Introduction
The natural product resveratrol was found to exhibit
a diverse range of biological activities in diseases associated
with oxidative stress (1). As a
polyphenolic natural product, resveratrol is automatically
synthesized by plants in response to fungal attack or exposure to
ultraviolet light (2). In the
numerous plants and organs resveratrol is produced by, it is mainly
localized to the skin and seeds of purple grapes and peanuts
(3). In particular, resveratrol is
an active polyphenolic component present in red wine and numerous
plants, which have multiple potential therapeutic benefits in the
treatment of cancer, inflammation, metabolic disorders and
neurological disorders. Studies have indicated that cognitive
degeneration may be attenuated by regular red wine consumption, in
which resveratrol contributes to the therapeutic effects (4,5).
Resveratrol is involved in anti-inflammatory,
anti-oxidant, anti-cancer and anti-aging processes in multiple
organisms. For example, resveratrol supplementation reduced aortic
atherosclerosis and calcification and attenuated loss of aerobic
capacity in a mouse model of uremia (6). In respiratory syncytial virus
infection, resveratrol was reported to inhibit the
Toll/interleukin-1 receptor-domain-containing adapter-inducing
interferon-β-dependent pathway by upregulating sterile alpha and
armadillo motif protein and thereby contributing to the
anti-inflammatory effects observed (7). In adipose tissue metabolism,
resveratrol increased brown adipose tissue thermogenesis markers by
increasing sirtuin 1 (SIRT1) expression and energy expenditure, and
decreasing fat accumulation in the adipose tissue of mice fed a
standard diet (8). Recently,
resveratrol has received attention in the field of neuroscience due
to its neuroprotective potential (2). In stroke and Huntington’s disease,
resveratrol was reported to exert neuroprotective effects (9). Resveratrol was also found to protect
neurons against 1-methyl-4-phenylpyridine ion, peroxide and β
amyloid (Aβ) injury (10–12). Furthermore, it was reported that in
a rat model of Alzheimer’s disease (AD), resveratrol was able to
prevent cognitive impairment (13). Therefore, resveratrol potentially
has a pivotal role in protecting neurons against damage.
p53, a known tumor suppressor, induces cell cycle
arrest and apoptotic cell death in response to DNA damage. p53
transcriptionally activates its downstream target genes, including
p21 for cell-cycle arrest and B-cell lymphoma-2 protein
(Bcl-2)-associated X protein (Bax) for apoptosis (14,15),
whereas in mitochondria, p53-mediated apoptosis influences its own
transcriptional activity as well as Bcl-2 family members (16). p53 is regulated by
post-translational modifications, including phosphorylation,
ubiquitination and acetylation (17), where the acetylation of p53
augments its DNA binding affinity (18). These results supported the
hypothesis that modulation of the deacetylation or acetylation of
p53 had a profound effect on p53 stability, as well as function.
The balance of acetylation and deacetylation of p53 may be an
important target in the prevention or treatment of disease.
The p53 protein has multiple acetylation sites, and
its hyperacetylation is stabilized and activated endogenously to
trigger apoptosis (17,19). In the present study, the
acetylation level of p53 in response to resveratrol treatment was
assessed. As a toxic factor, Aβ(25–35) triggers the development of
multiple degenerative diseases of the nervous system and its
aggregation has an important role in the initiation of the
pathogenesis of such diseases (20). In the present study, the
neuroprotective role of resveratrol in a toxic cell model using
PC12 cells that were exposed to Aβ(25–35) injury was assessed.
Subsequently, whether the neuroprotective role of resveratrol was
due to the inhibition of apoptosis in PC12 cells was evaluated.
Furthermore, the present study aimed to elucidate the role of p53
acetylation levels in resveratrol-mediated inhibition of apoptosis
in PC12 cells.
Materials and methods
Cells and cell culture
The PC12 cell line was obtained from the Cell Bank
at the Chinese Academy of Sciences (Shanghai, China). Cells were
maintained in Dulbecco’s modified Eagle’s medium (DMEM; HyClone, GE
Healthcare, Little Chalfont, UK) containing 10% fetal bovine serum
(FBS; HyClone) at 37°C in a humidified atmosphere of 5%
CO2.
Reagents
Primary antibodies against Bax, Bcl-2 and caspase-3
were all purchased from Santa Cruz Biotechnology Inc. (Dallas, TX,
USA). For the detection of transcriptional modification, primary
antibodies against p53 (100 μl, No. 9282S) were purchased from Cell
Signaling Technology, Inc. (Boston, MA, USA). Aβ(25–35),
resveratrol, pifithrin-α and dimethyl sulfoxide (DMSO) were
commercially obtained from Sigma-Aldrich (St. Louis, MO, USA).
Aβ(25–35) was prepared as described previously (21). In brief, resveratrol was dissolved
in DMSO at a concentration of 100 mM to produce a stock solution
and stored at −20°C. The stock solution was diluted to 5 mM in
serum-free DMEM prior to use and the working solution was further
diluted with DMEM to the required concentrations. Aβ(25–35) was
dissolved in deionized distilled water and subsequently filtered
(0.22 mm filter; EMD Millipore, Billerica, MA, USA). The solution
was aged by incubating at 37°C for one week and subsequently stored
at −20°C.
Experimental design
PC12 cells were cultured in 12-well plates at a
density of 5×104 cells/cm2 and then divided
into four distinct groups for treatment: i) PC12 cells
cultured in DMSO without Aβ(25–35) and resveratrol treatments
(control); ii) PC12 cells cultured in DMSO and treated with
20 mM final concentration of Aβ(25–35) [Aβ(25–35) group];
iii) PC12 cells cultured in DMSO and treated with
resveratrol (resveratrol group); iv) PC12 cells cultured in
DMSO and treated with 20 mM Aβ(25–35) and resveratrol [resveratrol
+ Aβ(25–35) group].
The cell culture medium was refreshed every three
days. The highest DMSO concentration, which had no impact on the
cell viability in the culture medium, was 0.1%. Forty-eight hours
after exposure to resveratrol treatment, cells were digested with
trypsin and washed with cold phosphate-buffered saline (PBS;
21–040-CM; Mediatech, Inc., Manassas, VA, USA) three times for
subsequent analysis.
Cell viability assay
Cell viability was assessed via colorimetric assay
using the Cell Counting kit-8 (CCK-8 kit; MAB5963; Abnova, Taipei,
Taiwan). Briefly, PC12 cells were washed with PBS and suspended at
a final concentration of 4×104 cells/ml in an assay
medium and dispensed into 96-well plates. CCK-8 solution was added
to cells in each well to a final concentration of 0.5 mg/ml and
incubated at 37°C for 5 h. Then the medium was gently aspirated and
DMSO was added to each well in order to dissolve the formazan
product at room temperature. The absorbance of each sample at a
wavelength of 490 nm (A490) was detected using a synergy 2
multi-mode microplate reader (Bio-Tek Instruments, Winooski, VT,
USA). Experiments were performed in triplicate and cell viability
was quantified based on the A490 value.
Western blot analysis
PC12 cells were harvested and lysed using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Nantong, China). Total proteins were extracted and
quantified using a bicinchoninic acid kit (Boster Biological
Technology, Wuhan, China). Subsequently, 50 μg proteins were
fractionated using 10% SDS-PAGE (GE Healthcare, Logan, UT, USA) and
electro-transferred onto nitrocellulose (NC) membranes (Bioleaf
Biotech, Shnghai, China; in an ice-water bath. NC membranes were
blocked with 5% skimmed milk in Tris buffer (Sigma-Aldrich)
containing 0.1% Tween-20 and then incubated with the following
rabbit monoclonal primary antibodies at 4°C overnight: Anti-Bax
(sc-526), anti-Bcl-2 (sc-492), anti-extracellular-signal-regulated
kinase (ERK; sc-292838), anti-phosphorylated (p)-ERK (sc-13073),
anti-caspase-3 (all Santa Cruz Biotechnology Inc.), and anti-p53
(9282S), anti-Akt (9272) and anti-p-Akt (9275) (Cell Signaling
Technology, Inc.) (all 1:1,000 dilution). Subsequently, the blots
were washed and incubated with goat anti-rabbit horseradish
peroxidase-conjugated immunoglobulin G secondary antibody (Santa
Cruz Biotechnology) at room temperature for 1 h. Finally, blots
were visualized with enhanced chemiluminescence reagent (EMD
Millipore).
Cell apoptosis analysis
PC12 cells in each group were stained with propidium
iodide at a concentration of 10 mg/ml for 20 min. Cells were then
labeled and observed under an LSM 780 Laser Scanning Confocal
Microscope (Carl Zeiss AG, Oberkochen, Germany). In order to
further distinguish early- from late-stage apoptosis and perform a
quantitative analysis, flow cytometry with Annexin V-fluorescein
isothiocyanate (FITC) staining was employed as previously described
(19). Briefly, PC12 cells were
diffused with 0.05% trypsin (Pierce Biotechnology Inc., Rockford,
IL, USA), centrifuged at 189 × g for 5 min and then washed twice
with sterile PBS. Subsequently, binding buffer (Fermentas, Thermo
Fisher Scientific, Waltham, MA, USA) was added to cells and
6×105 cells were re-suspended in the buffer, following
which they were stained with Annexin V-FITC (Bioteool, Houston, TX,
USA) for 15 min in the dark at room temperature. Finally, the
fluorescence of each group was determined by flow cytometry
(653158, BD Biosciences, Franklin Lakes, NJ, USA).
Statistical analysis
Values are expressed as the mean ± standard error of
the mean of three independent experiments. Student’s t-test was
used for quantitative data analysis. SPSS version 16.0 (SPSS, Inc.,
Chicago, IL, USA) was used for all statistical analysis. P<0.05
was considered to indicate a statistically significant difference
between values.
Results
Resveratrol prevents apoptotic cell death
induced by Aβ(25–35) in PC12 cells
The Aβ(25–35) peptide is a hallmark of degenerative
disorders, in particular AD (22).
As a toxic factor, abnormal deposits of Aβ(25–35) protein in the
brain have a critical role in the pathogenesis of multiple
diseases. To examine the role of resveratrol in preventing neurons
from undergoing cell death, a cell model of Aβ(25–35) injury was
constructed in PC12 cells. Cells were divided into four groups with
each group treated with DMSO, Aβ(25–35), resveratrol or Aβ(25–35)
in combination with resveratrol, respectively. The protective
effects of resveratrol against cell apoptosis of PC12 cells were
evaluated using flow cytometry with Annexin V-FITC staining, which
also allows efficient determination of early- and late-stage
apoptosis. When exposed to 20 mM Aβ(25–35), apoptosis was induced
in PC12 cells compared with the apoptotic rate of the normal
control group (P<0.05). Furthermore, Aβ(25–35) induced early- as
well as late-stage apoptosis as compared with apoptotic rates of
the control group [Fig. 1A; early
stage, 10.2% in Aβ(25–35) group vs. 2.38% in control group; late
stage, 0.937% in Aβ(25–35) group vs. 0.746% in control group]. Of
note, when resveratrol was added to PC12 cells, a significant
reduction in cell apoptosis was observed. Furthermore, early- and
late-stage apoptosis were markedly inhibited [Fig. 1A; early stage, 5.77% in resveratrol
+ Aβ(25–35) group vs. 10.2% in Aβ(25–35) group; late stage, 0.143%
in resveratrol + Aβ(25–35) group vs. 0.937% in Aβ(25–35)
group].
A CCK-8 assay was subsequently employed to evaluate
cell viability. The addition of Aβ(25–35) to PC12 cells
significantly decreased cell viability from nearly 100% to ~60%
[Fig. 1B; DMSO panels,
Aβ(25–35)(+) vs. Aβ(25–35)(−)]. However, when PC12 cells were
co-treated with resveratrol and Aβ(25–35), cell viability was
significantly increased from 60% in the Aβ(25–35) injury group to
nearly 90% in the resveratrol + Aβ(25–35) group (Fig. 1B; P<0.05). In addition, marked
survival of PC12 cells exposed to Aβ(25–35) was observed following
treatment with resveratrol (Fig.
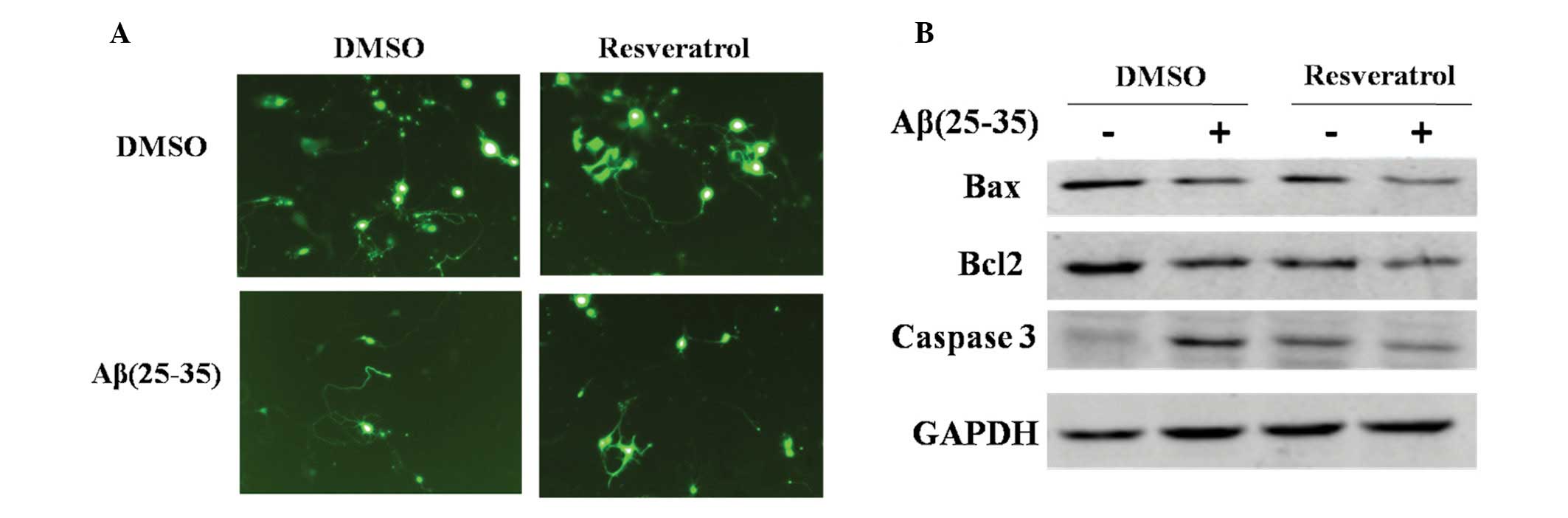
2A). As indicated in Fig. 2A,
PC12 cells developed long neurites following culture in DMSO
(Fig. 2A, left upper panel); when
exposed to Aβ(25–35), the neurites of cells retracted gradually and
cell death was apparent due to markedly decreased cell confluence.
The neurites gradually disappeared, while cell debris appeared
(Fig. 2A, left lower panel).
Following treatment with resveratrol, a protective effect on PC12
cells was observed, identified by the rescued cell growth and
morphology (Fig. 2A, right panel).
These results confirmed that resveratrol was able to inhibit
Aβ(25–35)-induced apoptotic cell death. In conclusion, resveratrol
prevented Aβ(25–35)-induced cell apoptosis in PC12 cells.
Resveratrol inhibits apoptotic inducers
and promotes apoptotic inhibitors
To further confirm that resveratrol was able to
prevent PC12 cells from apoptosis induced by Aβ(25–35), western
blot analysis was used to evaluate expression of apoptotic inducers
and inhibitors. Aβ(25–35) induced activation of the apoptotic
inducer, caspase-3 [Fig. 2B;
Aβ(25–35)(+) lane vs. Aβ(25–35)(−) lane in DMSO group].
Concomitantly, resveratrol decreased Bax and caspase-3 expression,
as well that of the apoptotic inhibitor, Bcl-2. These results
further confirmed that resveratrol inhibited Aβ(25–35)-induced cell
apoptosis in PC12 cells.
Inhibition of apoptosis by resveratrol is
associated with increased acetylation level of p53
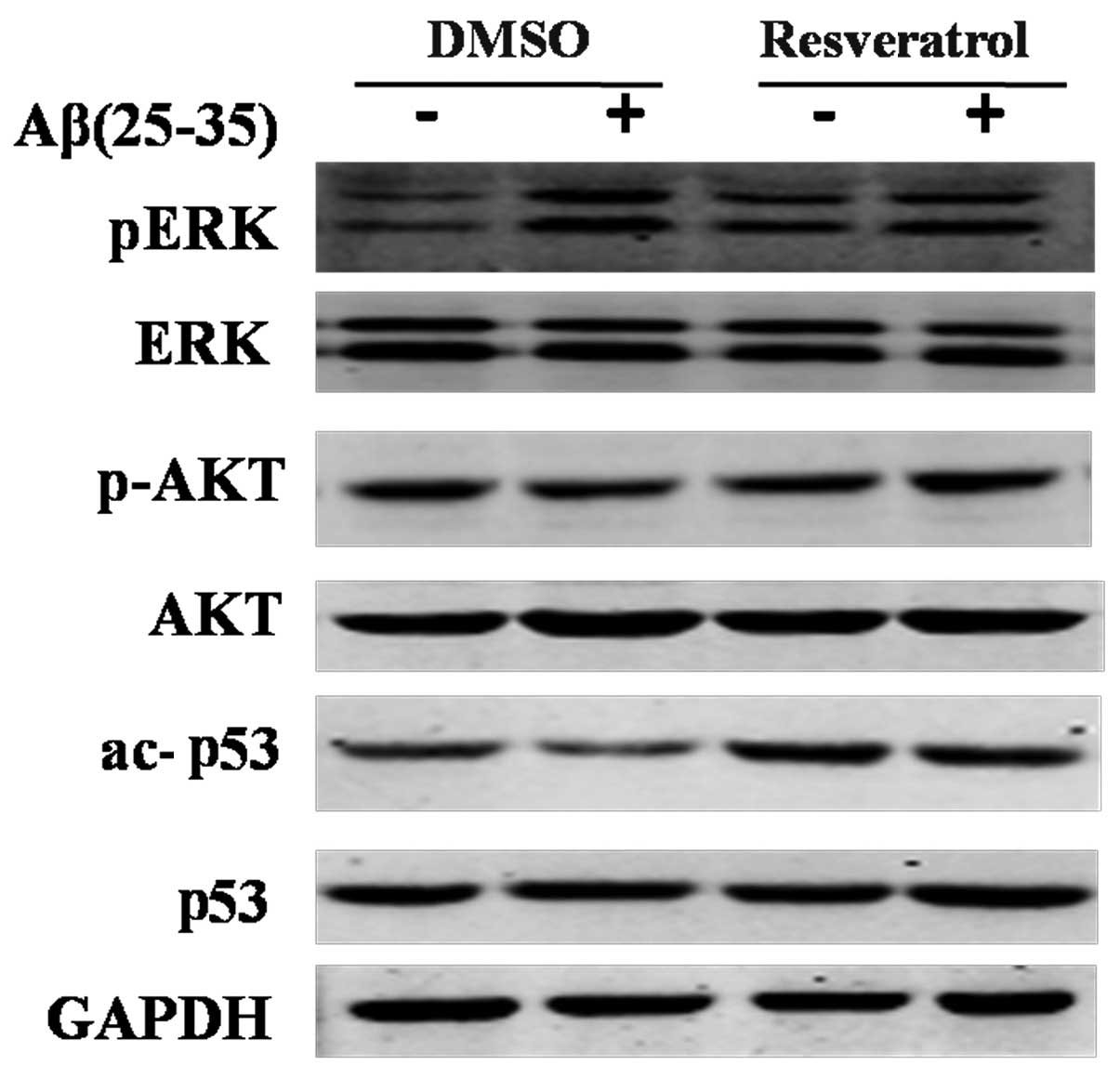
To assess the mechanism underlying the protective
effect of resveratrol in PC12 cells, the expression of proteins in
several common pathways, including ERK, Akt and p53, were analyzed.
p-ERK and p-Akt, as well as acetylated p53 (ac-p53) were also
assessed. Akt has an upstream function of p53. The activation of
Akt depends on its phosphorylation state, and phosphorylated Akt is
able to interrupt the stability and activity of p53 (23). As depicted in Fig. 3, no difference in the total protein
levels of ERK and p53 was detected amongst the groups. Similarly,
no significant difference was detected in p-Akt expression.
Expression levels of pERK were markedly increased in the Aβ(25–35)
injury group. However, no significant alteration in pERK expression
levels was detected in response to resveratrol treatment, which
indicated that the protective effects of resveratrol against
Aβ(25–35) likely had no association with ERK and Akt or their
phosphorylated modifications. In the DMSO-treated cells, Aβ(25–35)
significantly decreased ac-p53 expression levels, which suggested
that Aβ(25–35)-induced apoptosis was associated with decreased
acetylation of p53. Of note, it was demonstrated that ac-p53 levels
recovered and markedly increased following resveratrol treatment
(Fig. 3). Therefore, resveratrol
potentially inhibited Aβ(25–35)-induced apoptosis by association
with increased acetylation levels of p53.
Inhibition of p53 acetylation abrogates
resveratrol-mediated apoptosis inhibition
To further confirm the hypothesis that
resveratrol-mediated inhibition of apoptosis in PC12 cells was
positively associated with increased acetylation levels of p53,
pifithrin-α, an inhibitor of p53 acetylation, was applied to the
cells. Pifithrin-α is a chemical inhibitor of p53 that has been
shown to protect mice from the side effects of cancer therapy
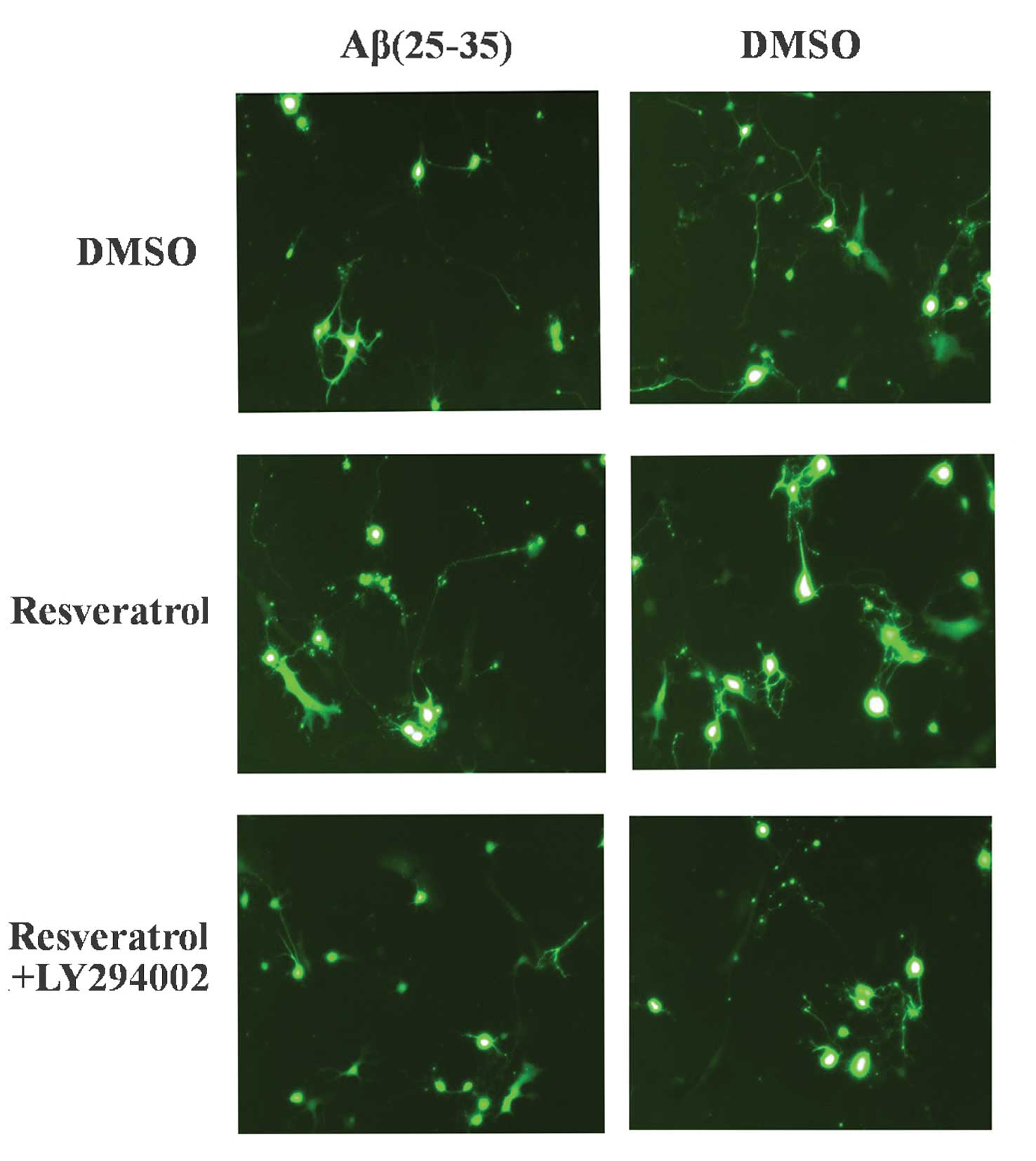
(24). PC12 cells were treated
with pifithrin-α to inhibit p53 expression, and subsequent
alterations in cell survival were evaluated. Aβ(25–35) treatment
induced cell death and caused retracted cell neurites, whereas
resveratrol abrogated this Aβ(25–35)-induced apoptotic effect
(Fig. 4; top and middle images).
When PC12 cells were co-treated with pifithrin-α and resveratrol,
cell growth was perturbed and neurites were no longer present. Cell
growth and confluence were markedly decreased as indicated by a
loss of cells. These results suggested that pifithrin-α attenuated
the protective effects of resveratrol in PC12 cells, indicating
that decreased acetylation levels of p53 may attenuate
resveratrol-mediated inhibition of cell apoptosis. It may therefore
be concluded that resveratrol-mediated inhibition of
Aβ(25–35)-induced apoptosis was associated with an increased
acetylation level of p53.
Discussion
In the present study, a neurotoxic cell model in
PC12 cells was established by administration of Aβ(25–35), which
provided novel evidence for the protective effects of resveratrol
against Aβ(25–35)-induced neurotoxicity. Resveratrol protected PC12
cells from neuronal damage through inhibition of apoptotic cell
death. Resveratrol reversed the Aβ(25–35)-induced decreased cell
viability and cell apoptosis. In particular, the underlying
mechanism that contributed to resveratrol-mediated inhibition of
apoptosis was examined. It was demonstrated that the
neuroprotective effects of resveratrol were associated with
increased acetylation levels of p53. Therefore, the neuroprotective
effects of resveratrol against Aβ(25–35) in PC12 cells may be
partially mediated by the acetylation of p53.
Resveratrol is a phytoestrogen, originally derived
from plants, with diverse anti-proliferative and pro-apoptotic
effects (25). The mechanisms
underlying these effects comprise downregulation of apoptosis
inhibitors, including survivin2 and Bcl-2, as well as upregulation
of apoptosis inducers, including Bax (25). In the central nervous system, it
was recently demonstrated that resveratrol decreased Bcl-2
expression and viability in GH3 pituitary adenoma cells of rats
(26). In the present study, it
was also confirmed that the protective effect of resveratrol in
preventing neuronal apoptosis was associated with
pro-apoptotic/anti-apoptotic factors. When the neurotoxic factor
Aβ(25–35) was added to PC12 cells, cell viability was significantly
decreased by ~40%. However, when PC12 cells were co-treated with
resveratrol, cell viability was significantly increased from 60% in
the Aβ(25–35) group to nearly 90% in that of the resveratrol +
Aβ(25–35) group. In addition, a marked increase in the survival of
PC12 cells was evident following resveratrol treatment. Resveratrol
treatment rescued PC12-cell survival, attenuating the neuronal
damage induced by Aβ(25–35). The evaluation of apoptosis-associated
protein expression revealed that resveratrol treatment inhibited
the expression of the pro-apoptotic protein, caspase-3, as well as
that of the anti-apoptotic protein, Bcl-2. These results confirmed
that resveratrol inhibited Aβ(25–35)-induced apoptotic cell death,
and that apoptosis-associated proteins Bax, Bcl-2 and caspase-3
were involved in mediating the resveratrol-induced inhibition of
apoptosis in PC12 cells.
Furthermore, it was demonstrated that the inhibition
of apoptosis by resveratrol was associated with an increased
acetylation level of p53. While total p53 remained stable in PC12
cells regardless of which treatment was administered, the
acetylation level of p53 varied between groups. Aβ(25–35) treatment
decreased the acetylation level of p53, which represented a process
underlying Aβ(25–35)-induced apoptosis. Of note, when PC12 cells
were co-treated with resveratrol, the acetylation level of p53
markedly increased, indicating that resveratrol may inhibit
Aβ(25–35)-induced apoptosis via an association with the
modification of p53 acetylation. In order to further analyze the
involvement of p53 in mediating the effects of resveratrol, p53
inhibitor pifithrin-α was introduced. Pifithrin-α prevented the
resveratrol-mediated recovery of Aβ(25–35)-induced cell growth
inhibition. This result supported the conclusion that resveratrol
inhibited Aβ(25–35)-induced apoptosis, potentially via the
regulation of acetylation of p53.
Previous studies revealed that resveratrol
upregulated SIRT1 expression in Aβ(25–35)-treated cells (27,28).
SIRT1 is a nicotinamide adenine dinucleotide-dependent histone
deacetylase, which has a critical role in regulating cellular
activities, including transcriptional silencing of telomeres and
life-span extension (29,30). Furthermore, p53 was found to be
regulated by SIRT1 (31,32), hence it may be possible that
resveratrol is able to cross-talk with SIRT1 and p53. Resveratrol
may influence the acetylation level of p53 via the regulation of
SIRT1 expression. Further study is required in order to investigate
this possible interaction.
In conclusion, the results of the present study
provided novel evidence which indicated that resveratrol, a natural
product, exerted a protective effect on PC12 cells in an
Aβ(25–35)-induced cell model of neurotoxic damage. Resveratrol
inhibited Aβ(25–35)-induced cell apoptosis and therefore promoted
cell viability. The inhibition of Aβ(25–35)-induced apoptosis by
resveratrol may be associated with an increased acetylation level
of p53. These results may provide a basis for elucidating the
therapeutic potential of resveratrol in treating degenerative
disorders of the brain.
References
|
1
|
Hosseinimehr SJ and Hosseini SA:
Resveratrol sensitizes selectively thyroid cancer cell to
131-iodine toxicity. J Toxicol. 2014:8395972014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Witte AV, Kerti L, Margulies DS and Flöel
A: Effects of resveratrol on memory performance, hippocampal
functional connectivity, and glucose metabolism in healthy older
adults. J Neurosci. 34:7862–7870. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pervaiz S: Resveratrol: from grapevines to
mammalian biology. FASEB J. 17:1975–1985. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang J, Ho L, Zhao W, et al: Grape-derived
polyphenolics prevent Abeta oligomerization and attenuate cognitive
deterioration in a mouse model of Alzheimer’s disease. J Neurosci.
28:6388–6392. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scalbert A, Manach C, Morand C, Rémésy C
and Jiménez L: Dietary polyphenols and the prevention of diseases.
Crit Rev Food Sci Nutr. 45:287–306. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tomayko EJ, Cachia AJ, Chung HR and Wilund
KR: Resveratrol supplementation reduces aortic atherosclerosis and
calcification and attenuates loss of aerobic capacity in a mouse
model of uremia. J Med Food. 17:278–283. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu T, Zang N, Zhou N, et al: Resveratrol
inhibits the TRIF-dependent pathway by upregulating sterile alpha
and armadillo motif protein, contributing to anti-inflammatory
effects after respiratory syncytial virus infection. J Virol.
88:4229–4236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Andrade JM, Frade AC, Guimarães JB, et al:
Resveratrol increases brown adipose tissue thermogenesis markers by
increasing SIRT1 and energy expenditure and decreasing fat
accumulation in adipose tissue of mice fed a standard diet. Eur J
Nutr. 53:1503–1510. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pasinetti GM, Wang J, Marambaud P, et al:
Neuroprotective and metabolic effects of resveratrol: therapeutic
implications for Huntington’s disease and other neurodegenerative
disorders. Exp Neurol. 232:1–6. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bournival J, Quessy P and Martinoli MG:
Protective effects of resveratrol and quercetin against MPP+
-induced oxidative stress act by modulating markers of apoptotic
death in dopaminergic neurons. Cell Mol Neurobiol. 29:1169–1180.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang TC, Lu KT, Wo YY, Wu YJ and Yang YL:
Resveratrol protects rats from Aβ-induced neurotoxicity by the
reduction of iNOS expression and lipid peroxidation. PLoS One.
6:e291022011. View Article : Google Scholar
|
|
12
|
Feng X, Liang N, Zhu D, et al: Resveratrol
inhibits β-amyloid-induced neuronal apoptosis through regulation of
SIRT1-ROCK1 signaling pathway. PLoS One. 8:e598882013. View Article : Google Scholar
|
|
13
|
Sharma M and Gupta YK: Chronic treatment
with trans resveratrol prevents intracerebroventricular
streptozotocin induced cognitive impairment and oxidative stress in
rats. Life Sci. 71:2489–2498. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luo J, Nikolaev AY, Imai S, et al:
Negative control of p53 by Sir2alpha promotes cell survival under
stress. Cell. 107:137–148. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vaziri H, Dessain SK, Ng Eaton E, et al:
hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell.
107:149–159. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mihara M, Erster S, Zaika A, et al: p53
has a direct apoptogenic role at the mitochondria. Mol Cell.
11:577–590. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brooks CL and Gu W: Ubiquitination,
phosphorylation and acetylation: the molecular basis for p53
regulation. Curr Opin Cell Biol. 15:164–171. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luo J, Li M, Tang Y, Laszkowska M, Roeder
RG and Gu W: Acetylation of p53 augments its site-specific DNA
binding both in vitro and in vivo. Proc Natl Acad Sci USA.
101:2259–2264. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Appella E and Anderson CW:
Post-translational modifications and activation of p53 by genotoxic
stresses. Eur J Biochem. 268:2764–2772. 2001. View Article : Google Scholar
|
|
20
|
Walsh DM and Selkoe DJ: A beta oligomers -
a decade of discovery. J Neurochem. 101:1172–1184. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jia LQ, Yang GL, Ren L, et al: Tanshinone
IIA reduces apoptosis induced by hydrogen peroxide in the human
endothelium-derived EA.hy926 cells. J Ethnopharmacol. 143:100–108.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang Y, Chen S, Zhang J, Li C, Sun Y,
Zhang L and Zheng X: Stimulation of autophagy prevents amyloid-β
peptide-induced neuritic degeneration in PC12 cells. J Alzheimers
Dis. 40:929–939. 2014.
|
|
23
|
Liao Y and Hung MC: Physiological
regulation of Akt activity and stability. Am J Transl Res. 2:19–42.
2010.PubMed/NCBI
|
|
24
|
Komarov PG, Komarova EA, Kondratov RV, et
al: A chemical inhibitor of p53 that protects mice from the side
effects of cancer therapy. Science. 285:1733–1737. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Athar M, Back JH, Kopelovich L, Bickers DR
and Kim AL: Multiple molecular targets of resveratrol:
Anti-carcinogenic mechanisms. Arch Biochem Biophys. 486:95–102.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Voellger B, Kirches E, Wilisch-Neumann A,
et al: Resveratrol decreases B-cell lymphoma-2 expression and
viability in GH3 pituitary adenoma cells of the rat. Onco Targets
Ther. 9:1269–1276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Akhter R, Sanphui P and Biswas SC: The
essential role of p53-up-regulated modulator of apoptosis (Puma)
and its regulation by FoxO3a transcription factor in
β-amyloid-induced neuron death. J Biol Chem. 289:10812–10822. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Renaud J, Bournival J, Zottig X and
Martinoli MG: Resveratrol protects DAergic PC12 cells from high
glucose-induced oxidative stress and apoptosis: effect on p53 and
GRP75 localization. Neurotox Res. 25:110–123. 2014. View Article : Google Scholar :
|
|
29
|
Guarente L: Sir2 links chromatin
silencing, metabolism, and aging. Genes Dev. 14:1021–1026.
2000.PubMed/NCBI
|
|
30
|
Lamming DW, Wood JG and Sinclair DA: Small
molecules that regulate lifespan: evidence for xenohormesis. Mol
Microbiol. 53:1003–1009. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lhee SJ, Song EK, Kim YR and Han MK: SIRT1
inhibits p53 but not NF-κB transcriptional activity during
differentiation of mouse embryonic stem cells into embryoid bodies.
Int J Stem Cells. 5:125–129. 2012. View Article : Google Scholar
|
|
32
|
Hori YS, Kuno A, Hosoda R and Horio Y:
Regulation of FOXOs and p53 by SIRT1 modulators under oxidative
stress. PLoS One. 8:e738752013. View Article : Google Scholar : PubMed/NCBI
|