Introduction
Hepatocellular carcinoma (HCC) accounts for 80–90%
of primary liver cancers and is one of the most prevalent malignant
tumors in the world (1). The
incidence of HCC has steadily increased in Western countries over
the past decade and the global incidence of HCC is predicted to
continue to rise over the next few years (2,3). HCC
has the third-highest rate of cancer-associated mortalities
(4) and therefore studies into the
effective treatment of HCC are essential.
Statins, cholesterol-lowering drugs, are one of the
most commonly prescribed types of medications. Previous studies
have focused on the use of statins as therapeutic agents for the
treatment of solid and hematological cancers (5,6,7).
Statins have been shown to elicit pleiotropic effects on various
cell types and differentially modulate cellular functions,
including cell migration, proliferation, survival and apoptosis, in
normal as well as malignant cells (8). Simvastatin, a member of the statin
family, was previously reported to regulate the migration,
proliferation, apoptosis and growth of tumor cells (9,10);
in addition, Wang et al (11) demonstrated that simvastatin induced
caspase-dependent apoptosis and activated p53 in OCM-1 cells.
Kochuparambil et al (10)
found that the inhibitory effect of simvastatin on prostate cancer
cell growth occurred via the inhibition of the Akt pathway.
Previous studies have suggested that simvastatin regulated
arteriogenesis following stroke and enhanced the differentiation of
bone marrow stromal cells into endothelial cells via the Notch
signaling pathway (12,13); this therefore indicated that the
pharmacological manipulation of Notch signaling may be a promising
novel strategy for the treatment of human disease.
Notch genes encode proteins which are activated via
interaction with certain families of ligands (14,15);
in mammals, there are four Notch receptors (Notch1-4), which have
five corresponding ligands, including δ-like 1, 3 and 4 as well as
Jagged 1 and 2. Interaction between Notch receptors and their
ligands induces a two-step proteolysis, resulting in activation of
the Notch receptors. Activated Notch receptors have a critical role
in maintaining the balance between cell proliferation,
differentiation and apoptosis (16); therefore perturbed Notch signaling
may contribute to tumorigenesis. Notch1 is the primary member of
the Notch family, which has been previously reported to regulate
the growth, apoptosis, migration and invasion of tumor cells,
including breast, pancreatic and cervical cancer cells (17–19).
Qi et al 20) demonstrated that Notch1 regulated HCC cell
proliferation and apoptosis via different mechanisms. Therefore,
the aim of the present study was to investigate the effect of
simvastatin on Akt signaling and, in turn, HCC cell proliferation
and apoptosis, as well as to determine whether the mechanism of
action of simvastatin proceeded via regulation of Notch1
expression.
Materials and methods
Cell culture
Human HCC HepG2 and Huh7 cells were obtained from
the American Type Culture Collection (Manassas, VA, USA) and
maintained in RPMI-1640 (Gibco-BRL, Carlsbad, CA, USA), as
previously described (21),
supplemented with 10% fetal bovine serum (FBS; HyClone
Laboratories, Logan, UT, USA).
Simvastatin treatment
Simvastatin (Merck, Darmstadt, Germany) was
dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis,
MO, USA) to obtain simvastatin concentrations of 0, 2, 4, 8 and 16
μM. HCC cells were plated and incubated with various concentrations
of simvastatin (50 μl) for 24, 48, and 72 h at 37°C in a humidified
atmosphere of 5% CO2 and 95% air. Following treatment,
cells were collected and washed with phosphate-buffered saline
(PBS; Sigma-Aldrich) prior to further use.
Trypan blue assay
A trypan blue exclusion assay was used to determine
the viability of HepG2 and Huh7 cells. In brief, HepG2 and Huh7
cells were stained using 0.04% (w/v) trypan blue solution
(Invitrogen Life Technologies, Carlsbad, CA, USA) at room
temperature for 7 min, during which irreversibly damaged cell
membranes take up the anionic dye trypan blue; cells which excluded
trypan were considered to have survived and trypan blue-positive
cells were identified as dead. HepG2 and Huh7 cells were then
visualized using microscopy (magnification, ×40). For each
experiment, 200 HepG2 and Huh7 cells were analyzed from ten
different fields of vision/dish.
MTT assay
HepG2 and Huh7 cells were seeded into 96-well plates
at 1,000 cells per well and then treated with various
concentrations of simvastatin. On the day cells were harvested, 100
μl spent culture medium was replaced with an equal volume of fresh
medium. MTT (Sigma-Aldrich) was added to the cells at a final
concentration of 0.5 mg/ml, and the plates were incubated for 4 h
at 37°C. DMSO (100 μl) was then added to each well and plates
incubated with agitation at room temperature for 10 min. The
absorbance was measured at 570 nm using a spectrophotometer
(Multiskan MK3; Thermo Fisher Scientific, Waltham, MA, USA).
Notch1 small interfering (si)RNA plasmid
transfection into HepG2 cells
Transfection of the Notch1 siRNA plasmid into HepG2
and Huh7 cells was performed as previously described (19). In brief, cells were plated in a
35-mm dish for 24 h prior to transfection in complete medium.
Transfection was performed using Lipofectamine 2000 (Invitrogen
Life Technologies) according to the manufacturer’s instructions.
The Notch1-specific siRNA plasmid for HepG2 (sense,
5′-AAGGUGUCUUCCAGAUCCUGA-3′) (22)
and the scrambled sequence (5′-AAAUGUGUGUACGUCUCCUCC-3′) were
inserted into the pGPU6/green fluorescent protein/Neo plasmid
(Shanghai GenePharma, Ltd, Shanghai, China). The efficiency of
transfection was confirmed using western blot analysis. Clones, in
which the expression of Notch1 was effectively suppressed, were
selected and used for the in vitro study.
Detection of apoptosis
Cell apoptosis was analyzed using flow cytometric
(FCM) analysis. In brief, following 48 h of simvastatin treatment
at various concentrations (0, 2, 4, 8 and 16 μM), HepG2 and Huh7
cells were trypsinized using trypsin (Mingzhu Chemical Co.,
Shanghai, China), washed with PBS and then suspended with binding
buffer. Cells apoptosis was detected by Annexin V-fluorescein
isothiocyanate (FITC)/propidium iodide (PI) kit (BD PharMingen, San
Diego, CA, USA). Cells were incubated with 5 μl PI (Sigma-Aldrich)
for 15 min at room temperature in the dark, prior to suspension in
500 μl binding buffer. The cells were then analyzed using FCM
(Beckman-Coulter, Brea, CA, USA) for relative quantitative
apoptosis.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from transfected and
non-transfected HepG2 cells using TRIzol® reagent (Life
Technologies, Inc., Rockville, MD, USA) according to the
manufacturer’s instructions. Reverse transcription was performed on
1 μg of total RNA from each sample using oligo (dT) primers and 200
units of SuperScript II (Life Technologies, Inc.) for extension.
PCR amplification was performed using 1.25 U Ex Taq polymerase
(Takara Bio, Inc., Dalian, China). All PCR products were resolved
on 1.8% agarose gels containing ethidium bromide (Sigma-Aldrich).
Primer pairs for human Notch1, p53, B cell lymphoma 2 (Bcl-2),
Bcl-2-associated X protein (Bax), phosphorylated Akt (p-Akt), Akt
and β-actin specific amplification of complementary DNA were as
follows: Notch1 forward, 5′-CCGCAGTTGTGCTCCTGAA-3′ and reverse,
5′-ACCTTGGCGGTCTCGTAGCT-3′; p53 forward,
5′-GACCCAGGTCCAGATGAAGCT-3′ and reverse,
5′-ACCGTAGCTGCCCTGGTAGGT-3′; Bcl-2 forward,
5′-AGTTCGCCGAGATGTCCAGGCA-3′ and reverse,
5′-ACTTGTGGCCCAGATAGGCACC-3′; Bax forward,
5′-ACAGGGTTTCACCAGGATC-3′ and reverse, 5′-GCTGCCACCCGCAAGAAGAC-3′;
p-Akt forward, 5′-GGAGAUCAUGCAGCAUCGC-3′ and reverse,
5′-GCGAUGCUGCAUGAUCUCC-3′; Akt forward, 5′-CTTTCCAGACCCACGACC-3′
and reverse, 5′-CTCCGAGTGCAGGTAGTCC-3′; β-actin forward,
5′-GAGGCACTCTTCCAGCCTTC-3′ and reverse,
5′-GGATGTCCACGTCACACTTC-3′.
Western blot analysis
Cells were homogenized and lysed using
radioimmunoprecipitation assay lysis buffer (100 mm NaCl; 50 mm
Tris-HCl, pH 7.5; 1% TritonX-100; 1 mm EDTA; 10 mm
β-glycerophosphate; 2 mm sodium vanadate; and protease inhibitor).
Protein concentrations were assayed using the micro-bicinchoninic
acid assay protein assay (Pierce Biotechnology, Inc., Rockford, IL,
USA). Proteins (20–30 μg per lane) were separated using 10%
SDS-PAGE and then electroblotted onto nitrocellulose membranes (GE
Healthcare, Little Chalfont, UK). Non-specific binding was blocked
by incubating with 5% non-fat milk in PBS with Tween 20 buffer
(Sigma-Aldrich) at room temperature for 1 h. Cells were then
incubated overnight at 4°C with a 1:1,000 dilution of primary
antibodies for Notch1 (monoclonal mouse anti-human), Akt
(polyclonal rabbit anti-human), p-Akt (polyclonal rabbit
anti-human), p53 (polyclonal rabbit anti-human), β-actin
(monoclonal mouse anti-human), Bcl-2 (monoclonal mouse anti-human)
and Bax (monoclonal mouse anti-human), all purchase from
Sigma-Aldrich, followed by incubation with the corresponding
horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin
(Ig)G and rabbit anti-mouse IgG (1:2,000; Sigma-Aldrich) at room
temperature for 1 h. Antigens were then detected using the standard
enhanced chemiluminescence kit (Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA).
Statistical analysis
All experiments were performed in triplicate, unless
otherwise stated. Statistical analyses were performed using SPSS
13.0 software (SPSS, Inc., Chicago, IL, USA). Values are presented
as the mean ± standard deviation. Statistical comparisons between
groups were performed using a one-way analysis of variance followed
by a Student’s t test. P<0.05 was considered to indicate a
statistically significant difference between values.
Results
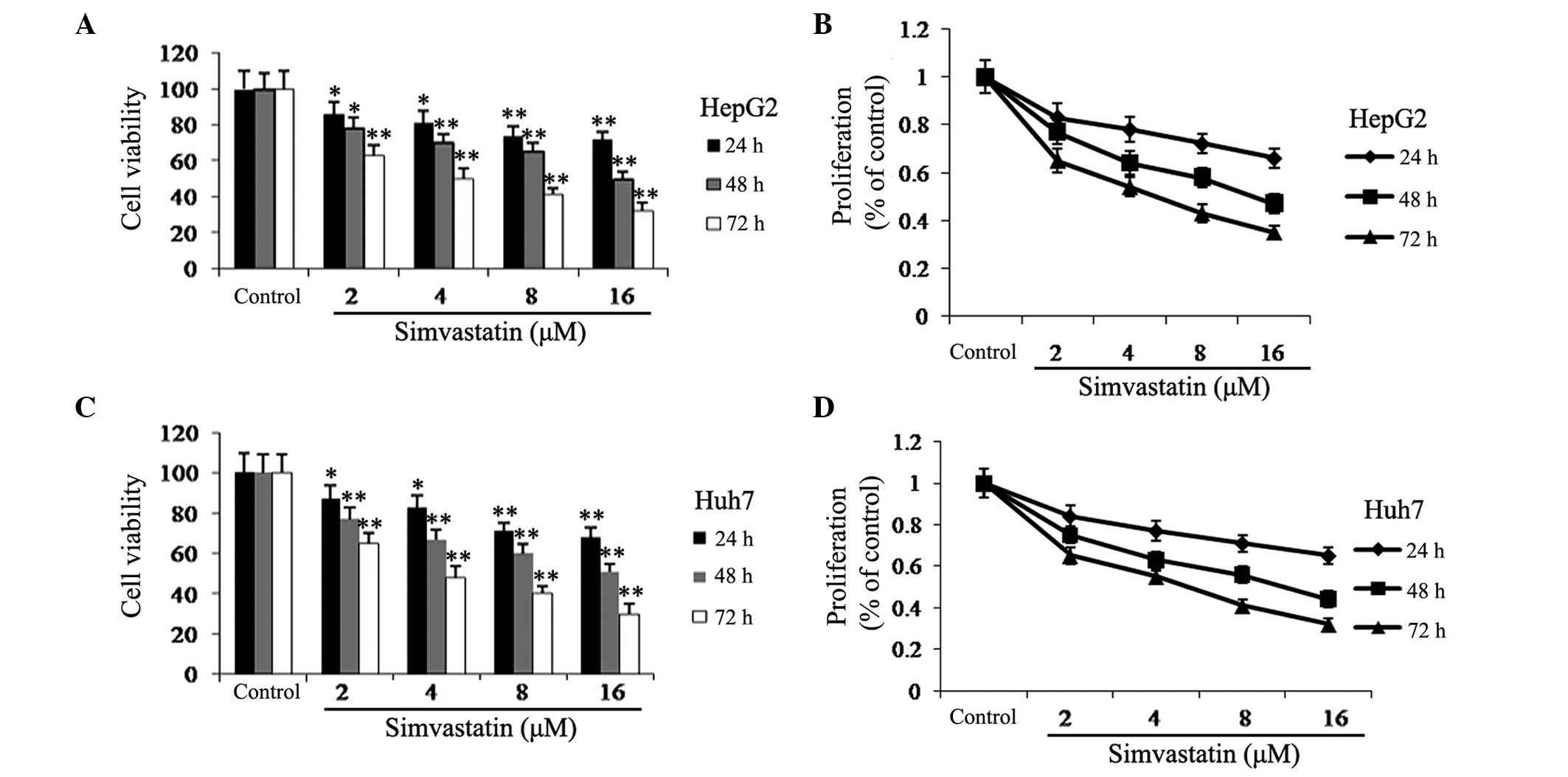
Simvastatin inhibits HCC cell viability
and proliferation
The cytotoxic effects of various concentrations (0,
2, 4, 8, and 16 μM) and incubation periods (24, 48, and 72 h) of
simvastatin was examined in HepG2 and Huh7 cells using a trypan
blue assay. As shown in Fig. 1,
simvastatin demonstrated high levels of cytotoxicity in HepG2 and
Huh7 cells in a time- and dose-dependent manner. Following 72 h of
simavastin treatment, even at the lowest dose (2 μM), the viability
and proliferation of HepG2 and Huh7 cells was significantly
decreased by >34 and 36%, respectively, compared to the
untreated group. These results therefore indicated that simvastatin
may be a promising anti-cancer drug for the treatment of HCC.
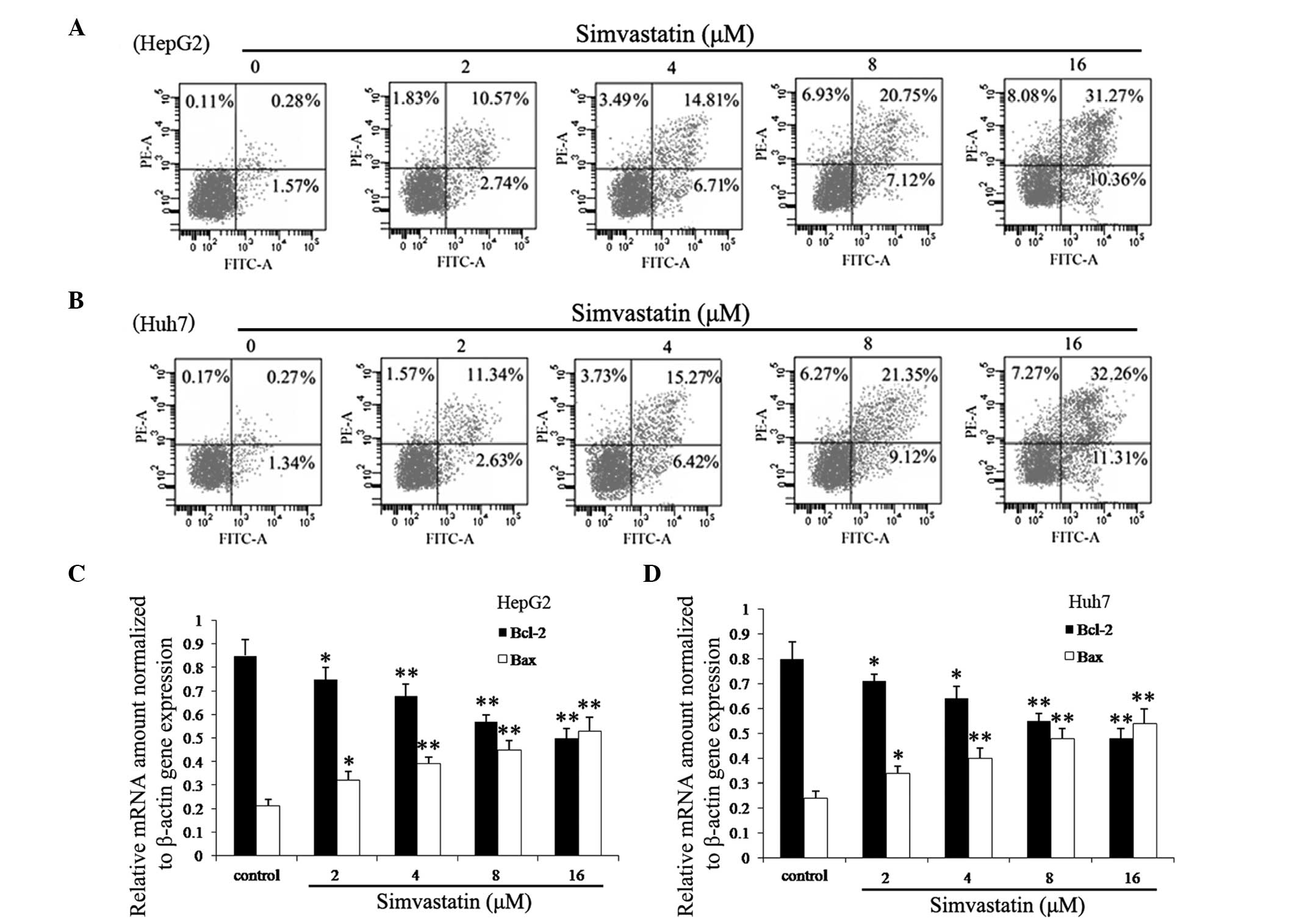
Simvastatin induces apoptosis in HepG2
and Huh7 cells
Bcl-2 family genes have an important role in the
regulation of apoptosis; Bcl-2 is an anti-apoptosis gene, while Bax
is a pro-apoptosis gene (23). In
normal cells the expression of Bcl-2 and Bax is maintained at an
equilibrium; however, during apoptosis, the expression of Bcl-2 is
downregulated, while Bax is overexpressed (24). In the present study FCM analysis
was performed in order to determine whether apoptosis contributed
to the simvastatin-induced reduction in HepG2 and Huh7 cell
viability. As shown in Fig. 2A and
B, HepG2 and Huh7 cells in the experimental groups demonstrated
a marked increase in apoptosis in a time- and dose-dependent
manner. In addition, Fig. 2C and D
showed that the apoptosis-associated genes Bcl-2 and Bax had
significantly altered expression in cells treated with simvastatin;
mRNA expression levels of Bcl-2 were significantly decreased and
expression levels of Bax were significantly increased in a time-
and dose-dependent manner.
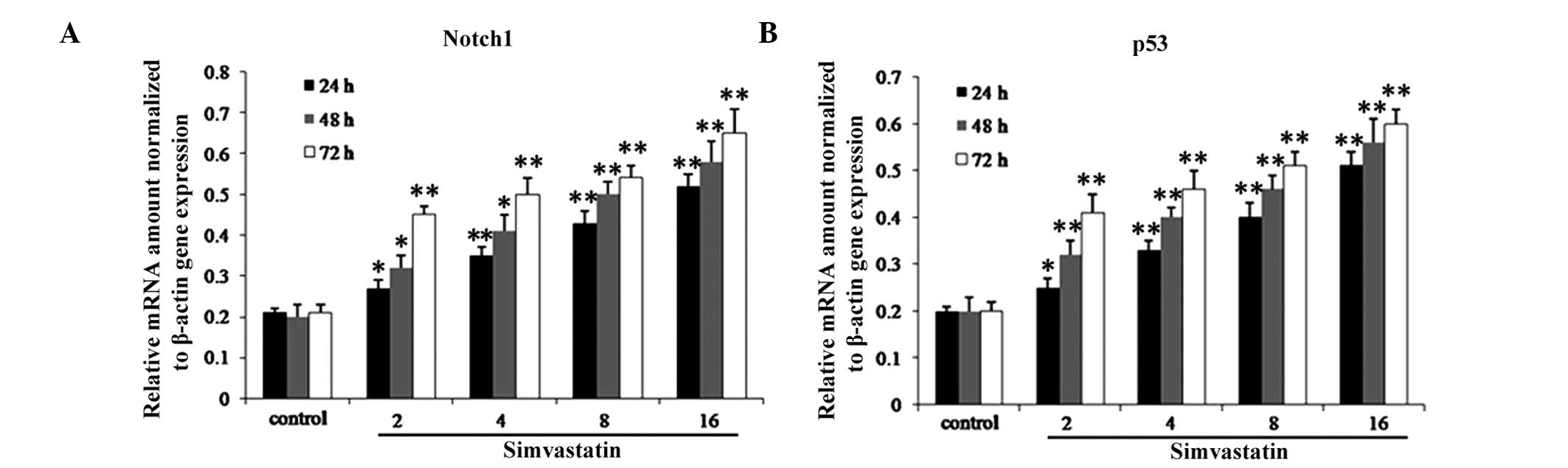
Simvastatin increases mRNA expression
levels of Notch1 and p53
RT-qPCR was used in order to investigate the effects
of simvastatin on the expression of Notch1 and p53 in HepG2 cells.
As shown in Fig. 3, following
incubation of HepG2 cells with various concentrations (0, 2, 4, 8
and 16 μM) of simvastatin for 48 h, mRNA expression levels of
Notch1 and p53 were significantly increased in a dose-dependent
manner.
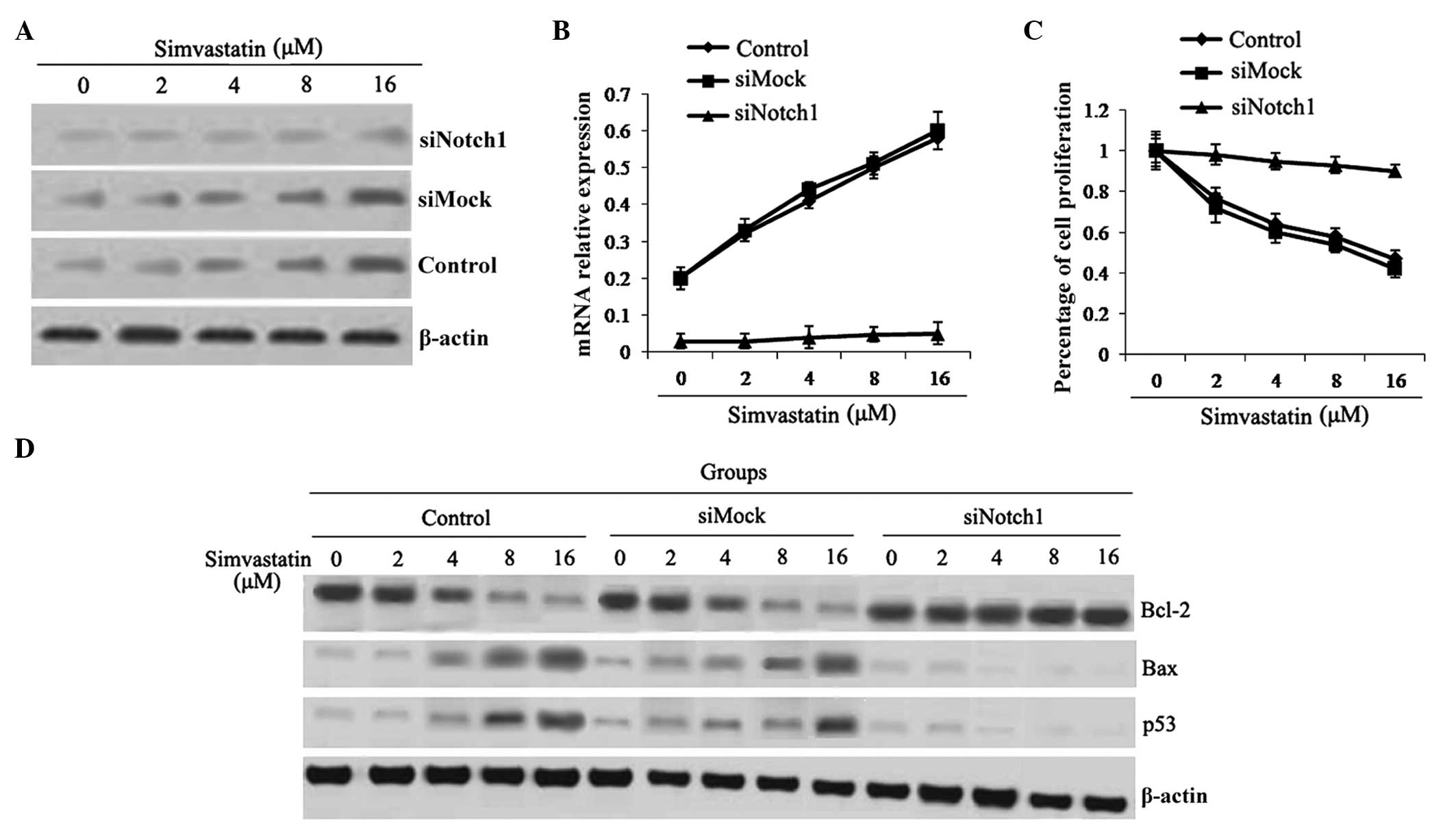
Simvastatin-induced apoptosis is
decreased following Notch1 gene knock-out
The results of the present study, as shown above,
demonstrated that simvastatin increased mRNA expression of Notch1;
therefore, Notch1 siRNA transfection experiments were performed
which knocked out the Notch1 gene in HepG2 cells, in order to
further investigate its role in simvastatin-induced apoptosis. As
shown in Fig. 4A and B, Notch1 was
successfully knocked out in HepG2 cells. Following Notch1 siRNA
transfection, the inhibitory effect of simvastatin on proliferation
in HepG2 cells was markedly decreased (Fig. 4C); in addition, mRNA expression
levels of p53 and Bax were downregulated compared to those of the
control and mock siRNA-transfected groups (P<0.05) (Fig. 4D).
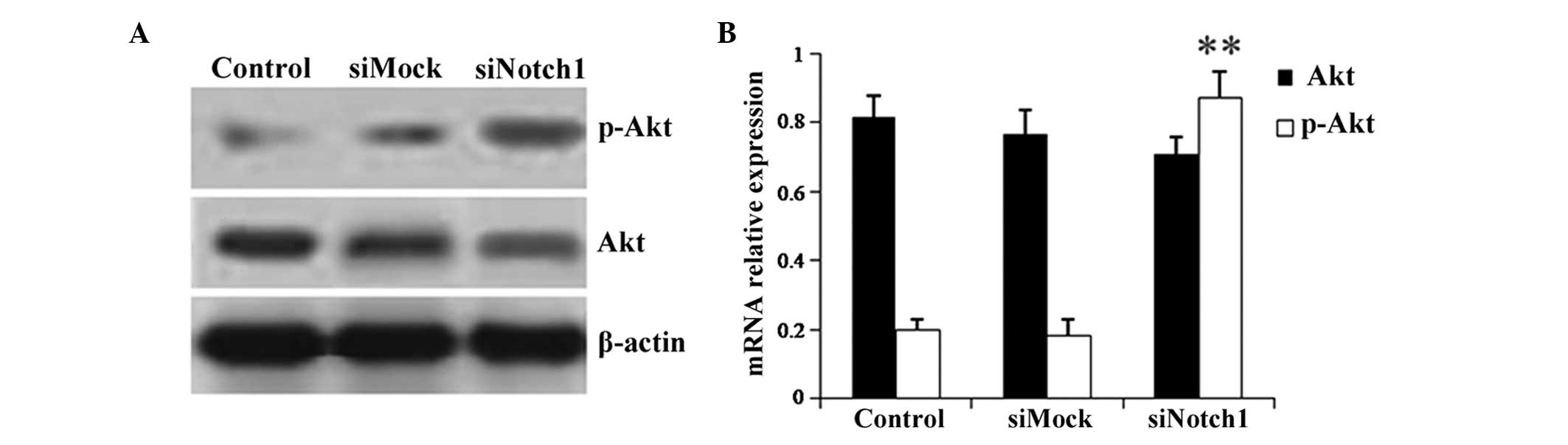
Simvastatin inhibits the phosphorylation
of Akt via upregulation of Notch1 expression in HepG2 cells
Western blot analysis and RT-qPCR were performed in
order to investigate the mechanism of simvastatin-induced HepG2
cell apoptosis by detecting the differential expression of the cell
survival-associated gene Akt. As shown in Fig. 5, simvastatin inhibited the
phosphorylation of Akt in the control and mock siRNA-transfected
groups; however, following Notch1 siRNA transfection, the
inhibitory effect of simvastatin on p-Akt levels was significantly
decreased (Fig. 5). This therefore
indicated that simvastatin regulated the phosphorylation of Akt via
increasing the expression of Notch1, which, in turn, resulted in
HCC cell apoptosis.
Discussion
Previous studies have indicated the potential use of
statins as cancer therapeutics; one study demonstrated simvastatin
was shown to decrease the cellular activity of thioredoxin
reductases in HepG2 cells, inhibiting cancer cell proliferation
(25). Cancer cells express
elevated levels of 3-hydroxy-3-methylglutaryl-coenzyme A reductase
and low-density lipid receptor in order to facilitate the increased
demand for isoprenoids and lipids (26); this therefore results in the
increased sensitivity of cancer cells to statins compared with that
of normal cells. However, the mechanisms by which the anti-cancer
effects of statins proceed remains to be elucidated. Numerous
studies have demonstrated that statins induced apoptosis in cancer
cells via various pathways in vitro (27–31);
however, there were discrepancy between the results of in
vivo and in vitro studies. The biological mechanisms by
which statins induce apoptosis and exert anti-proliferative effects
in HCC cells are complex. The results of the present study revealed
a novel mechanism by which simvastatin exerted cytotoxic effects in
the HepG2 and Huh7 cancer cell lines.
Notch1 protein is an essential molecule in the Notch
signaling pathway, which has an important role in maintaining the
balance between proliferation and differentiation. Previous studies
have shown that activated Notch1 inhibited growth in human
papillomavirus-positive cervical carcinoma cells and prostate
cancer cells, as well as induced cell cycle arrest in small cell
lung cancer cells (32–34). In addition, a study in
Notch1-deficient mice demonstrated that Notch1 functioned as a
tumor suppressor in mouse skin via inhibition of β-catenin
signaling (35). Another previous
study provided novel evidence for the potential role of Notch1
signaling in modulating the state of human liver carcinoma
(36,37). The results of the present study,
showed that simvastatin inhibited HepG2 and Huh7 cell viability and
proliferation, as well as promoted apoptosis; in addition, the
anti-tumor effect of simvastatin was found to be associated with
the Notch1 gene. This therefore indicated that simvastatin may
inhibit HCC cell growth via regulation of Notch1 expression. These
results were consistent with a previous study which showed that
Notch1 arrested the cell cycle of HCC via cyclin A, cyclin D1,
cyclin E and cdk4 (38).
The aim of the present study was to investigate the
role of Notch1 signaling in simvastatin-induced apoptosis in HepG2
and Huh7 cell by assessing the effect of Notch1 signaling on the
expression of the apoptosis-associated proteins p53, Bax, and
Bcl-2. The results showed that expression levels of p53 and Bax
expression were significantly increased, whereas Bcl-2 expression
was significantly decreased in a time- and dose-dependent manner.
Previous studies have demonstrated that the overexpression of
mutant or wild-type p53 may downregulate Bcl-2 expression,
resulting in apoptotic cell death (39). The results of the present study
therefore indicated that following cell cycle arrest, Notch1
signaling induced apoptosis via a p53-dependent reduction in Bcl-2
pathway signaling.
Numerous studies have indicated that Akt kinase may
be a noval target for cancer therapy (40–43).
Experimental models have revealed an association between the
regulation of survivin expression and increased Akt activity
(44). In prostate cancer cells,
survivin expression was shown to be regulated by insulin-like
growth factor 1 (IGF-1)-stimulated Akt-mTOR signaling (45), which was impaired following
simvastatin treatment (10).
Therefore, the present study investigated whether
simvastatin-induced apoptosis in HepG2 and Huh7 cells was
associated with the Akt pathway. The results demonstrated that
simvastatin reduced levels of phosphorylated Akt in HCC cells.
However, following Notch1 gene knock out, simvastatin had no effect
on p-Akt expression. These results suggested that the mechanism of
simvastatin-induced HCC cell apoptosis may proceed via regulation
of the expression of the Notch1 gene, which has an important role
in the Akt-dependent signaling pathway.
In conclusion, the results of the present study
demonstrated that simvastatin treatment induced apoptosis in human
HCC cell lines HepG2 and Huh7; in addition, simvastatin decreased
cell viability and proliferation in a time- and dose-dependent
manner. The anti-tumor effect of simvastatin was found to be
associated with p53, Bcl-2 and Bax expression levels, as well as
the Akt-dependent signaling pathway. The present study reinforced
the anti-tumor effect of statins and provided evidence for the
potential beneficial effects of simvastatin in HCC therapy in
humans.
Acknowledgements
The present study was supported by the Science and
Technology Plan Projects of Social Development Plans for Public
Relations of Shaanxi Province (nos. S2009SF471 and 2012SF2-13).
References
|
1
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsochatzis E, Meyer T, O’Beirne J and
Burroughs AK: Transarterial chemoembolisation is not superior to
embolisation alone: The recent European Association for the Study
of the Liver (EASL)-European Organisation for Research and
Treatment of Cancer (EORTC) guidelines. Eur J Cancer. 49:1509–1510.
2013. View Article : Google Scholar
|
|
3
|
Cabibbo G, Rolle E, De Giorgio M, et al:
Management of cirrhotic patients with hepatocellular carcinoma
treated with sorafenib. Expert Rev Anticancer Ther. 11:1807–1816.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parkin DM: Global cancer statistics in the
year 2000. Lancet Onco. 2:533–543. 2001. View Article : Google Scholar
|
|
5
|
Jakobisiak M and Golab J: Statins can
modulate effectiveness of antitumor therapeutic modalities. Med Res
Rev. 30:102–135. 2010.
|
|
6
|
Jakobisiak M and Golab J: Potential
antitumor effects of statins (Review). Int J Oncol. 23:1055–1069.
2003.PubMed/NCBI
|
|
7
|
Graaf MR, Richel DJ, van Noorden CJ and
Guchelaar HJ: Effects of statins and farnesyltransferase inhibitors
on the development and progression of cancer. Cancer Treat Rev.
30:609–641. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liao JK and Laufs U: Pleiotropic effects
of statins. Annu Rev Pharmacol Toxicol. 45:89–118. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Freed-Pastor WA, Mizuno H, Zhao X, et al:
Mutant p53 disrupts mammary tissue architecture via the mevalonate
pathway. Cell. 148:244–258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kochuparambil ST, Al-Husein B, Goc A,
Soliman S and Somanath PR: Anticancer efficacy of simvastatin on
prostate cancer cells and tumor xenografts is associated with
inhibition of Akt and reduced prostate-specific antigen expression.
J Pharmacol Exp Ther. 336:496–505. 2011. View Article : Google Scholar
|
|
11
|
Wang Y, Xu SL, Wu YZ, et al: Simvastatin
induces caspase-dependent apoptosis and activates p53 in OCM-1
cells. Exp Eye Res. 2013. View Article : Google Scholar
|
|
12
|
Xu J, Liu X, Chen J, et al: Simvastatin
enhances bone marrow stromal cell differentiation into endothelial
cells via notch signaling pathway. Am J Physiol Cell Physiol.
296:C535–C543. 2009. View Article : Google Scholar :
|
|
13
|
Zacharek A, Chen J, Cui X, Yang Y and
Chopp M: Simvastatin increases notch signaling activity and
promotes arteriogenesis after stroke. Stroke. 40:254–260. 2009.
View Article : Google Scholar
|
|
14
|
Aster JC, Xu L, Karnell FG, et al:
Essential roles for ankyrin repeat and transactivation domains in
induction of T-cell leukemia by notch1. Mol Cell Biol.
20:7505–7515. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bresnick EH, Chu J, Christensen HM, Lin B
and Norton J: Linking Notch signaling, chromatin remodeling, and
T-cell leukemogenesis. J Cell Biochem Suppl. 35(Suppl): 46–53.
2000. View Article : Google Scholar
|
|
16
|
Wael H, Yoshida R, Kudoh S, et al: Notch
signaling controls cell proliferation, apoptosis and
differentiation in lung carcinoma. Lung Cancer. 85:131–140. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ramdass B, Maliekal TT, Lakshmi S, et al:
Coexpression of Notch1 and NF-κB signaling pathway components in
human cervical cancer progression. Gynecol Oncol. 104:352–361.
2007. View Article : Google Scholar
|
|
18
|
Wang Z, Banerjee S, Li Y, et al:
Down-regulation of notch-1 inhibits invasion by inactivation of
nuclear factor-κB, vascular endothelial growth factor, and matrix
metalloproteinase-9 in pancreatic cancer cells. Cancer Res.
66:2778–2784. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, Fu L, Gu F and Ma Y: Notch1 is
involved in migration and invasion of human breast cancer cells.
Oncol Rep. 26:1295–1303. 2011.PubMed/NCBI
|
|
20
|
Qi R, An H, Yu Y, et al: Notch1 signaling
inhibits growth of human hepatocellular carcinoma through induction
of cell cycle arrest and apoptosis. Cancer Res. 63:8323–8329.
2003.PubMed/NCBI
|
|
21
|
Yu Y, Zhou XD, Liu YK, et al: Platelets
promote the adhesion of human hepatoma cells with a highly
metastatic potential to extracellular matrix protein: involvement
of platelet P-selectin and GP IIb-IIIa. J Cancer Res Clin Oncol.
128:283–287. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu H, Zhao X, Huang S, et al: Blocking
Notch1 signaling by RNA interference can induce growth inhibition
in HeLa cells. Int J Gynecol Cancer. 17:511–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Singh L, Pushker N, Saini N, et al:
Expression of pro-apoptotic Bax and anti-apoptotic Bcl-2 proteins
in human retinoblastoma. Clin Experiment Ophthalmol. Jul
31–2014.(Epud ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao J, Yan Q, Liu S and Yang X: Knockdown
of EpCAM enhaces the chemosensitivity of breast cancer cells to
5-fluorouracil by downregulating the antiapoptotic factor Bcl-2.
PLoS One. 9:e1025902014. View Article : Google Scholar
|
|
25
|
Ekström L, Johansson M, Monostory K, et
al: Simvastatin inhibits the core promoter of the TXNRD1 gene and
lowers cellular TrxR activity in HepG2 cells. Biochem Biophys Res
Commun. 430:90–94. 2013. View Article : Google Scholar
|
|
26
|
Liao JK: Isoprenoids as mediators of the
biological effects of statins. J Clin Invest. 110:285–288. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shimada T, Takeshita Y, Murohara T, et al:
Angiogenesis and vasculogenesis are impaired in the
precocious-aging klotho mouse. Circulation. 110:1148–1155. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Seeger H, Wallwiener D and Mueck A:
Statins can inhibit proliferation of human breast cancer cells in
vitro. Exp Clin Endocrinol Diabetes. 111:47–48. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mandal CC and Ghosh-Choudhury N, Yoneda T,
Choudhury GG and Ghosh-Choudhury N: Simvastatin prevents skeletal
metastasis of breast cancer by an antagonistic interplay between
p53 and CD44. J Biol Chem. 286:11314–11327. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hwang KE, Na KS, Park DS, et al: Apoptotic
induction by simvastatin in human lung cancer A549 cells via Akt
signaling dependent down-regulation of survivin. Invest New Drugs.
29:945–952. 2011. View Article : Google Scholar
|
|
31
|
Hussain B, Saleh GM, Sivaprasad S and
Hammond CJ: Changing from Snellen to LogMAR: debate or delay? Clin
Experimental Ophthalmol. 34:6–8. 2006. View Article : Google Scholar
|
|
32
|
Sriuranpong V, Borges MW, Ravi RK, et al:
Notch signaling induces cell cycle arrest in small cell lung cancer
cells. Cancer Res. 61:3200–3205. 2001.PubMed/NCBI
|
|
33
|
Shou J, Ross S, Koeppen H, de Sauvage FJ
and Gao WQ: Dynamics of notch expression during murine prostate
development and tumorigenesis. Cancer Res. 61:7291–7297.
2001.PubMed/NCBI
|
|
34
|
Talora C, Sgroi DC, Crum CP and Dotto GP:
Specific down-modulation of Notch1 signaling in cervical cancer
cells is required for sustained HPV-E6/E7 expression and late steps
of malignant transformation. Genes Dev. 16:2252–2263. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nicolas M, Wolfer A, Raj K, et al: Notch1
functions as a tumor suppressor in mouse skin. Nature Genet.
33:416–421. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ahn S, Hyeon J and Park CK: Notch1 and
Notch4 are markers for poor prognosis of hepatocellular carcinoma.
Hepatobiliary Pancreat Dis Int. 12:286–294. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou L, Zhang N, Song W, et al: The
significance of Notch1 compared with Notch3 in high metastasis and
poor overall survival in hepatocellular carcinoma. PLoS One.
8:e574822013.
|
|
38
|
Masaki T, Shiratori Y, Rengifo W, et al:
Cyclins and cyclin-dependent kinases: Comparative study of
hepatocellular carcinoma versus cirrhosis. Hepatology. 37:534–543.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Basu A and Haldar S: The relationship
between BcI2, Bax and p53: consequences for cell cycle progression
and cell death. Mol Hum Reprod. 4:1099–1109. 1998. View Article : Google Scholar
|
|
40
|
Somanath P, Vijai J, Kichina J, Byzova T
and Kandel E: The role of PAK-1 in activation of MAP kinase cascade
and oncogenic transformation by Akt. Oncogene. 28:2365–2369. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Goc A, Al-Husein B, Kochuparambil ST, et
al: PI3 kinase integrates Akt and MAP kinase signaling pathways in
the regulation of prostate cancer. Int J Oncol. 38:267–277.
2011.
|
|
42
|
Crowell JA, Steele VE and Fay JR:
Targeting the AKT protein kinase for cancer chemoprevention. Mol
Cancer Ther. 6:2139–2148. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Davies MA: Regulation, role, and targeting
of Akt in cancer. J Clin Oncol. 29:4715–4717. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Amaravadi R and Thompson CB: The survival
kinases Akt and Pim as potential pharmacological targets. J Clin
Invest. 115:2618–2624. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vaira V, Lee CW, Goel HL, et al:
Regulation of survivin expression by IGF-1/mTOR signaling.
Oncogene. 26:2678–2684. 2006. View Article : Google Scholar : PubMed/NCBI
|