Introduction
Acute respiratory distress syndrome (ARDS)/acute
lung injury (ALI) are severe clinical respiratory failure disorders
associated with refractory hypoxemia, which require patients to be
kept in intensive care (1). The
primary pathogenic risk factors for ARDS/ALI include serious
infections or traumatic injury of the respiratory tract, shock,
toxic gas inhalation and toxicity (2). The pathogenesis of ARDS/ALI has been
well documented by previous studies; however, it still remains a
significant problem that results in long-term illness and
disability with a high mortality rate of 40–60% (3). The progression of ARDS/ALI consists
of three overlapping stages: Exudative, proliferative and fibrotic
(4). Detection of pulmonary
fibrosis is used as a marker to indicate poor prognosis of ARDS/ALI
patients (5). ARDS/ALI therapy
currently focuses on repairing alveolar epithelial cells and
reducing the severity of pulmonary fibrosis (6,7).
Bleomycin-induced lung fibrosis injury was demonstrated to be
analogous to ALI in internal medicine diseases, and may therefore
be used to study novel therapeutic approaches to the treatment of
ALI in animal models.
Previous studies have indicated that mesenchymal
stem cells (MSCs) may have potential as a novel therapeutic
strategy for the treatment of ALI/ARDS (3). MSCs are multipotent cells derived
from adult tissues, which are capable of self-renewal and
multi-directional differentiation (into cell types including
chondrocytes, osteocytes and adipocytes) (8). Bone marrow mesenchymal stem cells
(BMSCs) as well as human umbilical cord mesenchymal stem cells
(uMSCs) were reported to differentiate into pulmonary epithelial
cells in vivo and in vitro; these cells were shown to
exhibit characteristics specific to lung epithelial cells (9,10).
uMSCs may be an ideal and more practical source of MSCs than BMSCs
due to their accessibility, pain-free procurement and lack of
ethical concerns (11). However,
the effectiveness of uMSC treatment for epithelial restitution and
reducing fibrosis in ARDS patients requires improvement.
Angiotensin-converting enzyme 2 (ACE2) is a human
homologue of ACE (12). ACE2 is
primarily involved in the degradation of angiotensin (Ang) II,
which results in the formation of Ang 1–7 which opposes the effects
of Ang II (13). In brief, ACE2
hydrolyzes Ang II into Ang 1–9, which can be transformed into Ang
1–7 via ACE. Ang 1–7 was reported to block the action of Ang II
through the Ang 1–7 G protein-coupled receptor, Mas (14). ACE2 was reported to prevent lung
injury resulting from acid inhalation, endotoxin shock and
septicemia; by contrast, Ang II promoted fibrosis in mice with
bleomycin-induced lung fibrosis injury (15). Current studies into the therapeutic
use of ACE2 include increasing its expression using ACE2
adenoviruses, recombinant ACE2 or compounds specific to ARDS/ALI,
thereby affording a certain level of organ protection (16–18).
In the present study, ACE2 was transfected into
uMSCs via lentiviral vectors. ACE2-modified uMSCs were injected
into mice with bleomycin-induced lung injury. The effects of
ACE2-uMSCs on lung injury restitution and reduction of fibrosis
were subsequently evaluated.
Materials and methods
Ethical approval
All human and animal experiments performed
throughout the present study were approved by the Human and Animal
Research Ethics Committees of the First People’s Hospital
affiliated to Shanghai Jiao Tong University, (Shanghai, China).
Isolation and culture of human uMSCs
Human umbilical cords were obtained, with informed
consent, from mothers following normal vaginal delivery at term
(n=12). Cords were dissected, washed with D-Hank’s buffer
(containing penicillin 100 mg/l and streptomycin 100 U/ml;
Invitrogen Life Technologies, Carlsbad, CA, USA), and the blood
vessels were removed. The remaining tissue was cut into small
pieces (1 mm2) and placed in six-well plates at
(2.5–5.0)x103/cm2 cell density in the
presence of Dulbecco’s modified Eagle’s medium supplemented with
10% fetal calf serum (DMEM/F12; Gibco-BRL, Carlsbad, CA, USA). The
cell plates were incubated at 37°C in 5% CO2 in a
humidified incubator. The medium was replaced every three days and
images were captured using an inverted microscope (Leitz Labovert
FS, Foster City, CA, USA) in order to observe the morphology. The
tissue was removed from culture and the fibroblast cells were
cultured until they reached 80% confluence in DMEM/F12. Cells were
then trypsinized (0.25% trypsin-0.02% EDTA solution, preheated to
37°C; Invitrogen Life Technologies) and re-seeded into culture
flasks at 1×104/cm2 cell density.
Determination of cell surface antigen
expression
At passage three of the logarithmic phase, cells
were harvested using 0.25% trypsin-0.02% EDTA solution. Digestion
was terminated by adding fetal calf serum (FCS; Gibco-BRL,
Carlsbad, CA, USA). Following centrifugation (1,000 rpm, 10 min),
the supernatant was discarded. Cell pellets were washed in
phosphate-buffered saline (precooled to 4°C) three times and then
suspended evenly. Cells were then incubated with primary
monocloncal antibodies, including mouse anti-human CD29, CD34,
CD44, CD45, CD86 and CD105 (BD Biosciences, San Jose, CA, USA) at a
dilution of 1:50 for 30 min, stained using mouse anti-human
fluorescein isothiocyanate (FITC) secondary monoclonal antibodies
against CD29-FITC, CD34-FITC, CD44-FITC, CD45-FITC, CD86-FITC and
CD105-FITC (BD Biosciences) at a dilution of 1:200 for 30 min, and
then analyzed using a flow cytometer (FACSCalibur; Becton
Dickinson, Franklin Lakes, NJ, USA).
Differentiation assays of human
uMSCs
At passage five of the logarithmic phase, cells were
harvested for preparation of the single-cell suspension and
re-seeded into six-well plates at 2×103/ml cell density.
Cells were propagated to 60% confluence and then the medium was
supplemented with the osteogenic induction medium, containing
β-glycerol sulfate (0.5 mmol/l), ascorbic acid (50 mg/l),
dexamethasone (0.1 μmol/l; Sigma-Aldrich, St. Louis, MO, USA) and
DMEM/F12 (Gibco-BRL), which was replaced every two days. Following
three weeks in culture, the cells were subjected to Alizarin Red
staining (ARS), Oil red O and immunofluorescent staining to detect
the expression of collagen type II (COL II) during the
differentiation into osteoblasts, adipocytes and chondrocytes.
ACE2 gene transfection into uMSCs via
lentiviral vectors
The ACE2 complementary DNA (cDNA) sequence was
amplified from total RNA with the following primers: Forward:
5′-AAGCTAGCATAGCCAGGTCCTCCTGGCTCCTTC-3′ and reverse:
5′-AAGTCGACCTAAAAGGAAGTCTGAGCATCATCACTG-3′. The amplification of
ACE2 was conducted using the following procedure: Denaturation at
98°C for 30 sec, 35 cycles at 98°C for 10 sec, 55°C for 30 sec,
72°C for 90 sec and a final step at 72°C for 10 min, using the
Reverse Transcription System. The sequences were then directionally
cloned into the lentiviral vector (Invitrogen Life Technologies).
The ACE2 lentiviral vector was co-transfected with packaging
plasmid (Invitrogen Life Technologies) and envelope plasmid into
293 T cells (Invitrogen Life Technologies).
During the logarithmic phase, uMSCs were trypsinized
and adjusted to a suitable cell density using DMEM/F12. The cells
were reseeded into 25-ml culture flasks and then cultured in the
incubator until cell density reached 2×105/ml. Cells
were subsequently transfected via viral vectors. In addition, green
fluorescent protein (GFP) was simultaneously run as a control to
label uMSCs and its expression was analyzed by fluorescence
microscopy (Nikon Eclipse TE200; Nikon Corporation, Tokyo,
Japan).
Establishment of bleomycin-induced lung
fibrosis-injury models in mice
A total of 100 C57BL/6 mice (specific pathogen-free
grade) were purchased from the Animal Laboratory of FuDan
University (Shanghai, China) and randomly divided into five groups
(n=20) as follows: Control group (received saline infusion), BLM
group (bleomycin pretreatment, received saline infusion), ACE2
group (bleomycin pretreatment, injected with ACE2-cells), uMSC
group (bleomycin pretreatment, injected with uMSCs) and ACE2-uMSC
group (bleomycin pretreatment, injected with ACE2-uMSCs). Bleomycin
(40 mg/ml/kg; Nippon Kayaku Co. Ltd., Tokyo, Japan) was
administered intratracheally in order to induce pulmonary fibrosis.
Animals were sacrificed following 7, 14 or 28 days of treatment and
all parameters were evaluated.
Pathological observation
Following 7, 14 and 28 days of treatment, five mice
from each group were sacrificed. The right lobes of the lung tissue
were fixed in 4% paraformaldehyde (Bogoo Biomart, Shanghai, China),
embedded in paraffin (Bogoo Biomart), and then stained with
hematoxylin-eosin (HE; Bogoo Biomart). Pathological changes of the
lung were observed using a light microscope (Nikon, Eclipse E200;
Nikon Corporation). Slides were scored for fibrosis using the
Ashcroft method (19).
Examination of oxidative damage
Following 14 days of treatment, five mice from each
group were sacrificed and the left lobe of the lung tissue was
dissected, weighed and ground into homogenate using saline (1:5).
Following centrifugation (3,300 × g, 10 min), the supernatant of
the lung homogenate was obtained and frozen until further use. The
levels of glutathione (GSH), glutathione disulfide (GSSG) and
malondialdehyde (MDA) were determined using the modified
fluorescence method. Briefly, the total GSH level was measured
using the method of DNTB-GSSG recycling assay, as previously
described (20). The GSSG activity
was determined by the same method of total GSH assay, once the
supernatant was pretreated with solution provided by the
manufacturers in order to remove the reduced GSH. The GSH activity
was determined by subtracting the GSSG from the total GSH. Results
are expressed as molar concentration per mg of protein (nmol/mg).
MDA activity was measured by analyzing the reaction of MDA with
thiobarbituric acid (TBA), which forms an MDA-TBA2 adduct that
absorbs strongly at 535 nm. Results are expressed as (nmol/mg).
Superoxide dismutase (SOD) levels were detected using the modified
hydroxylamine hydrochloride method as previously reported (21). SOD activity is expressed as unit
activity per ml of protein in the sample(U/ml). Commercial assay
kits were used to detect GSH, GSSG (GSH and GSSG assay kit), SOD
(superoxide dismutase assay kit) and MDA (lipid peroxidation MDA
assay kit)levels and were purchased from Westang Co., Ltd,
(Shanghai, China). The ratio of GSH/GSSG was then calculated. All
procedures were performed according to the manufacturer’s
instructions.
Expression of fibrosis factors and
pro-inflammatory factors
Following 14 days of treatment, 30 mg lung tissue
was dissected and ground into a homogenate as described above. The
supernatant of lung homogenate was used to detect the protein
levels of fibrosis factors [tumor necrosis factor (TNF)-α,
interferon (IFN)-γ and transforming growth factor (TGF)-β] and
inflammatory factors [Interleukin (IL)-1, IL-2, IL-6 and IL-10]
using an ELISA kit (ExCellBiology, Inc., Shanghai, China). All
procedures were performed according to the manufacturer’s
instructions.
ACE2 expression in lung tissue
Following 7, 14 and 28 days of treatment, 100 mg
lung tissue was dissected and ground into powder using liquid
nitrogen. The powder was lysed in an Eppendorf tube and agitated
using an oscillator every 10 min (total three times), then left to
stand for 30 min. Following centrifugation (13,900 × g, 5 min) the
supernatant was collected and stored at −80°C for further use for
western blot analysis of protein expression.
Collagen type 1 mRNA expression in lung
tissue was measured using quantitative polymerase chain reaction
(qPCR)
Total RNA was extracted from lungs of mice following
7, 14 and 28 days of treatment. The Taq-Man probe was obtained from
Applied Biosystems (Foster City, CA, USA) for the detection of
mouse collagen type 1. Cycling parameters used for qPCR were as
follows: Denaturation at 95°C for 7 sec, annealing at 60°C for 7–15
sec and extension at 72°C for 15 sec, for 40 cycles. Data were
normalized to β-actin and the expression of the control group was
calculated.
MMP activity and TIMP expression
Following 14 and 28 days of treatment, total RNA was
extracted from lung tissue. Matrix metalloproteinase (MMPs; MMP-2
and MMP-9) and tissue inhibitors of matrix metalloproteinase (TIMP;
TIMP-1, TIMP-2, TIMP-3 and TIMP-4) mRNA expression in lung tissue
was measured using qPCR. Reagents, primers and Taq-Man probes were
purchased from Applied Biosystems. Data were normalized to β-actin
and the expression of the control group was calculated. The
expression of the MMP protein was detected by immunohistochemistry.
Lung tisssue were fixed with 4% paraformaldehyde and embedded in
paraffin wax, following rinsing and dehydrated. The specimens were
sectioned and incubated with mouse anti-MMP-2 and MMP-9 antibody
(1:100 dilutions, Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
Then the specimen were incubated with peroxidase-conjugated rabbit
anti-mouse secondary antibody (Santa Cruz Biotechnology, Inc.) at a
dilution of 1:400.
Statistical analysis
Statistically significant differences between groups
were assessed using one-way ANOVA followed by the Bonferroni
post-hoc test using SPSS13.0 software for statistical analysis
(SPSS Inc., Chicago, IL, USA). Data are presented as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference between values.
Results
uMSC phenotype
At passage three of the logarithmic growth phase,
adherent fibroblast-like cells were harvested and used to detect
the expression of cell surface antigens. Fluorescence activated
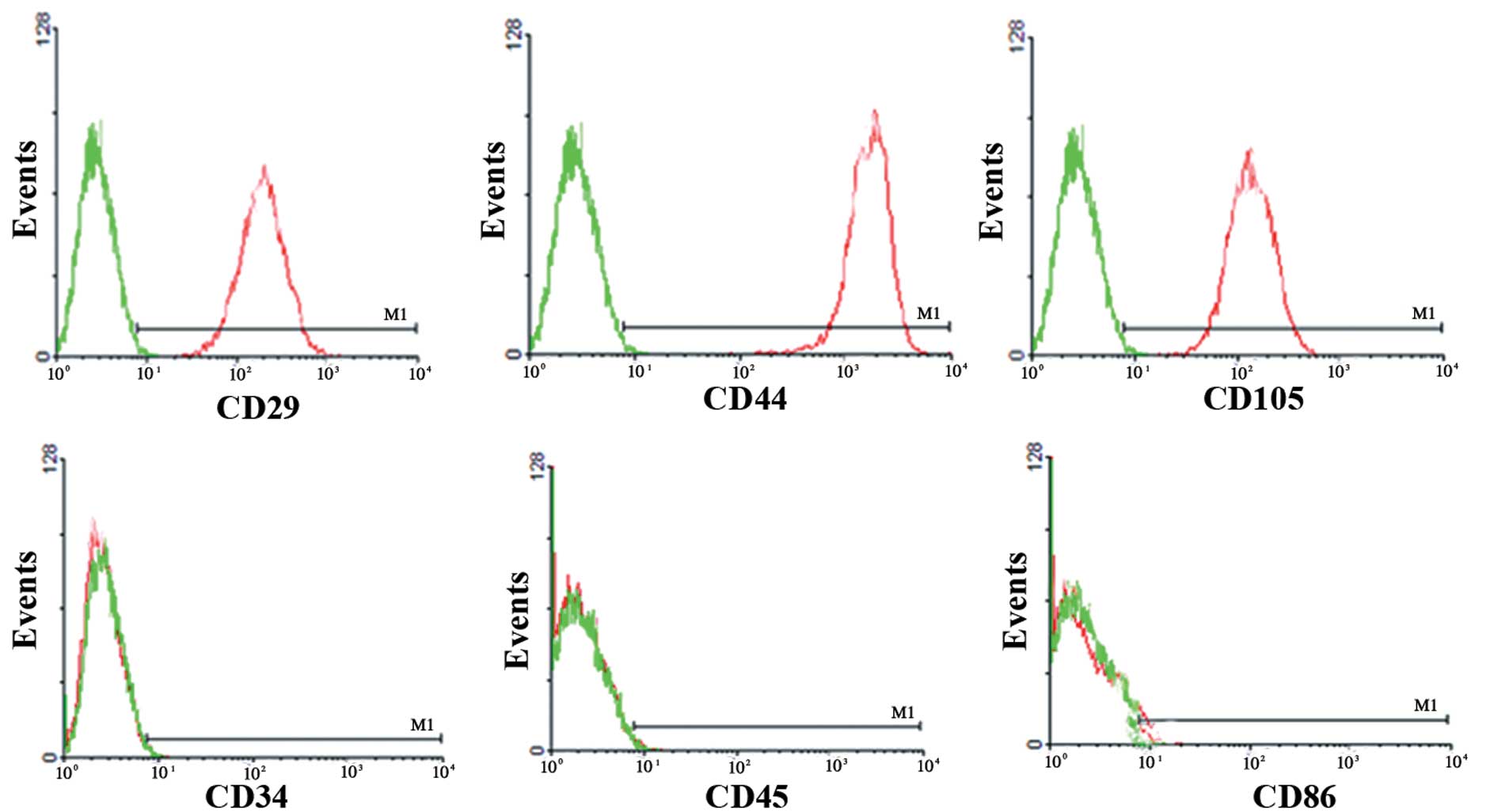
cell sorting demonstrated that uMSCs expressed the typical
mesenchymal pattern of markers, including CD29(+), CD44(+),
CD105(+), CD34(−), CD45(−), CD86(−) (Fig. 1).
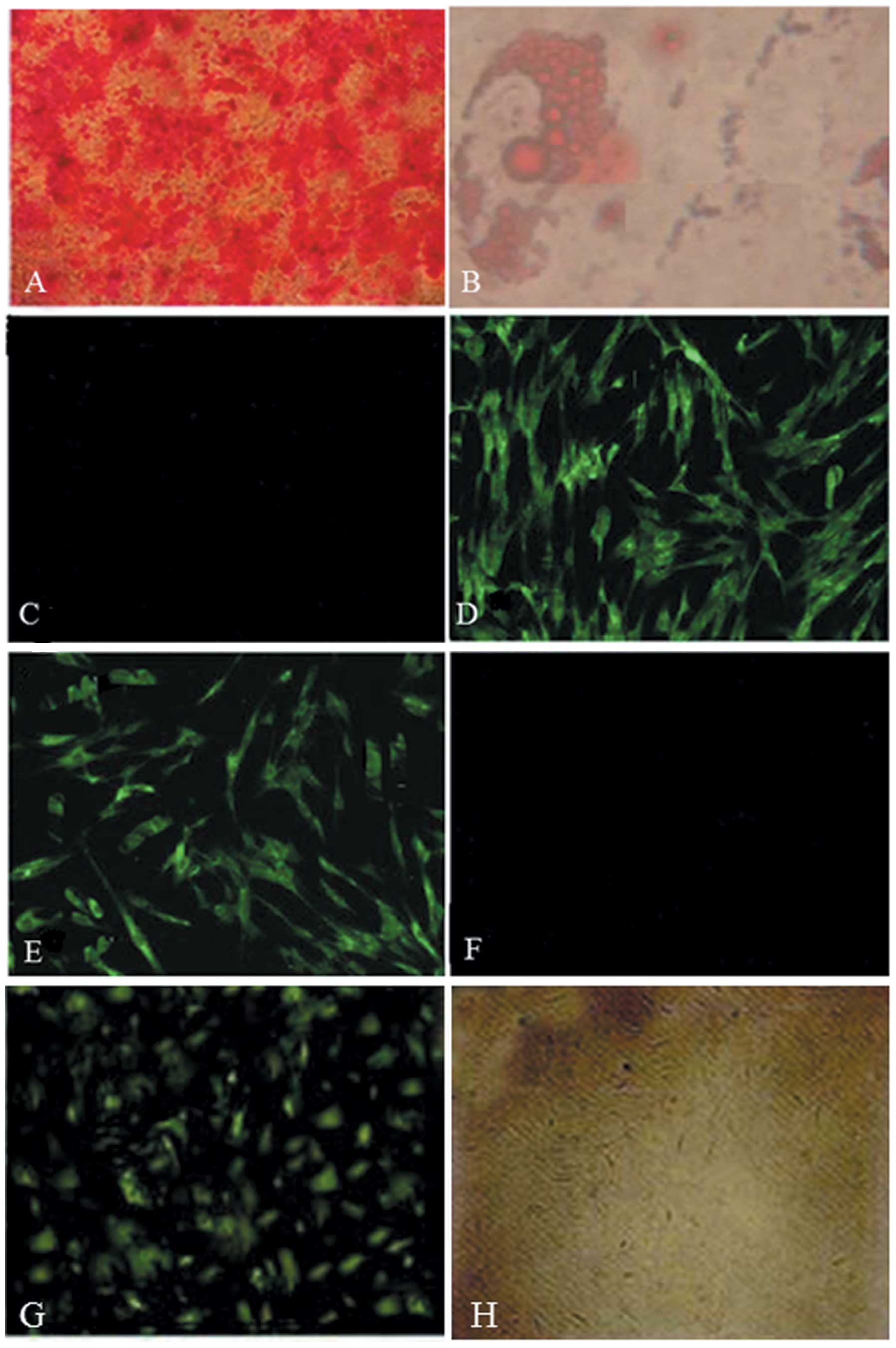
Following 3 weeks in osteogenic induction medium,
ARS revealed orange regions that represent calcium deposition and
therefore demonstrated bone nodule formation (Fig. 2A). Oil red O staining showed red
regions of intracellular lipid droplets, which indicated adipogenic
differentiation (Fig. 2B).
Immunofluorescent staining of chondrocytes demonstrated a high
expression of COL II expression following induction with osteogenic
supplements (Fig. 2C–F).
Efficiency of ACE2 gene transfection into
uMSCs via lentiviral vectors
Following 96 h of transfection, GFP detection
demonstrated the highest fluorescence intensity (Fig 2G). When the multiplicity of
infection (MOI) reached 10, the percentage of GFP-positive cells
was >90%. No lesions were detected.
ACE2-uMSCs significantly alleviate the
symptoms of bleomycin-induced lung injury
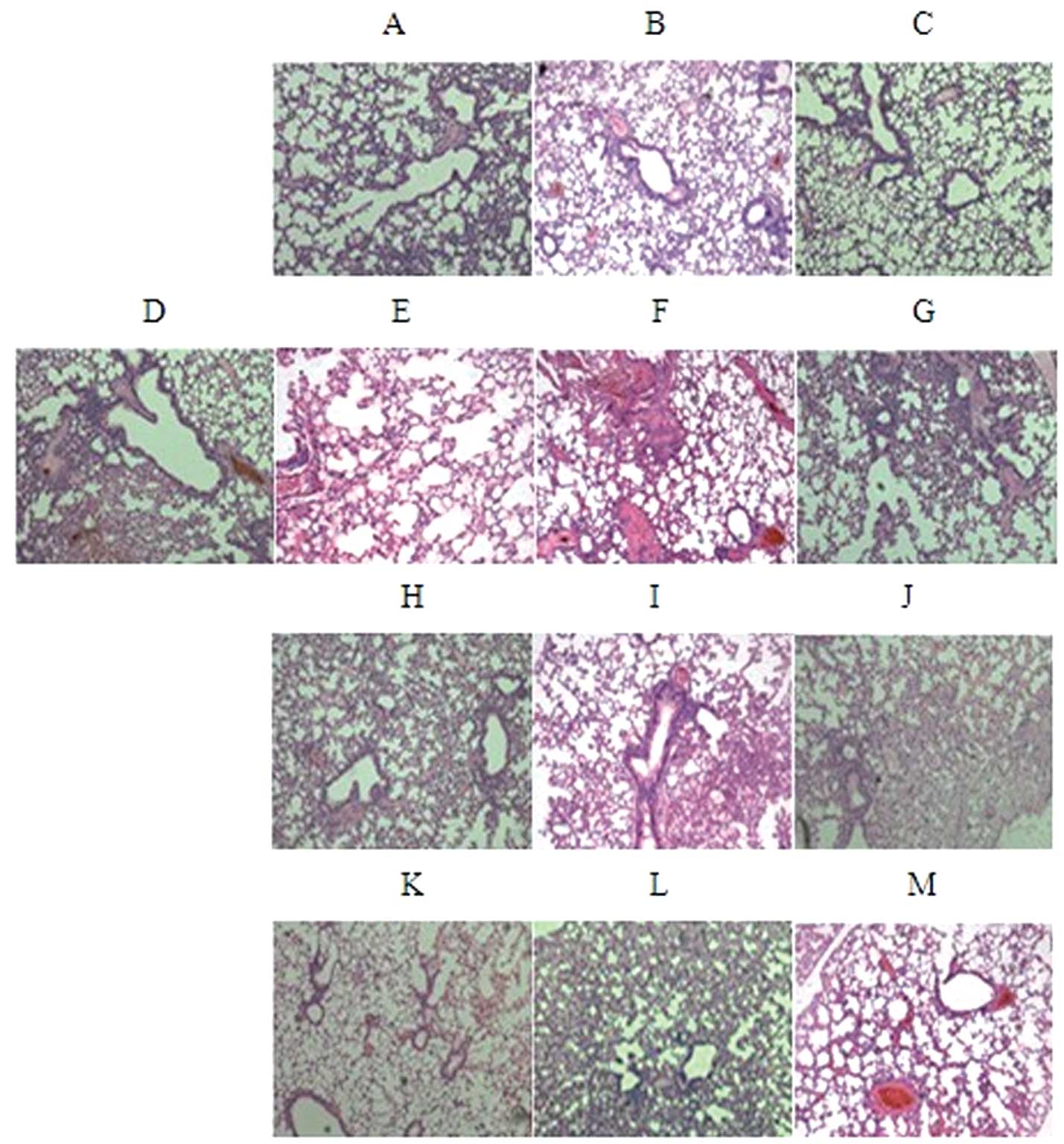
Bleomycin-induced lung fibrosis was established in
C57BL/6 mice via pretreatment with bleomycin. In each group, at 7,
14 and 28 days following therapy, HE staining of lung tissue
revealed pathological changes, including significant pneumonitis,
inflammatory exudates, fibroblastic foci and distortion of the
normal architecture of the lung (Fig
3).
The ACE2-uMSC group showed significantly alleviated
symptoms (Fig. 2A–C) compared with
those of the BLM group (Fig.
3E–G); at day 7, inflammation, hyporrhea and edema were visible
but improved; at day 14, stem cells were observed, the structure
was less distorted and collagen deposition was reduced; at day 28,
the structure of the lung tissues was clear, other symptoms were
significantly alleviated and stem cells were not observed. The
ACE2-uMSC group also showed significant therapeutic effects
compared to those of the ACE2 (Fig.
3H–J) and uMSC (Fig. 3K–M)
groups, including reduced inflammation at day 7, reduced collagen
deposition at day 14 and clear lung structure at day 28 (Fig. 3A–C). This therefore indicated that
factors from uMSCs had significantly contributed to the therapeutic
effects of ACE2-uMSCs.
In addition, the Ashcroft score of fibrosis was
progressively elevated as lung injury evolved from 7 to 28 days
following bleomycin injury (Fig.
4A). The levels of fibrosis at 14 and 28 days following
treatment were significantly reduced in the ACE2-uMSC group
compared to those of the BLM group.
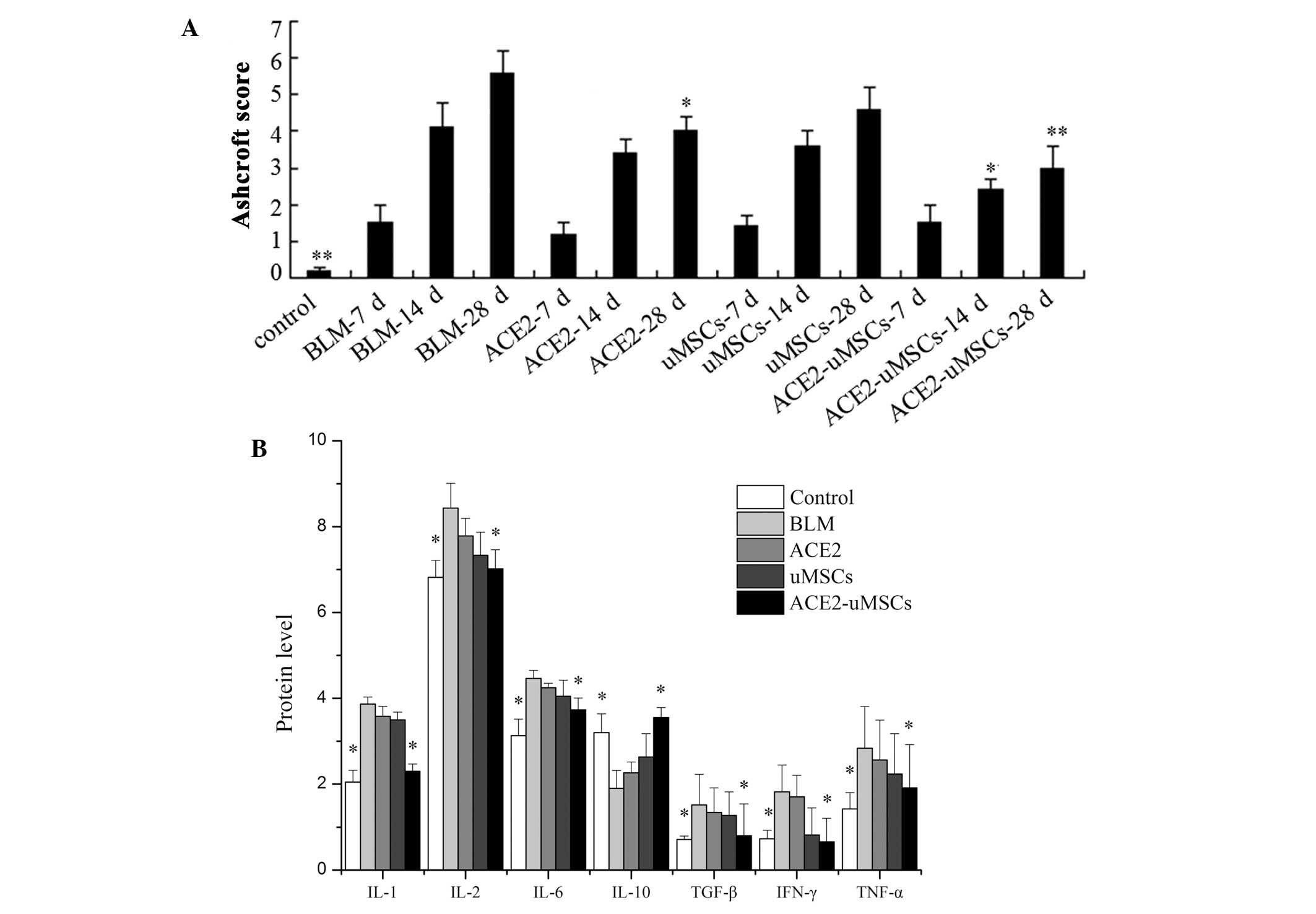 | Figure 4Evaluation of pulmonary fibrosis in
the different groups. (A) Ashcroft score of fibrosis for each group
at 7, 14 and 28 days following bleomycin-induced lung injury and
the control group. (B) Protein expression levels, as determined by
ELISA, of profibrotic and proinflammatory factors for each group 14
days following treatment. *P<0.05 and
**P<0.01 compared with BLM group. uMSCs, group
injected with human umbilical cord mesenchymal stem cells; ACE2,
group injected with angiotensin-converting enzyme 2 gene;
ACE2-uMSCs, group injected with uMSCs transfected with ACE2; BLM,
group with bleomycin-induced lung injury only; IL, interleukin;
TGF, transforming growth factor; TNF, tumor necrosis factor; IFN,
interferon. |
ACE2-uMSCs significantly alter oxidation
indexes
Following 14 days of treatment, levels of MDA, SOD,
GSH and GSSG were detected in the lung tissue of mice from each
group (Table I). The ACE2-uMSC
group showed significantly reduced MDA and GSSG levels as well as
increased levels of SOD and GSH compared to those of the BLM and
ACE2 groups (P<0.05). The ACE2-uMSC group also demonstrated
significantly reduced MDA levels and increased GSH levels compared
to those of the uMSC group (P<0.05); however, the levels of SOD
and GSSG were not significantly different. These results therefore
indicated that ACE2 alone had no significant effect on preventing
oxidative damage and that ACE2 combined with uMSCs had
statistically significant effects on the indexes of oxidative
damage compared with the BLM and ACE groups, but only altered MDA
and GSH levels significantly compared to those in the uMSC
group.
 | Table IMDA, SOD, GSH and GSSG levels of lung
tissues after 14 days of different treatments. |
Table I
MDA, SOD, GSH and GSSG levels of lung
tissues after 14 days of different treatments.
| Group | MDA (nmol/mg) | SOD (U/ml) | GSH nmol/mg) | GSSG (nmol/mg) | GSH/GSSG |
|---|
| Control | 3.1±0.05a,b,c | 30.1±2.36a,b,c | 664±20.55a,b,c | 309±10.22a,b,c | 2.14±0.04a,b,c |
| BLM | 8.3±0.11 | 16.9±1.43 | 382±12.32 | 523±18.37 | 0.73±0.02 |
| ACE2 | 7.5±0.09 | 19.3±1.33 | 412±15.87 | 501±17.79 | 0.82±0.02 |
| uMSC | 7.1±0.08 | 21.4±1.92 | 463±17.76a.b | 458±16.93a | 1.01±0.03a |
| ACE2-uMSC | 5.7±0.06a,b,c | 25.8±2.05a,b | 577±19.06a,b,c | 401±11.46a,b | 1.43±0.04a,b |
ACE2-uMSCs significantly alter the
expression of fibrosis and inflammatory factors
IL-1, IL-2, TNF-α, IFN-γ, TGF-β and IL-6 protein
levels were significantly decreased in the ACE2-uMSC group compared
with those of the BLM group (P<0.05); whereas, IL-10 was
significantly increased (P<0.05) (Fig. 4B). No significant differences in
expression of inflammation or fibrosis factors were observed in the
ACE2 and uMSC groups compared to those of the BLM group.
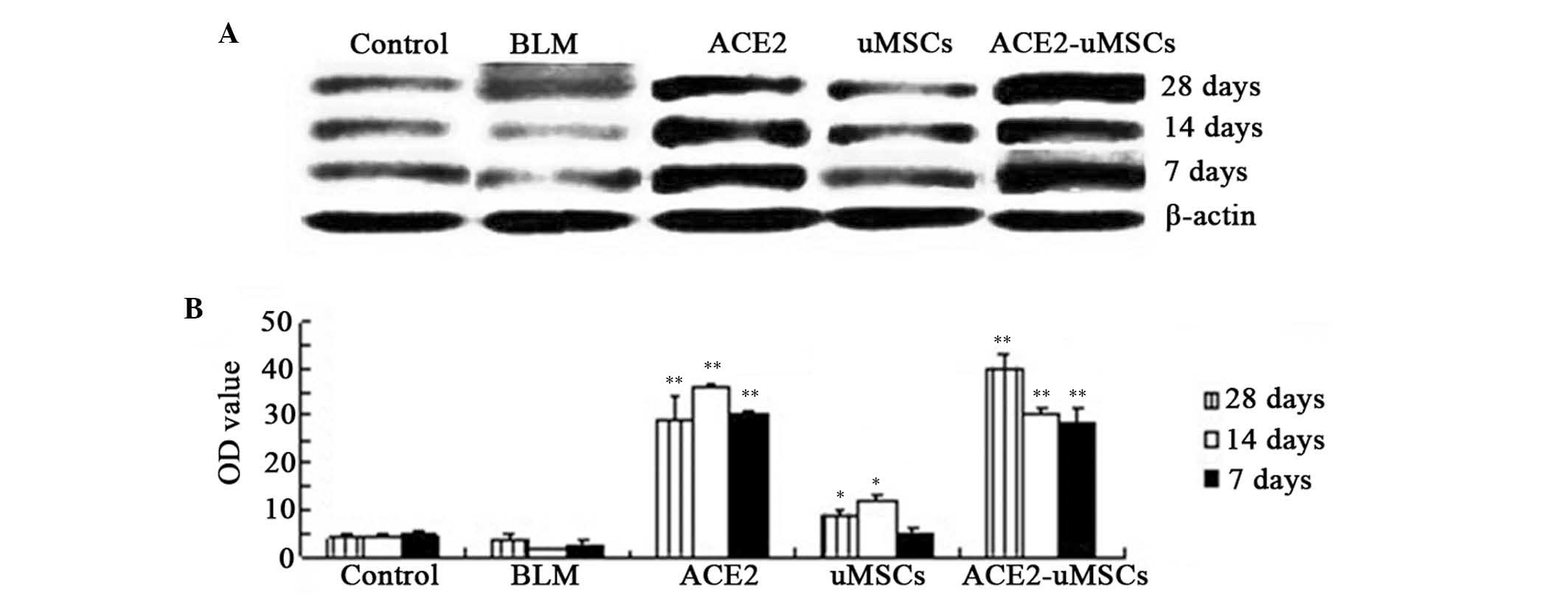
ACE2 expression in lung tissue is
significantly increased in the ACE2 and ACE2-uMSC groups
Western blot and densitometric analyses were used to
detect the protein expression levels of ACE2 in lung tissue from
each group at 7, 14 and 28 days following treatments (Fig. 5). ACE2 expression levels were
significantly increased at 7, 14 and 28 days in the ACE2 group as
well as the ACE2-uMSC group compared to those of the BLM group
(P<0.01) (Fig. 5A and B). Of
note, the uMSC group demonstrated significantly increased ACE2
expression levels at 14 and 28 days compared to those of the BLM
group (P<0.05). In the ACE2-uMSC group, ACE2 expression levels
at each time-point were higher than those of the BLM group and also
had a larger increase than those in the ACE2 and uMSC groups at 28
days.
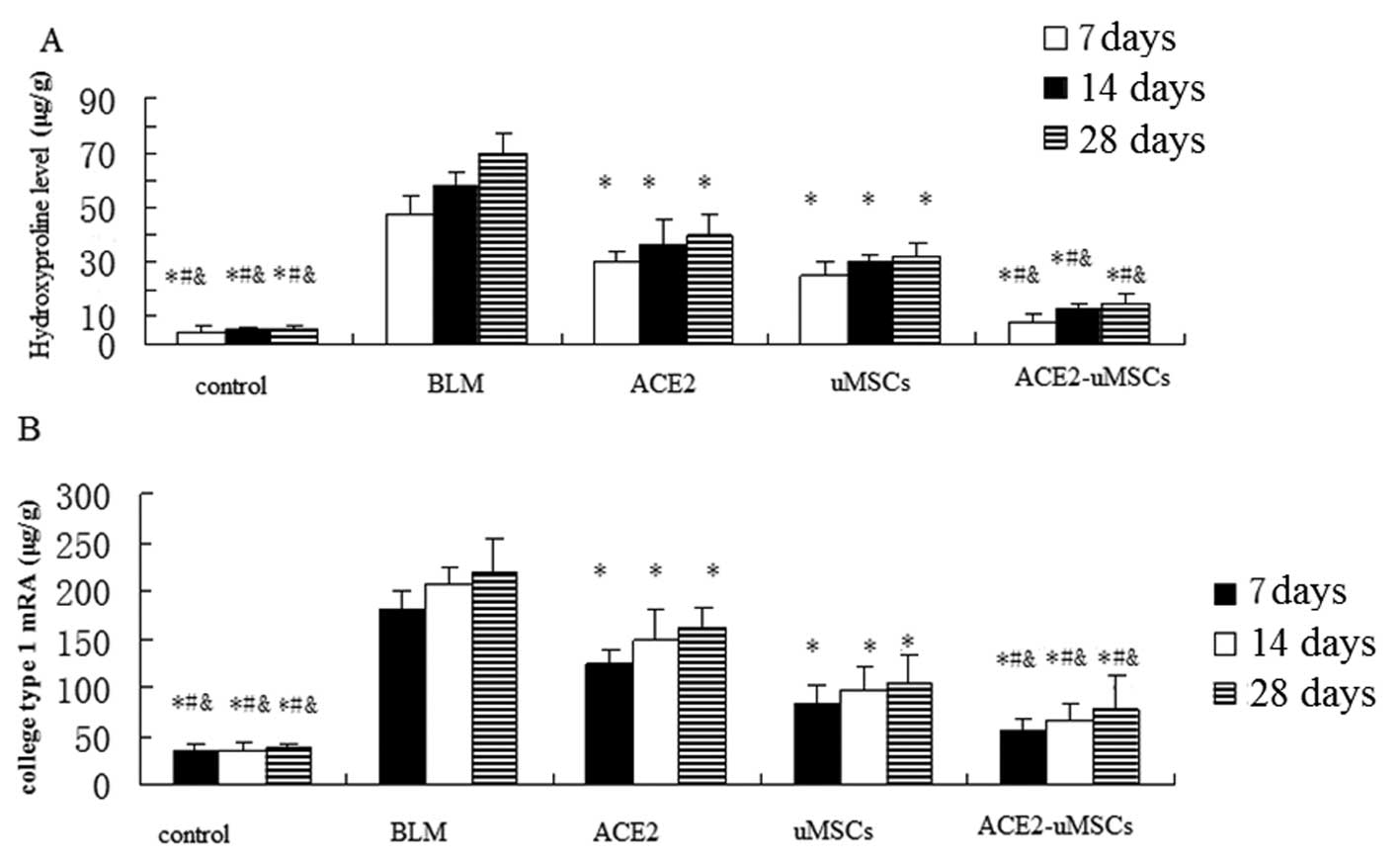
Collagen deposition is significantly
reduced by ACE2 and uMSC treatment individually and in
combination
Collagen deposition was assessed using a
hydroxyproline assay performed on lung tissues at 7, 14 and 28 days
following treatment (Fig. 6A).
Compared with the BLM, ACE2 and uMSC groups, the administration of
ACE2-uMSC decreased expression of hydroxyproline and reduced
collagen deposition. In addition, the ACE2 and uMSC groups
significantly attenuated collagen deposition compared with the BLM
group (P<0.05).
Collagen type 1 mRNA was analyzed using qPCR in
order to determine that collagen downregulation was due to reduced
synthesis. As shown in Fig. 6B,
collagen type 1 mRNA was significantly reduced in mice from the
ACE-uMSC group compared to that of the BLM, ACE2 and uMSC groups;
furthermore, the ACE2 and uMSC groups significantly attenuated
collagen type 1 mRNA expression compared with that of the BLM
group. This confirmed that the reduction of lung hydroxyproline
levels was due to reduced collagen type 1 synthesis.
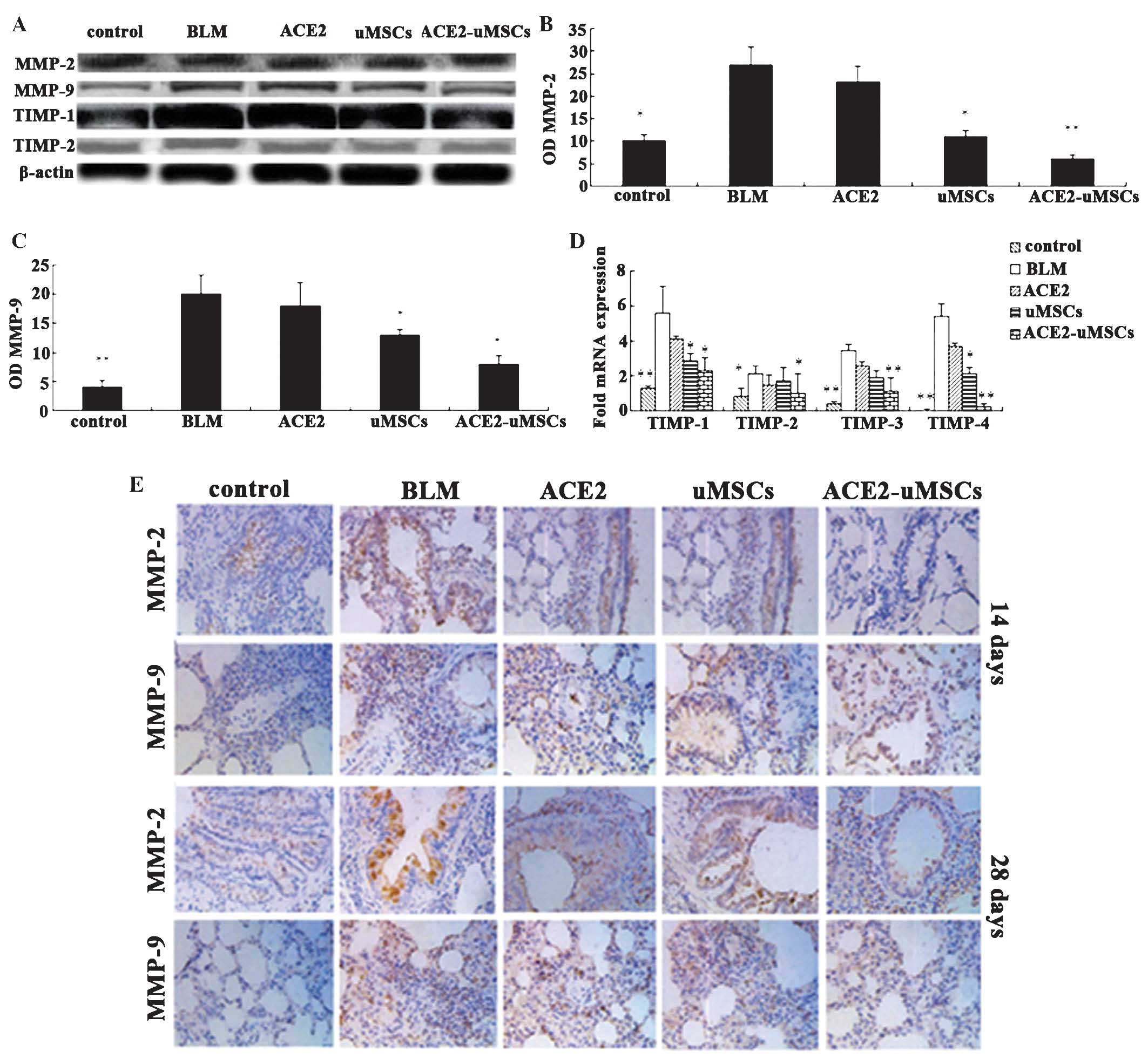
Regulation of MMPs and TIMPs
The expression of MMPs (MMP-2 and MMP-9) in whole
lung homogenates was determined at 14 and 28 days following
treatment (Fig. 7A–C). MMP-2
expression levels were significantly decreased in the ACE2-uMSC and
uMSC groups compared to those of the BLM group (P<0.01 and
P<0.05, respectively). MMP-9 expression in the five groups was
comparable with MMP-2 expression. MMP-9 expression in the ACE2-uMSC
and uMSC groups was significantly decreased compared to that of the
BLM group (P<0.05).
Immunohistochemical analysis demonstrated different
histological expression of MMP-2 and MMP-9 (Fig. 7E). Expression of MMPs was
significantly increased in the BLM group compared to that of the
control group. Following ACE2-uMSC treatment, MMP expression was
significantly decreased compared with that in the BLM group. MMP
expression levels at 28 days were higher than those at 14 days.
The corresponding expression of TIMPs was
examined in lung tissues
Transcripts of TIMPs 1–4 were quantified relative to
the β-actin control for each group (Fig. 7A and D). uMSC and ACE2-uMSC
treatments resulted in reduced expression of TIMP-1 (P<0.05) and
TIMP-4 (P<0.05 and P<0.01, respectively) compared with that
of the BLM group. Additionally, ACE2-uMSC treatment significantly
downregulated the expression of TIMP-2 and TIMP-3 compared to that
of the BLM group (P<0.05 and P<0.01, respectively). Western
blot analysis of TIMP-1 and TIMP-2 expression levels produced
comparable results (Fig. 7A).
TIMP-1 and TIMP-2 expression levels were significantly increased in
the BLM group compared to those of the control group; by contrast,
ACE2-uMSC treatment reduced TIMP-1 and TIMP-2 expression levels
significantly.
Discussion
MSCs, first proposed by Cohnheim (22) in 1867, are a type of adult stem
cells from bone marrow and named by Friedenstein et al
(23). Following delivery of an
infant, uMSCs are easily collected and cultured from the umbilical
cord (24). To date, the clinical
application of uMSCs for the treatment of ARDS/ALI is limited and
the therapeutic effect of uMSCs on epithelial restitution and
fibrosis reduction of ARDS has not yet been elucidated, to the best
of our knowledge.
Previous studies have demonstrated that ACE2 was
involved in the pathological processes of numerous lung diseases
(25,26). Following the induction of an
identical pathogenic environment in vivo, ACE2 deletion
caused severe ALI in mice, which was reported to be alleviated by
injection of ACE2. This therefore indicated that ACE2 acted as a
negative regulator of ALI and significantly protected lung tissues
(27,28). Microinjection of purified,
recombinant ACE2 was reported to reduce collagen deposition,
indicating that ACE2 inhibited the formation of pulmonary fibrosis
(29). However, to the best of our
knowledge, the effect of uMSCs in combination with ACE2 on lung
injury and pulmonary fibrosis has not yet been studied. The present
study aimed to explore a novel approach for the effective treatment
of lung injury and pulmonary fibrosis by transfecting ACE2 into
uMSCs.
The results of the present study showed that
following bleomycin-induced lung injury, uMSCs were only detected
in fibrotic regions at 14 days post-uMSC injection (not detected at
28 days); this therefore indicated that damaged tissues attracted
and retained these uMSCs. A previous study reported the transient
presence of uMSCs, verifying that the repair of lung injury was
consistent with that of other organs and highlighted the importance
of trophic factors produced by uMSCs in tissue repair (30). Following ACE2-uMSC injection,
expression levels of MDA, GSSG, SOD and GSH were significantly
altered compared to those of the BLM group as well as the ACE2 and
uMSC groups. The indexes of oxidative damage showed that ACE2-uMSCs
effectively reduced the elevated levels of inflammatory oxygen
radicals following bleomycin-induced lung injury and demonstrated
protective effects on lung tissues. This therefore indicated that
ACE2 and uMSCs had a synergistic effect, which was significantly
more effective than using ACE2 or uMSCs individually.
To explore the molecular mechanism of the protective
ffect of ACE2-uMSC treatment, levels of fibrosis factors (TNF-α,
IFN-γ, TGF-β) and inflammatory factors (IL-1, IL-2, IL-6 and IL-10)
were determined. In the ACE2 and uMSC groups, the expression levels
of inflammatory and fibrosis factors was not significantly
different to those of the BLM group. However, previous studies have
reported the downregulation of TGF-β by uMSCs, which was associated
with reduced collagen deposition (31). The ACE2-uMSC group demonstrated
significantly altered expression of all factors examined;
therefore, it was suggested that ACE2-uMSC injection inhibited
pulmonary fibrosis through downregulation of fibrosis inflammatory
factors. Furthermore, the degree of collagen deposition (collagen
type 1 mRNA and hydroxyproline) and fibrosis were significantly
reduced 28 days following ACE2-uMSC injection compared to that of
the BLM group. Individual injections of ACE2 and uMSCs also reduced
collagen deposition compared to that of the BLM group; however,
injection of ACE2-uMSCs was found to be significantly more
effective. The reduction of TGF-β levels and collagen deposition is
comparable to that of a previous study, which reported an
associated between increased TGF-β levels and the pathogenesis of
fibrosis (32).
MMPs and their endogenous inhibitors (TIMPs) are
primarily responsible for the degradation of extracellular matrix
proteins such as collagen (33).
MMP-2 and MMP-9 were downregulated following ACE2-uMSC treatment in
bleomycin-injured mice, contrary to previous studies (31). This difference may be due to the
source of MSCs and the mouse strain (C57BL/6) used. In addition,
Ortiz et al (25) reported
the downregulation of MMP-2 by murine BMSCs following
bleomycin-induced fibrosis. In the present study MMP-2 expression
levels were significantly decreased in the ACE2-uMSC group,
compared to those of the BLM and uMSC groups. Previous studies have
indicated MSCs may reduce pulmonary fibrosis via inhibition of MMP
expression (34,35). In addition, ACE2-uMSCs
significantly reduced the expression of TIMP1–4, which was
concurrent with the results reported by a previous study (36). In addition, ACE2-uMSCs were found
to be more effective in the reduction of TIMP expression than ACE2
or uMSCs alone. In the ACE2-uMSC group, the MMP-2/TIMP-2 ratio was
relatively balanced, which may have promoted the protection of
injury-induced pulmonary fibrosis.
In conclusion, injection of ACE2-uMSC demonstrated
significantly more effective results in the treatment of
bleomycin-induced pulmonary fibrosis in vivo compared to
those of the ACE2 and uMSC treatments alone. The results of the
present study therefore suggested that the synergistic effect of
ACE2 and uMSCs may be used as a promising novel treatment for lung
injury.
References
|
1
|
Bakowitz M, Bruns B and McCunn M: Acute
lung injury and the acute respiratory distress syndrome in the
injured patient. Scand J Trauma Resusc Emerg Med. 20:542012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wada T, Jesmin S, Gando S, et al: The role
of angiogenic factors and their soluble receptors in acute lung
injury (ALI)/acute respiratory distress syndrome (ARDS) associated
with critical illness. J Inflamm (Lond). 10:62013. View Article : Google Scholar
|
|
3
|
Hayes M, Curley G, Ansari B and Laffey JG:
Clinical review: Stem cell therapies for acute lung injury/acute
respiratory distress syndrome - hope or hype? Crit Care.
16:2052012. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Walkey AJ, Summer R, Ho V and Alkana P:
Acute respiratory distress syndrome: epidemiology and management
approaches. Clin Epidemiol. 4:159–169. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kumar P, Goldstraw P, Yamada K, et al:
Pulmonary fibrosis and lung cancer: risk and benefit analysis of
pulmonary resection. J Thorac Cardiovasc Surq. 125:1231–1237.
2003.
|
|
6
|
Perkins GD, Gao F and Thickett DR: In vivo
and in vitro effects of salbutamol on alveolar epithelial repair in
acute lung injury. Thorax. 63:215–220. 2008. View Article : Google Scholar
|
|
7
|
Corbel M, Belleguic E, Biochot V and
Lagente V: Involvement of gelatinases (MMP-2 and MMP-9) in the
development of airway inflammation and pulmonary fibrosis. Cell
Biol Toxicol. 18:51–61. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hayes M, Curley G and Laffey JG:
Mesenchymal stem cells - a promising therapy for acute respiratory
distress syndrome. F1000 Med Rep. 4:22012.PubMed/NCBI
|
|
9
|
Krause DS, Theise ND, Collector MI, et al:
Multi-organ, multi-lineage engraftment by a single bone
marrow-derived stem cell. Cell. 105:369–377. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li J, Li D, Liu X, Tang S and Wei F: Human
umbilical cord mesenchymal stem cells reduce systemic inflammation
and attenuate LPS-induced acute lung injury in rats. J Inflamm
(Lond). 9:332012. View Article : Google Scholar
|
|
11
|
Fong CY, Gauthaman K, Cheyyatraivendran S,
Lin HD, Biswas A and Bongso A: Human umbilical cord Wharto’s jelly
stem cells and its conditioned medium support hematopoietic stem
cell expansion ex vivo. J Cell Biochem. 113:658–668. 2012.
View Article : Google Scholar
|
|
12
|
Donoghue M, Hsieh F, Baronas E, et al: A
novel angiotensin-converting enzyme-related carboxypeptidase (ACE2)
converts angiotensin I to angiotensin 1–9. Circ Res. 87:E1–E9.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tikellis C and Thomas M:
Angiotensin-converting enzyme 2 (ACE2) is a key modulator of the
renin angiotensin system in health and disease. Int J Pept.
2012:2562942012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Uhal BD, Li X, Xue A, Gao X and
Abdul-Hafez A: Regulation of alveolar epithelial cell survival by
the ACE-2/angiotensin 1–7/Mas axis. Am J Physiol Lung Cell Mol
Physiol. 301:L269–L274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oudit GY, Kassiri Z, Patel MP, et al:
Angiotensin II-mediated oxidative stress and inflammation mediate
the age-dependent cardiomyopathy in ACE2 null mice. Cardiovasc Res.
75:29–39. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lovren F, Pan Y, Quan A, et al:
Angiotensin converting enzyme-2 confers endothelial protection and
attenuates atherosclerosis. Am J Physiol Heart Circ Physiol.
295:H1377–H1384. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Treml B, Neu N, Kleinsasser A, et al:
Recombinant angiotensin-converting enzyme 2 improves pulmonary
blood flow and oxygenation in lipopolysaccharide-induced lung
injury in piglets. Crit Care Med. 38:596–601. 2010. View Article : Google Scholar
|
|
18
|
Imai Y, Kuba K, Rao S, et al:
Angiotensin-converting enzyme 2 protects from severe acute lung
failure. Nature. 436:112–116. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ashcroft T, Simpson JM and Timbrell V:
Simple method of estimating severity of pulmonary fibrosis on a
numerical scale. J Clin Pathol. 41:467–470. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baker MA, Cerniglia GJ and Zaman A:
Microtiter plate assayfor the measurement of glutathione and
glutathione disulfide in large numbers of biological samples. Anal
Biochem. 190:360–365. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shimoda-Matsubayashi S, Hattori T,
Matsumine H, et al: Mn SOD activity and protein in a patient with
chromosome 6-linked autosomal recessive parkinsonism in comparison
with Parkinson’s disease and control. Neurology. 49:1257–1262.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cohnheim J: Ueber die endigung der
sensiblen nerven in der hornhaut. Virchows Arch. 38:343–386. 1867.
View Article : Google Scholar
|
|
23
|
Friedenstein AJ, Chailakhyan RK and
Gerasimov UV: Bone marrow osteogenic stem cells: in vitro
cultivation and transplantation in diffusion chambers. Cell Tissue
Kinet. 20:263–272. 1987.PubMed/NCBI
|
|
24
|
Secco M, Zucconi E, Vieira NM, et al:
Multipotent stem cells from umbilical cord: cord is richer than
blood! Stem Cells. 26:146–150. 2008. View Article : Google Scholar
|
|
25
|
Imai Y, Kuba K and Penninger JM:
Angiotensin-converting enzyme 2 in acute respiratory distress
syndrome. Cell Mol Life Sci. 64:2006–2012. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamamoto K, Ohishi M, Katsuya T, et al:
Deletion of angiotensin-converting enzyme 2 accelerates pressure
overload-induced cardiac dysfunction by increasing local
angiotensin II. Hypertension. 47:718–726. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li X, Molina-Molina M, Abdul-Hafez A, Uhal
V, Xaubet A and Uhal BD: Angiotensin converting enzyme-2 is
protective but downregulated in human and experimental lung
fibrosis. Am J Physiol Lung Cell Mol Physiol. 295:L178–L185. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zisman LS, Keller RS, Weaver B, et al:
Increased angiotensin-1–7-forming activity in failing human heart
ventricles: evidence for upregulation of the angiotensin-converting
enzyme homologue ACE2. Circulation. 108:1707–1712. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rey-Parra GJ, Vadivel A, Coltan L, et al:
Angiotensin converting enzyme 2 abrogates bleomycin-induced lung
injury. J Mol Med (Berl). 90:637–647. 2012. View Article : Google Scholar
|
|
30
|
Horwitz EM, Prockop DJ, Fitzpatrick LA, et
al: Transplantability and therapeutic effects of bone
marrow-derived mesenchymal cells in children with osteogenesis
imperfecta. Nat Med. 5:309–313. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moodley Y, Atienza D, Manuelpillai U, et
al: Human umbilical cord mesenchymal stem cells reduce fibrosis of
bleomycin-induced lung injury. Am J Pathol. 175:303–313. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Verrecchia F and Mauviel A: Transforming
growth factor-beta and fibrosis. World J Gastroenterol.
13:3056–3062. 2007.PubMed/NCBI
|
|
33
|
Parks WC: Matrix metalloproteinases in
lung repair. Eur Respir J. Suppl 44:36s–38s. 2003. View Article : Google Scholar
|
|
34
|
Ortiz LA, Gambelli F, McBride C, et al:
Mesenchymal stem cell engraftment in lung is enhanced in response
to bleomycin exposure and ameliorates its fibrotic effects. Proc
Natl Acad Sci USA. 100:8407–8411. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yaguchi T, Fukuda Y, Ishizaki M and
Yamanaka N: Immunohistochemical and gelatin zymography studies for
matrix metalloproteinases in bleomycin-induced pulmonary fibrosis.
Pathol Int. 48:954–963. 1998. View Article : Google Scholar
|
|
36
|
Oggionni T, Morbini P, Inghilleri S, et
al: Time course of matrix metalloproteases and tissue inhibitors in
bleomycin-induced pulmonary fibrosis. Eur J Histochem. 50:317–325.
2006.
|