyson M, Egger M, Heller RF
and Zwahlen M: Body-mass index and incidence of cancer: a
systematic review and meta-analysis of prospective observational
studies. Lancet. 371:569–578. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rader DJ: Effect of insulin resistance,
dyslipidemia, and intra-abdominal adiposity on the development of
cardiovascular disease and diabetes mellitus. Am J Med. 120(Suppl
1): S12–S18. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Leal VO and Mafra D: Adipokines in
obesity. Clin Chim Acta. 419:87–94. 2013. View Article : Google Scholar
|
|
4
|
van der Weerd K, Dik WA, Schrijver B, et
al: Morbidly obese human subjects have increased peripheral blood
CD4+ T cells with skewing toward a Treg- and
Th2-dominated phenotype. Diabetes. 61:401–408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de Heredia FP, Gómez-Martinez S and Marcos
A: Obesity, inflammation and the immune system. Proc Nutr Soc.
71:332–338. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wagner NM, Brandhorst G, Czepluch F, et
al: Circulating regulatory T cells are reduced in obesity and may
identify subjects at increased metabolic and cardiovascular risk.
Obesity (Silver Spring). 21:461–468. 2013. View Article : Google Scholar
|
|
7
|
Lindau D, Gielen P, Kroesen M, Wesseling P
and Adema GJ: The immunosuppressive tumour network: myeloid-derived
suppressor cells, regulatory T cells and natural killer T cells.
Immunology. 138:105–115. 2013. View Article : Google Scholar :
|
|
8
|
Umansky V and Sevko A: Overcoming
immunosuppression in the melanoma microenvironment induced by
chronic inflammation. Cancer Immunol Immunother. 61:275–282. 2012.
View Article : Google Scholar
|
|
9
|
Irving BA, Chan AC and Weiss A: Functional
characterization of a signal transducing motif present in the T
cell antigen receptor zeta chain. J Exp Med. 177:1093–1103. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Irving BA and Weiss A: The cytoplasmic
domain of the T cell receptor zeta chain is sufficient to couple to
receptor-associated signal transduction pathways. Cell. 64:891–901.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Srivastava MK, Andersson Å, Zhu L, et al:
Myeloid suppressor cells and immune modulation in lung cancer.
Immunotherapy. 4:291–304. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao F, Hoechst B, Duffy A, et al: S100A9
a new marker for monocytic human myeloid-derived suppressor cells.
Immunology. 136:176–183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schouppe E, Van Overmeire E, Laoui D, et
al: Modulation of CD8(+) T-cell activation events by monocytic and
granulocytic myeloid-derived suppressor cells. Immunobiology.
218:1385–1391. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kono K, Ressing ME, Brandt RM, et al:
Decreased expression of signal-transducing zeta chain in peripheral
T cells and natural killer cells in patients with cervical cancer.
Clin Cancer Res. 2:1825–1828. 1996.PubMed/NCBI
|
|
15
|
Fatty Liver and Alcoholic Liver Disease
Study Group of Chinese Liver Disease Association. Diagnostic
criteria of nonalcoholic fatty liver disease. Zhoonghua Gan Zang
Bing Za Zhi. 11:712003.(In Chinese).
|
|
16
|
Kusmartsev S, Su Z, Heiser A, et al:
Reversal of myeloid cell-mediated immunosuppression in patients
with metastatic renal cell carcinoma. Clin Cancer Res.
14:8270–8278. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ozao-Choy J, Ma G, Kao J, et al: The novel
role of tyrosine kinase inhibitor in the reversal of immune
suppression and modulation of tumor microenvironment for
immune-based cancer therapies. Cancer Res. 69:2514–2522. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang L, Chang EW, Wong SC, et al:
Increased myeloid-derived suppressor cells in gastric cancer
correlate with cancer stage and plasma S100A8/A9 proinflammatory
proteins. J Immunol. 190:794–804. 2013. View Article : Google Scholar
|
|
19
|
Filipazzi P, Valenti R, Huber V, et al:
Identification of a new subset of myeloid suppressor cells in
peripheral blood of melanoma patients with modulation by a
granulocyte-macrophage colony-stimulation factor-based antitumor
vaccine. J Clin Oncol. 25:2546–2553. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gebhardt C, Németh J, Angel P and Hess J:
S100A8 and S100A9 in inflammation and cancer. Biochem Pharmacol.
72:1622–1631. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Foell D, Wittkowski H, Ren Z, et al:
Phagocyte-specific S100 proteins are released from affected mucosa
and promote immune responses during inflammatory bowel disease. J
Pathol. 216:183–192. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Auffray C, Sieweke MH and Geissmann F:
Blood monocytes: development, heterogeneity, and relationship with
dendritic cells. Annu Rev Immunol. 27:669–692. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hansmann L, Groeger S, von Wulffen W, Bein
G and Hackstein H: Human monocytes represent a competitive source
of interferon-alpha in peripheral blood. Clin Immunol. 127:252–264.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin Y, Gustafson MP, Bulur PA, et al:
Immunosuppressive CD14+HLA-DR(low)/− monocytes in B-cell
non-Hodgkin lymphoma. Blood. 117:872–881. 2011. View Article : Google Scholar :
|
|
25
|
Najjar YG and Finke JH: Clinical
perspectives on targeting of myeloid derived suppressor cells in
the treatment of cancer. Front Oncol. 3:492013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Monneret G, Lepape A, Voirin N, et al:
Persisting low monocyte human leukocyte antigen-DR expression
predicts mortality in septic shock. Intensive Care Med.
32:1175–1183. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mentula P, Kylänpää-Bäck ML, Kemppainen E,
et al: Decreased HLA (human leucocyte antigen)-DR expression on
peripheral blood monocytes predicts the development of organ
failure in patients with acute pancreatitis. Clin Sci (Lond).
105:409–417. 2003. View Article : Google Scholar
|
|
28
|
Antoniades CG, Berry PA, Davies ET, et al:
Reduced monocyte HLA-DR expression: a novel biomarker of disease
severity and outcome in acetaminophen-induced acute liver failure.
Hepatology. 44:34–43. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zimmermann HW, Trautwein C and Tacke F:
Functional role of monocytes and macrophages for the inflammatory
response in acute liver injury. Front Physiol. 3:562012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Trayhurn P and Wood IS: Adipokines:
inflammation and the pleiotropic role of white adipose tissue. Br J
Nutr. 92:347–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Trayhurn P, Bing C and Wood IS: Adipose
tissue and adipokines - energy regulation from the human
perspective. J Nutr. 136(Suppl 7): 1935S–1939S. 2006.
|
|
32
|
Rahimi RS and Landaverde C: Nonalcoholic
fatty liver disease and the metabolic syndrome: clinical
implications and treatment. Nutr Clin Pract. 28:40–51. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Höchst B, Schildberg FA, Sauerborn P, et
al: Activated human hepatic stellate cells induce myeloid derived
suppressor cells from peripheral blood monocytes in a
CD44-dependent fashion. J Hepatol. 59:528–535. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Baniyash M: TCR zeta-chain downregulation:
curtailing an excessive inflammatory immune response. Nat Rev
Immunol. 4:675–687. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Whiteside TL: Down-regulation of
zeta-chain expression in T cells: a biomarker of prognosis in
cancer? Cancer Immunol Immunother. 53:865–878. 2004.PubMed/NCBI
|
|
36
|
Sinha P, Okoro C, Foell D, et al:
Proinflammatory S100 proteins regulate the accumulation of
myeloid-derived suppressor cells. J Immunol. 181:4666–4675. 2008.
View Article : Google Scholar : PubMed/NCBI
|