Introduction
White matter damage (WMD) is the most common
manifestation of brain injury in premature infants. Periventricular
leukomalacia (PVL), as the most serious manifestation of WMD,
influences the development and maturation of myelin, and may also
result in severe neurologic deficits. The etiology of PVL is
complex, but the major cause is hypoxia-ischemia (HI), which is
also a primary contributor to brain injury in premature infants
(1). No effective agent for the
prevention and treatment of PVL has been developed, and the
pathogenesis of PVL remains unclear. Thus, there is an urgent need
to further study the pathogenesis of PVL, with the aim of
identifying new targets for its treatment and prevention.
PVL is pathologically characterized by diffuse
injury of the oligodendrocytes (OLs) and dysmyelination (2,3).
Oligodendrocytes contribute to the myelination of the central
nervous system, and have received much attention regarding their
role in PVL-related injuries. Current studies on the role of PVL
have focused on the abnormal secretion of inflammatory factors, the
damage produced by oxyradicals, and hemodynamics (4,5), but
interpretation of the results of these studies has been less than
satisfactory.
The expression of the OL transcription factor OLIG1
was detected in immature oligodendrocyte precursor cells (OPCs) of
the nervous system (6). OLIG1 can
promote not only the differentiation of nerve stem cells (NSCs) to
OLs (7), but also, the development
and maturation of OLs and myelin (8,9).
However, the association between OLIG1 and OLs or myelin in
association with PVL has not been well established. This study
aimed to further explore the effects of OLIG1 on OLs and myelin,
which are affected by the processes occuring during PVL.
Previous studies have shown that OLIG1 not only
regulates myelin, but is also involved in the repair of injured
myelin (10,11). Xin et al (10) reported that OLIG1 is present in the
nuclei of rat OPCs at the embryonic stage, and then translocates to
the cytoplasm after birth. However, OLIG1 translocates back into
the nuclei of OPCs following a demyelinating injury. Nuclear OLIG1
also plays an important role in the activation of other
transcription factors, such as Sox10, Sox9 and OLIG2 (12). Furthermore, an association between
OLIG1 expression and certain disorders of the central nervous
system, e.g., the repair of myelin in multiple sclerosis, was
previously reported (11);
however, it is still unknown whether OLIG1 is associated with PVL
in premature infants. An explanation for the important role of the
nuclear translocation of OLIG1 in the repair and regeneration of
myelin following HI-induced PVL would be highly valuable, for both
the prevention and the development of new therapies for PVL. In
this study, the effects of OLIG1 on PVL-affected OLs and myelin are
discussed in detail.
Materials and methods
Animals and experimental groups
Eighty rats (3 days-old) of either gender,
spontaneously delivered by pregnant rats on days 21–23 of
pregnancy, were randomly divided into the HI group (n=40) and the
normoxia group (n=40). The animals were provided by the Animal
Department Experiment Center, of the Shengjing Hospital of China
Medical University. The present study was approved by the Ethical
Committee of China Medical University (Shenyang, China). An
HI-induced animal model for PVL was established for the HI group
according to the method reported by Mizuno et al (13). Ligation of the right common carotid
artey was then performed following inhalation anesthesia, in order
to reduce the pain. Rats in the HI group were then subjected to 2 h
of exposure to hypoxic conditions (using a mixture of 8%
O2 and 92% N2), while rats in the normoxia
group were only subjected to isolation of their right common
carotid artery. Samples of brain tissue were collected at 1, 3, 7,
14 and 21 days after HI exposure.
Hematoxylin and eosin (H&E)
staining
Samples of brain tissues were resected at locations
within ±5 mm of the optic chiasma, embedded in paraffin blocks, cut
into continuous coronal sections of 5 μm thickness, and finally
stained with H&E (Beijing Zhongshan Goldenbridge Biotechnology,
Beijing,China). Six sections performed at each time-point were
randomly selected from each group, and then, the periventricular
alba was observed for pathological changes in 5 random fields of a
light microscope (magnification, ×400; Olympus Corporation, Tokyo,
Japan).
Transmission electron microscope
(TEM)
The brain tissues were initially fixed in 2.5%
glutaral at 4°C for 24 h, then treated in 1% osmic acid, dehydrated
with acetone, and embedded in epoxy resin. The embedded tissues
were cut into ultrathin sections and double-stained with uranyl
acetate and lead nitrate. The ultrastructure of OLs and myelin was
observed using a JEOL JEM-1200EX TEM (magnification, ×25,000;
Hitachi High Technologies Corp., Tokyo, Japan).
Immunohistochemistry
Sections of brain tissue were deparaffinized in
graded alcohol solutions and xylene. The sections were then blocked
with 3% H2O2 (37°C, 30 min) and goat serum
(37°C, 20 min), followed by addition of rabbit anti-rat monoclonal
anti-OLIG1 (1:300 dilution; Abcam, San Francisco, CA, USA) and
incubation overnight at 4°C. The negative control tissues were
incubated with phosphate-buffered saline (PBS) instead of the
primary antibody, and also incubated with the biotin-labeled goat
anti-rabbit IgG secondary antibody (37°C, 30 min), similarly to the
tissues of interest. All sections were then incubated with a
horseradish peroxidase (HRP) marker (37°C, 30 min), stained with
3′-diaminobenzidine (DAB), re-stained with hematoxylin, dehydrated,
vitrified, and mounted. Ten different sections from each time-point
were randomly selected from each group, and the periventricular
alba was observed in 5 random fields of a light microscope
(magnification, ×400).
Immunofluorescence staining
Sections of brain tissue were infiltrated with 4%
paraformaldehyde, soaked in 3% paraformaldehyde for 3 h at 4°C,
cryoprotected in 30% sucrose for 12 h at 4°C, and frozen at 80°C.
The frozen sections (10 μM thick) were air-dried and washed 3 times
with PBS, incubated with 0.5% Triton X-100 for 5 min at room
temperature, and again washed 3 times with PBS. The sections were
then blocked with 10% goat serum for 30 min at 37°C, and incubated
with the mouse monoclonal primary antibodies anti-oligodendrocyte
marker O4 and anti-OLIG1 (both at 1:100 dilution; R&D Systems,
Minneapolis, MN, USA) overnight at 4°C. Sections were incubated in
the absence of the primary antibodies served as negative controls.
The tissue sections were washed 4 times with PBS-Triton X-100. The
secondary antibodies were anti-mouse Alexa Fluor-fluorescein
isothiocyanate (FITC)-conjugated IgM (green fluorescence) and
anti-rabbit Alexa Fluor-Cy3-conjugated IgG (red fluorescence;
Beijing Zhongshan Goldenbridge Biotechnology), and were incubated
for 60 min at 37°C. The tissue sections were washed 3 times with
PBS, and the nuclei were stained for 2 min with
4′,6-diamidino-2-phenylindole (DAPI, 1:2,000 dilution), purchased
from Sigma-Aldrich (Santa Clara, CA, USA). Following additional
washes, images of the tissues were captured by confocal laser
scanning microscopy (MTC-600; Bio-Rad, Hercules, CA, USA).
Western blotting
At each time point for each group, five samples were
obtained, with 100 mg per sample. Each solid tissue sample was
broken up using ultrasonication, then 0.5–1 ml pyrolysis liquid
(Nanjing KeyGEN Biotech, Nanjing, China) was added, mixed
thoroughly and span at 14,000 g at 4°C for 30 min. The top layer
was then transferred into new tubes and the mixture was centrifuged
at 14,000 × g, at 4°C for 10 min and the supernatant was carefully
discarded. A mixture of 200 μl buffer and 2 μl protease inhibitors
(Nanjing KeyGEN Biotech) were added and centrifuged at 14,000 g for
10min at 4°C. The supernatants were then transferred to new tubes
and stored at −80°C. Determination of the protein concentrations
was performed using the bicinchoninic acid (Nanjing KeyGen Biotech)
method. Subsequently, equal amounts 40 μg) of protein extract were
mixed in 1X Laemmli buffer (Nanjing KeyGEN Biotech)., boiled for 5
min, and electrophoresed on precast 7.5% sodium dodecyl
sulfate-polyacrylamide gels (80 V for 120 min). The proteins were
then electrophoretically transferred onto polyvinylidene difluoride
membranes (100 V for 90 min; EMD Millipore, Bedford, MA, USA). The
membranes were incubated for 1 h in normal donkey serum (1:10) to
block nonspecific binding, and then incubated overnight at 4°C with
primary antibodies targeting OLIG1 (1:500 dilution), myelin basic
protein (MBP; rabbit monoclonal antibody at 1:1,000 dilution;
Abcam), and β-actin (1:2,000 dilution; Abcam), diluted in PBS-0.02%
Tween-20. Samples incubated in this solution without primary
antibodies served as the negative controls. After washing 3 times
in PBS, the membranes were incubated at room temperature for 90 min
with the HRP-conjugated secondary antibody. The membranes were then
washed in PBS and impregnated with an enhanced chemiluminescence
(ECL) substrate (Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA) to expose the radiographic film. Protein bands were scanned
with the ChemiImager 5500 v2.03 software (AlPha InnCh, Miami, FL,
USA), integrated digital values were calculated using a
computerized image analysis system (Fluor Chen 2.0; Alpha Innotech,
San Jose, CA, USA) and normalized to the value of β-actin.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The RNAiso Plus kit, manufactured by Takara Bio,
Inc. (Dalian, China), was used to extract total RNA. Briefly, 1 ml
Trizol reagent (Takara Bio, Inc.) was added to 1–2 g fresh
material, mixed thoroughly, cooled on ice for 5 min and centrifuged
at 12,000 × g,4°C for 10 min. The supernatants were then
transferred to new tubes, 400 μl phenol/chloroform (1:1v) was added
and the mixture was vortexed for 15 sec. The samples were then
centrifuged at 12,000 × g,4°C for 10 min and the top (aqueous)
layer was transferred into new tubes. Isopropanol (1:1v) was added
to each tube and samples were centrifuged at 12,000 × g for 10 min
at 4°C. The supernatant was discarded and the pellet washed with 1
ml 75% ethanol. Samples were centrifuged again at 7,500 × g for 5
min. Residual ethanol was removed with a pipet. Samples were
air-dried for 4 min and the RNA was then redissolved in 30 μl DEPC
(diethyl pyrocarboanate, Takara Bio, Inc.). cDNA was synthesized by
reverse transcription using the PrimeScript RT reagent kit (Takara
Bio, Inc.), and then amplified with a LightCycler real-time PC
amplifier (Roche Diagnostics, Mannheim, Germany). The sequences of
primers, provided by Takara Bio, Inc., were the following: OLIG1
forward (F), 5′-CCA CAG CAA GGC AGC TGA AG-3′ and reverse (R),
5′-TGC TAA CGC TAA TCA CAA GCC AAG-3′; β-actin F, 5′-GGA GAT TAC
TGC CCT GGC TCC TA-3′ and R, 5′-GAC TCA TCG TAC TCC TGC TTG CTG-3′.
Reactions were carried out in a total volume of 20 μl, and the
amplification conditions were: 95°C for 15 sec and 60°C for 1 min,
for a total of 45 cycles. The relative expression level of the
OLIG1 mRNA was calculated with the ΔΔCt method.
Statistical analysis
Data were summarized as mean ± standard deviation
(SD). Statistical analysis was performed by two-way analysis of
variance (ANOVA), followed by multiple comparison least significant
difference (LSD) tests, using theSPSS 18.0 software (SPSS Inc.,
Chicago, IL, USA). A difference was considered to be statistically
significant at a value of P<0.05.
Results
Morphological changes in the brain
tissues
At days 1, 3, 7, 14 and 21, brain tissues in the
normoxia group showed a normal morphology and structure; no
significant differences were observed among the different
time-points (Fig. 1A–E). In the HI
group, karyopyknosis and apoptosis were observed in only a few
cells at day 1 (Fig. 1F), while at
day 3, necrosis following karyopyknosis was observed in numerous
cells (indicated by →), and brain tissues had a porous, malacotic
structure, in which numerous areas of cystic necrosis vacuoles were
observed (Fig. 1G). At day 7, the
cribriform structure disappeared, and the number of vacuoles was
significantly reduced (Fig. 1H).
At day 14, there were progressively increasing numbers of normal
cells and much fewer vacuoles (Fig.
1I); at day 21, the total size and number of vacuoles were both
significantly decreased, but brain tissues still had a porous,
irregular structure (Fig. 1J).
 | Figure 1Malacotic periventricular brain
tissues caused by hypoxia-ischemia (HI), as observed with
hematoxylin and eosin (H&E) staining (magnification, ×400).
Brain tissues of the normoxia group exhibited a normal morphology,
and uniform, intensive cell distribution at all time-points (A–E).
In the HI group, karyopyknosis was observed in brain tissues, with
necrosis of a few cells at day 1 (F). At day 3, the focus of
malacia began to appear in the alba, and necrosis was observed in a
high number of cells, along with a cribriform change (G). At day 7,
the number of cystic necroses was reduced compared to day 3, when
the necroses first appeared (H). At days 14 and 21 (I,J), the
number of cystic necroses was smaller, and cribriform necroses
gradually disappeared or were transformed into areas of cluster or
punctiform necroses, although brain tissues still had a porous,
malacotic structure. |
Ultrastructural changes in the brain
tissues
HI disrupts the development of
OLs
Evaluation of OLs under the electron microscope
showed that in the normoxia group, OLs demonstrate a regular
morphology and structure, a complete cell membrane structure, a
visible nuclear membrane, and a large and visible nucleus,
containing uniformly distributed particles of chromatin (Fig. 2A and C). In the HI group, OLs
showed a highly irregular morphology, an invisible, damaged nuclear
membrane and a vague nucleus, and necrosis was observed in a high
number of cells at day 3 (Fig.
2B). At day 21, the nuclear structure had vanished, karyolysis
was observed in a few cells, and necrosis of OLs was also observed
(Fig. 2D).
 | Figure 2Observation of the effects of
hypoxia-ischemia (HI) on oligodendrocytes (OLs) by transmission
electron microscopy (magnification, ×25,000). (A and C) In the
normoxia group, the morphology and structure of OLs is visible,
with a large, full nucleus, and a complete nuclear membrane,
indicated by arrows. (B) In the HI group, the cell morphology and
structure is highly irregular, with cell atrophy observed, altered
nuclear structure, and cell necrosis at day 3. (D) By day 21, the
OLs have obtained an irregular structure and show serious cell
degeneration and necrosis. |
HI disrupts the maturation of
myelin
In the normoxia group, myelin had a visible
morphology and a compact inter-layer structure, in which developing
axons were observed (arrows in Fig. 3A
and D). In the HI group at day 3, there were numerous small
vacuoles formed in the myelin, with a cribriform change, and the
development of myelin was clearly altered (Fig. 3B and C). At day 21, an obvious
abnormality was still detectable in the structure of myelin, the
inter-layer structure was porous, and there was visible
stratification in certain layers (Fig.
3E and F).
Measurement of OLIG1 protein expression
in brain tissues by immunohistochemistry
At days 1, 3, 7, 14 and 21, a high numbers of cells
with their cytoplasm stained brown was observed in brain tissues of
the normoxia group, which indicated that numerous cells were
expressing the OLIG1 protein (Fig.
4A–E). Compared to the normoxia group, a decreased number of
cells with brown staining in the cytoplasm was observed at all
time-points in the HI group (Fig.
4F–J), while a high number of nuclei stained brown was
observed. These data indicate that the expression of OLIG1 was
partially transferred to the nucleus following the HI-induced brain
tissue injury.
 | Figure 4OLIG1 protein expression in brain
tissues, as measured by immunohistochemistry (magnification, ×500).
At days 1, 3, 7, 14 and 21, the cytoplasm was stained brown
(indicating that numerous cells were expressing the OLIG1 protein)
in the brain tissues of the normoxia group (A–E). In the
hypoxia-ischemia group, only a sparse distribution of cells
expressing OLIG1 was observed, with numerous nuclei stained brown
at day 1 (F). At day 3 (G), there were substantially fewer
positively-stained cells, and at days 7, 14 and 21 (H–J) the
positively-stained cells were more extensively distributed,
although there remained a high number of yellow-stained nuclei. |
Effects of HI on OLIG1 localization in
the brain tissues
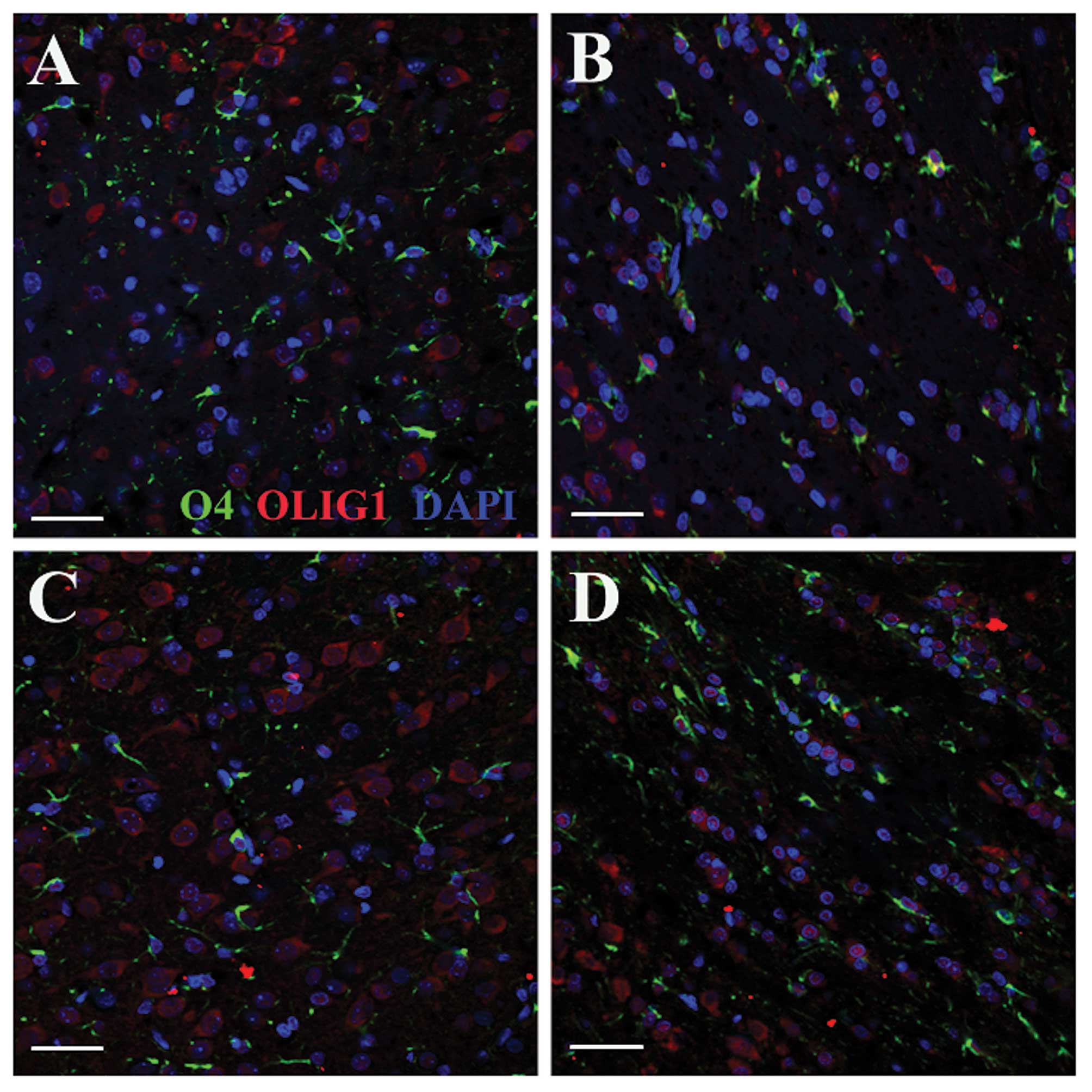
Immunofluorescent double staining revealed
co-expression of OLIG1 and O4 (important proteins in mature OPCs)
in the cytoplasm of the same cells (Fig. 5A and C); expression of OLIG1 was
observed in the cell nuclei of alba cells following HI, but it was
reduced compared with that in the normoxia groups (Fig. 5B and D). In the normoxia group,
OLIG1 was consistently expressed in the cytoplasm, with no nuclear
translocation observed (Fig. 5A and
C). These results indicate that OLIG1 is a protein normally
expressed in the cytoplasm of healthy OPCs after birth, while under
hypoxic and ischemic conditions, it translocates to the
nucleus.
Effects of HI on the protein expression
of OLIG1 and MBP in brain tissues
Western blot analysis showed that the protein
expression levels of OLIG1 and MBP in the HI group were reduced
compared to the normoxia group. No significant difference
(P>0.05) in the expression level of OLIG1 was found in the
normoxia group at any time-point (Fig.
6C). In the HI group, the expression level of OLIG1 in brain
tissue gradually decreased at days 1 and 3, but then increased at
days 7, 14 and 21 (Fig. 6B). In
the normoxia group, the expression level of MBP progressively and
significantly (P<0.01) increased (Fig. 7F). In the HI group, the expression
level of MBP reached a peak at day 7 (Fig. 6D) (P<0.01), and then increased
at days 14 and 21 (Fig. 6E)
(P<0.01). Despite the late increase, the MBP and OLIG1
expression levels were still lower (P<0.01) than those of the
normoxia group (Fig. 6C and
F).
Decreased expression of OLIG1 mRNA
following HI-induced brain injury
At all time-points, there was no significant
difference in the expression level of the OLIG1 mRNA in the
normoxia group (P>0.05), but there was a significant decrease in
the HI group as compared to the normoxia group (P<0.01). In the
HI group, this decrease began at day 1 (P<0.01), and reached a
peak at day 3 (P<0.01); then, a slight increase was observed at
days 7 and 14, but the difference between days 3 and 7 or day 14
was not statistically significant (P>0.05). The increase in the
level of the OLIG1 mRNA observed at day 21 was statistically
significant compared to the levels at days 1 and 3 (P<0.01), but
was not significant when compared to the levels at days 7 and 14
(P>0.05).
Discussion
PVL is the most serious sign of WMD in premature
infants. Its incidence in this group is 8–26% in the developing
countries, and 5–17% in premature infants weighing <1,500 g,
with >66% of these infants developing cerebral paralysis
(13). In developed countries
however, only 10% of PVL patients develop cerebral paralysis, and
25–50% of these patients suffer from complicated cognitive
disorders, behavioral deficiencies, and mild dyskinesia (14). At present, PVL is the most common
disease seriously affecting the quality of life and long-time
survival in premature infants. The current hypotheses on the
mechanisms underlying PVL include the abnormal secretion of
inflammatory factors and the deleterious effects of oxyradicals
(15,16). However, most studies have rarely
focused on HI as the molecular mechanism of PVL. A study conducted
by Craig et al (17),
showed that newborn rats aged 2–5 days show immature nerve
development equivalent to that of a human fetus at a gestation age
of 28–32 weeks, which is consistent with the time period when alba
injury occurs in humans during the perinatal period. The PVL model
we used in our study is very similar to PVL in premature infants
with respect to the pathology and clinical manifestations, and we
therefore investigated the effects of OLIG1 on PVL-related WMD in a
3-day newborn rat model of HI-induced PVL.
PVL is frequently observed during the peak of the
myelin period (18), and when
there is an abundance of advanced-stage OPCs (accounting for ~90%
of OLs) in newborn rat brain tissues. The OPCs will further develop
and form axon-enclosing myelin, and are highly sensitive to HI
(19). The stimulation of OPCs by
HI at 2–5 days after birth disrupts myelin and causes PVL (20). OLs are currently considered the
major cell target of PVL (21),
and the deposition of myelin is not complete in the above-mentioned
period; thus the selective vulnerability of OPCs is the main
contributor to cerebral paralysis, which is a severe sequela
of PVL (22). It was reported that
HI not only disrupts the development and maturation of OLs
(23), but also results in changes
in OLIG1 expression (24,25). French et al (24) showed that oxidative stress
following HI reduces the expression of OLIG1 and hinders the
differentiation and maturation of nerve stem cells to OLs.
Furthermore, Tanaka et al (25) found that the expression of OLIG1
and other transcription factors in adult monkey brain tissue is
decreased, followed by an increase during the process of ischemic
brain injury. This study showed that the expression of OLIG1 in
newborn rat brain tissues is decreased at the early stage, but then
increases to levels lower than the normal at the late stage
following HI exposure. Therefore, the dynamic changes in OLIG1
expression in the presence of HI-induced WMD and the role of OLIG1
in promoting the repair and regeneration of OLs and myelin
following PVL brain injury need both to be confirmed by future
studies.
In this study, we observed porous, malacotic brain
tissues, karyolysis followed by OL necrosis, formation of small
vacuoles, and a loosened inter-layer myelin structure following HI
exposure. Furthermore, we observed a significant decrease in the
expression of OLIG1 and MBP after HI, which is consistent with OL
and myelin injury. These findings reveal that the abnormal protein
expression of OLIG1 and MBP following PVL brain injury caused by HI
plays an essential role in disrupting the development and
maturation of OLs and myelin.
The current study showed that the downregulation of
OLIG1 during the process of HI-induced PVL brain injury may affect
the maturation and development of myelin. OLIG1 is an important
indicator of OPCs (9,26), and it can stimulate their
development to mature OLs (27);
myelin is then formed by the development of OLs (17). OPCs are highly sensitive to the
effects of HI (28,29), and we found nearly parallel changes
in OLIG1 and MBP expression levels, which is consistent with the
developmental disorders affecting OLs and myelin. Therefore,
downregulation of the OLIG1 gene in hypoxic-ischemic brain
tissues will lead to a decrease in the expression of certain
proteins that are required to form myelin (e.g., MBP), and thus
delay the development of myelin.
Our findings demonstrate that brain tissues have a
certain ability of self-repair following HI; however, myelin cannot
completely be repaired. The expression level of OLIG1 in brain
tissues showed a decreasing trend at the early stage, and an
increasing trend at the late stage following HI, but failed to
reach the levels observed in the normoxia group. In addition, a
change in the localization of OLIG1 was also observed; i.e.,
similar to other studies (26,30),
OLIG1 was expressed in the cytoplasm of OLs in normoxic brain
tissues after birth. OLIG1 was also highly expressed in the nuclei
of OPCs after the initiation of developmental disorders that were
secondary to myelin injury caused by HI (31). Thus, OLIG1 promoted the development
of immature OPCs to mature OLs, and eventually contributed to the
migration of mature OLs to newly formed myelin. This change in the
nuclear translocation of OLIG1 may enhance the regeneration of
myelin. Following HI, the protein expression level of MBP was
increased at the late compared to the early stage, which is a
pattern very similar to that of OLIG1; this suggests that OLIG1 has
a certain promoting role in the repair of myelin. However, despite
this repair effect of OLIG1, myelin was not fully repaired to a
normal level.
The earliest manifestation of HI-induced PVL is
degeneration and necrosis in a few cells, with brain tissues
maintaining a visible structure, and intensively arranged nerve
fibers. This is followed by liquefactive necrosis in a higher
number of cells, a porous structure of brain tissues with even
visible small vacuoles left by liquefactive necrosis, and
irregularly arranged nerve fibers. The vacuoles left by cell
necrosis of the brain tissue eventually become reduced in number
and size with the progressive development of brain tissue. At 21
days following HI, TEM observations revealed serious damage in a
considerable number of OLs and myelin in brain tissues of the HI
group, as compared to the normoxia group. The downregulation of
OLIG1 following hypoxic-ischemic WMD can also result in the reduced
expression of myelin-related proteins such as MBP, and thus cause
delayed myelination and development of PVL. OLIG1 can promote
myelination by stimulating nuclear transcription, but this effect
is insufficient for the complete repair of the injured myelin.
Additional studies need to be conducted to determine whether
OLIG1 gene therapy may efficiently prevent and treat PVL in
premature infants with PVL-related WMD.
Acknowledgements
We gratefully acknowledge professors Jianhua Fu and
Xindong Xue for invaluable help during the research and Dr Jianhua
Fu for valuable comments and corrections on this manuscript. We
would like to thank the Center Laboratory and Department of Animal
of Shengjing Hospital of China Medical University for expert
assistance. This study was partially supported by the Natural
Science Foundation of China (grant no., 30872781).
References
|
1
|
Welin AK, Svedin P, Lapatto R, Sultan B,
Hagberg H, Gressens P, et al: Melatonin reduces inflammation and
cell death in white matter in the mid-gestation fetal sheep
following umbilical cord occlusion. Pediatr Res. 61:153–158. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haynes RL, Folkerth RD, Keefe RJ, Sung I,
Swzeda LI, Rosenberg PA, et al: Nitrosative and oxidative injury to
premyelinating oligodendrocytes in periventricular leukomalacia. J
Neuropathol Exp Neur. 62:441–450. 2003.
|
|
3
|
Burton A: Olig1 needed for remyelination.
Lancet Neurol. 4:802005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fullerton HJ, Ditelberg JS, Chen SF, Sarco
DP, Chan PH, Epstein CJ and Ferriero DM: Copper/zinc superoxide
dismutase transgenic brain accumulates hydrogen peroxide after
perinatal hypoxia ischemia. Ann Neurol. 44:357–364. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
De Felice C, Toti P, Laurini RN, Stumpo M,
Picciolini E, Todros T, et al: Early neonatal brain injury in
histologic chorioamnionitis. J Pediatr. 138:101–104. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou Q and Anderson DJ: The bHLH
transcription factors OLIG2 and OLIG1 couple neuronal and glial
subtype specification. Cell. 109:61–73. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu Y, Jiang P and Deng W: OLIG gene
targeting in human pluripotent stem cells for motor neuron and
oligodendrocyte differentiation. Nat Protoc. 6:640–655. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takebayashi H, Yoshida S, Sugimori M,
Kosako H, Kominami R, Nakafuku M and Nabeshima Y: Dynamic
expression of basic helix-loop-helix Olig family members:
implication of Olig2 in neuron and oligodendrocyte differentiation
and identification of a new member, Olig3. Mech Dev. 99:143–148.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu QR, Sun T, Zhu Z, Ma N, Garcia M,
Stiles CD and Rowitch DH: Common developmental requirement for Olig
function indicates a motor neuron/oligodendrocyte connection. Cell.
109:75–86. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xin M, Yue T, Ma Z, Wu FF, Gow A and Lu
QR: Myelinogenesis and axonal recognition by oligodendrocytes in
brain are uncoupled in Olig1-null mice. J Neurosci. 25:1354–1365.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arnett HA, Fancy SP, Alberta JA, Zhao C,
Plant SR, Kaing S, et al: bHLH transcription factor Olig1 is
required to repair demyelinated lesions in the CNS. Science.
306:2111–2115. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li H, Lu Y, Smith HK and Richardson WD:
Oligl and Soxl0 interact synergistically to drive myelin basic
protein transcription in oligodendrocytes. J Neurosci.
27:14375–14382. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mizuno K, Hida H, Masuda T, Nishino H and
Togari H: Pretreatment with low doses of erythropoietin ameliorates
brain damage in periventricular leukomalacia by targeting late
oligodendrocyte progenitors: a rat model. Neonatology. 94:255–266.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kinney HC: The near-term (late preterm)
human brain and risk for periventricular leukomalacia: a review.
Semin Perinatol. 30:81–88. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yoon BH, Romero R, Yang SH, Jun JK, Kim
IO, Choi JH and Syn HC: Interleukin-6 concentrations in umbilical
cord plasma are elevated in neonates with white matter lesions
associated with periventricular leukomalacia. Am J Obstet Gynecol.
174:1433–1440. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murata Y, Itakura A, Matsuzawa K, Okumura
A, Wakai K and Mizutani S: Possible antenatal and perinatal related
factors in development of cystic periventricular leukomalacia.
Brain Dev. 27:17–21. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Craig A, Ling LN, Beardsley DJ,
Wingate-Pearse N, Walker DW, Hohimer AR and Back SA: Quantitative
analysis of perinatal rodent oligodendrocyte lineage progression
and its correlation with human. Exp Neurol. 181:231–240. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Iida K, Takashima S and Ueda K:
Immunohistochemical study of myelination and oligodendrocyte in
infants with periventricular leukomalacia. Pediatr Neurol.
13:296–304. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Back SA, Han BH, Luo NL, Chricton CA,
Xanthoudakis S, Tam J, et al: Selective vulnerability of late
oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci.
22:455–463. 2002.PubMed/NCBI
|
|
20
|
Yoshioka H, Goma H, Nioka S, Ochi M,
Miyake H, Zaman A, et al: Bilateral carotid artery occlusion causes
periventricular leukomalacia in neonatal dogs. Brain Res Dev Brain
Res. 78:273–278. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kinney HC and Back SA: Human
oligodendroglial development: relationship to periventricular
leukomalacia. Semin Pediatr Neurol. 5:180–189. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Levine JM, Reynolds R and Fawcett JW: The
oligodendrocyte precursor cell in health and disease. Trends
Neurosci. 24:39–47. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thordstein M, Bågenholm R, Thiringer K and
Kjellmer I: Scavengers of free oxygen radicals in combination with
magnesium ameliorate perinatal hypoxic-ischemic brain damage in the
rat. Pediatr Res. 34:23–26. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
French HM, Reid M, Mamontov P, Simmons RA
and Grinspan JB: Oxidative stress disrupts oligodendrocyte
maturation. J Neurosci Res. 87:3076–3087. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tanaka K, Nogawa S, Suzuki S, Dembo T and
Kosakai A: Upregulation of oligodendrocyte progenitor cells
associated with restoration of mature oligodendrocytes and
myelination in peri-infarct area in the rat brain. Brain Res.
989:172–179. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ross SE, Greenberg ME and Stiles CD: Basic
helix-loop-helix factors in cortical development. Neuron. 39:13–25.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Meijer DH, Kane MF, Mehta S, Liu H,
Harrington E, Taylor CM, et al: Separated at birth? The functional
and molecular divergence of OLIG1 and OLIG2. Nat Rev Neurosci.
13:819–831. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Baud O, Daire JL, Dalmaz Y, Fontaine RH,
Krueger RC, Sebag G, et al: Gestational hypoxia induces white
matter damage in neonatal rats: a new model of periventricular
leukomalacia. Brain Pathol. 14:1–10. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rezaie P and Dean A: Periventricular
leukomalacia, inflammation and white matter lesions within the
developing nervous system. Neuropathology. 22:106–132. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ligon KL, Fancy SP, Franklin RJ and
Rowitch DH: Olig gene function in CNS development and disease.
Glia. 54:1–10. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kotter MR, Li WW, Zhao C and Franklin RJ:
Myelin impairs CNS remyelination by inhibiting oligodendrocyte
precursor cell differentiation. J Neurosci. 26:328–332. 2006.
View Article : Google Scholar : PubMed/NCBI
|