Introduction
Due to technical advancements, the application of
radiation therapy (RT) in treating hepatic tumors is rapidly
increasing. However, the frequent association of RT with concurrent
liver cirrhosis is a major challenge in radiotherapy. Irradiation
of the non-tumor compartment of the liver may cause cell damage,
changes in laboratory assessments and/or clinical signs of liver
dysfunction. This is termed radiation-induced liver disease,
typically emerging between 4 and 8 weeks after the completion of RT
and is accompanied by fatigue, rapid weight gain and ascites
(1). In the majority of cases, the
course of the disease is stable or transient, however, certain
patients develop overt liver insufficiency and treatment-associated
mortality (2). No pharmacological
therapy is currently available to relieve radiation-induced liver
disease and it is important to develop techniques to minimize the
toxicity or to identify the toxicity early using biomarkers.
Radiation-induced liver damage (RILD) has not been fully
investigated in parallel with its clinical development, the
pathophysiology of RILD remains to be elucidated and systemic
investigation of the biomarkers of RILD has not been performed
(3). The present study observed
alterations in the expression levels of certain molecules, which
may be involved in the pathogenesis of RILD, the potential
mechanisms underlying RILD and potential targets for its
treatment.
Materials and methods
Animals
Male Sprague-Dawley (SD) rats (6-weeks-old, weighing
220±10 g) were used. The rats were housed in the animal breeding
house at Xinjiang Medical University, (Urumqi, China) and were
maintained in a 12 h light-dark cycle at a constant temperature and
humidity. The care and use of the laboratory animals were based on
the Guidelines and Regulations for the Use and Care of Animals
provided by the Ministry of Science and Technology of the People’s
Republic of China. The present study complied with the Principles
of Laboratory Animal Care (NIH publication No. 85-23, revised
1985), the Office for Protection from Research Risks Public Health
Service Policy on the Humane Care and Use of Laboratory Animals
(revised 1986) and the U.S. Animal Welfare Act. The procedures were
approved by the ethics committee of Xinjiang Medical University
(permit no. IACUC-20121122004).
RILD model
The 36 SD rats were anesthetized by intraperitoneal
injection of ketamine and xylazine (60 and 10 mg/kg, respectively;
Jiangsu Henrui Medicine Co., Ltd, Lianyungang, China), prior to
irradiation with 6 MV photons at a dose of 300 cGy/min in one
fraction to the right upper quadrant of the abdomen (2×2 cm) using
a Varian Clinac CX accelerator (Varian Medical Systems, Inc., Palo
Alto, CA, USA). The one-time total dose of irradiation was 20 Gy.
The rats were then sacrificed by decapitation 3 days and 1, 2, 4, 8
and 12 weeks after irradiation. At each time point, six rats were
sacrificed and their liver tissues and blood samples were harvested
for analysis. Rats, which were not exposed to irradiation were used
as controls.
Evaluation of liver injury by serum
analysis
To evaluate liver injury following irradiation, the
weight of the rats were monitored at each time point. The serum
levels of alanine aminotransferase (ALT), aspartate
aminotransferase (AST), alkaline phosphatase (ALP), total bilirubin
(TB) and direct bilirubin (DB) were measured to assess liver
function. The serum levels of hyaluronic acid (HA), laminin (LN),
type III procollagen (PCIII) and type IV collagen (IV-C) were
measured to assess liver fibrosis.
Histological analysis
Liver sections of ~0.5×0.5 cm were collected for
histological analysis by hematoxylin and eosin (H&E; Tianjing
Zhiyuan Chemical Agents Co., Ltd, Tianjing, China) and Masson’s
trichrome (MT; cat. no. MST-8003/8004; Maixin Biotech Co., Ltd.,
Fuzhou, China) staining. The extent of liver fibrosis and the
assessment of the histological features were performed by two
pathologists in a blinded-manner. The area of liver fibrosis was
quantified using a microscope (Olympus CX41, Olympus, Tokyo, Japan)
equipped with a CCD camera by computer-assisted image analysis
using Meta-Morph software version 6.06r (Universal Imaging
Corporation, Downingtown, PA, USA).
Apoptotic analysis
The levels of apoptosis were assessed in the liver
sections using terminal deoxynucleotidyl transferase dUTP nick end
labeling (TUNEL) with a TUNEL test kit (cat. no. MK1020; Boster
Co., Ltd., Wuhan, China). Briefly, the liver sections were digested
with proteinase K and incubated with 0.2% Triton X-100 in
phosphate-buffered saline (PBS)-Tween 20 (ZSGB-Bio Co., Ltd,
Beijing, China) for 30min. The sections were then washed twice
using PBS-Tween 20 (2 min each), prior to incubation with 3%
H2O2 in PBS for 10 min at 4°C to block
endogenous peroxidase activity. The labeling was performed
according to the manufacturer’s instructions. Briefly, 18 μl
labeling buffer, 1 μl TdT and 1 μl DIG-d-UTP were added to each
slide. The slides were put in a box and left at 37°C for 2 h. The
slides were subsequently washed with 0.01 M PBS-Tween 20 three
times for 2 min. Blocking buffer was added (50 μl/slide) and
incubated at room temperature for 30 min. Anti-DIG-Biotin (1:100)
was added and slides were incubated in a box at 37°C for 30 min;
The slides were subsequently washed three times with 0.01 M
PBS-Tween 20 for 2 min. Finally SABC (1:100) was added to the
slides (all reagents were purchased from Tianjing Zhiyuan Chemical
Agents Co. Ltd). The sections were then incubated with
diaminobenzidine (DAB) and counterstained with Gill’s hematoxylin
prior to being dehydrated, cleared in xylene (all Tianjing Zhiyuan
Chemical Agents Co. Ltd) and placed under coverslips using a
xylene-based mounting medium (Poly-Mount xylene; Polysciences,
Inc., Warrington, PA, USA). The apoptotic cells were identified by
brown staining in the nuclei. Images were captured from 10 randomly
selected fields on each slide section at a magnification of ×400
(Leica DM300; Leica Microsystems, Heerbrugg, Switzerland) and, in
each field, the apoptotic bodies were expressed as the percentage
of 1,000 nuclei.
Immunohistochemistry
The liver sections (4 μm) were immunohistochemically
stained. The primary antibodies used were as follows: Rabbit
monoclonal antibodies against transforming growth factor-β1
(TGF-β1; 1:250), nuclear factor (NF)-κB65 (1:100), mothers against
decapentaplegic homolog 4 (Smad4) (1:40), Smad3 (1:250), Smad7
(1:30), tumor necrosis factor-α (TNF-α; 1:200) and connective
tissue growth factor (CTGF; 1:200), all purchased from Boster Co.,
Ltd. The secondary antibody used was the Two-Step IHC Detection
reagent (cat. no. PV-6001/6002; ZSGB-BIO, Beijing, China). The DAB
kit (cat. no. ZLI-9017/9018/9019; ZSGB-BIO) was then used. The
immunohistochemical analysis was performed in paraffin sections
using a microwave-based (MYE-1870MEG; Haier, Qingdao, China)
antigen retrieval technique (4).
Following immunostaining, the sections were counterstained with
hematoxylin and images were captured using a Leica DM300 microscope
and analyzed using Leica Application Suite V3. 35.0 (Leica,
Mannheim, Germany). The percentage of the positive staining areas
were quantified using Image-Pro plus software (Media Cybernetics,
Bethesda, MD, USA).
RNA extraction and reverse transcription
quantitative polymerase chain reaction (RT-qPCR)
The total RNA was isolated from the liver tissues
using TRIzol reagent (Roche Diagnostics, Basel, Switzerland)
according to the manufacturer’s instructions. RT-qPCR was performed
for TGF-β1, NF-κB65, Smad4, Smad3, Smad7, TNF-α and CTGF using a
PrimeScriptTM one-step RT-PCR kit (Takara Bio, Inc.,
Shiga, Japan) with GAPDH as an endogenous control and Bio-Rad iQ
SYBR Green supermix with Opticon2 (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The primers used were as follows: GAPDH,
forward 5′-AGTGCCAGCCTCGTCTCATAG-3′ and reverse
5′-CGTTGAACTTGCCGTGGGTAG-3′; TGF-β1, forward
5′-GTGGCTGAACCAAGGAGACG-3′ and reverse 5′-CAGGTGTTGAGCCCTTTCCAG-3′;
TNF-α, forward 5′-ACAAGGCTGCCCCGACTAT-3′ and reverse
5′-CTCCTGGTATGAAGTGGCAAATC-3′; CTGF, forward
5′-TGTGTGATGAGCCCAAGGAC-3′ and reverse 5′-AGTTGGCTCGCATCATAGTTG-3′;
NF-κB65, forward 5′-ATCTGCCGAGTGAACCGAAACT-3′ and reverse
5′-CCAGCCTGG TCCCGTGAAA-3′; Smad3, forward
5′-AGGCAGGTCTGGGCTTTATT-3′ and reverse 5′-CGTATCCACAAAGCTGAGCA-3′;
Smad4, forward 5′-CACTCGAGGGATCCGAATTC-3′ and reverse
5′-GCGTCGACAAGCTTTCTAGA-3′; Smad7, forward
5′-GAAGTCAAGAGGCTGTGTTGC-3′ and reverse 5′-CAGGCTCCAGAAGAAGTTGG-3′.
The oligonucleotide primers for RT-qPCR were purchased from Sangong
Biotech Co., Ltd. (Shanghai, China). Relative quantification was
performed using the ΔΔCt method [ΔΔCt = ΔCt (treated) - Ct
(control)] (5). The ratio of the
mRNA expression levels of interest were normalized with the
internal control GAPDH.
Western blot analysis
The protein from the liver tissues were extracted
using radioimmunoprecipitation buffer and western blot analysis was
performed. The liver tissue samples were homogenized in
radioimmunoprecipitation buffer (Thermo Fisher Scientific, Inc.,
Rockford, IL, USA). The lysates were clarified by centrifugation at
12,000 × g for 10 min and the protein concentrations were
determined using a bicinchoninic acid protein assay kit (Thermo
Fisher Scientific, Inc.). The samples were mixed with loading
buffer and boiled for 5 min at 100°C prior to separation using 10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed
by transferring onto polyvinylidene fluoride membranes (Roche
Diagnostics). The membranes were blocked in Tris-buffered saline
(TBS) containing 5% non-fat milk and 0.1% Tween-20 for 1 h at room
temperature. The membranes were then incubated at 4°C overnight
with the following primary antibodies: Mouse polyclonal anti-TGF-β1
(cat. no. ab92486; 1:20; Abcam, Cambridge, MA, USA), rabbit
polyclonal anti-CTGF (1:1,000; Thermo Fisher Scientific, Inc.),
rabbit polyclonal anti-NF-κB65 (1:100; Boster Co., Ltd.), rabbit
polyclonal anti-TNF-α (1:200; Boster Co., Ltd.), rabbit monoclonal
anti-Smad3 (1:100; Boster Co., Ltd.), rabbit monoclonal anti-Smad4
(1:10,000; Boster Co., Ltd.), rabbit monoclonal anti-Smad 7 (1:500;
Boster Co., Ltd.) and mouse monoclonal anti-GAPDH (1:5,000; Boster
Co., Ltd.). The membranes were then washed three times for 10 min
each in TBS containing 0.1% Tween-20, prior to incubation with the
corresponding peroxidase-conjugated goat anti-rabbit immunoglobulin
G/horseradish peroxidase secondary antibody (PV-6001, ZSGB-Bio Co.,
Ltd) and washed, as previously. The protein bands were detected
using a chemiluminescence detection system (WesternBreeze;
Invitrogen Life Technologies, Carlsbad, CA, USA) and
autoradiography film (Biomax XAR film; Kodak, Shenzhen, China).
Statistical analysis
The data are presented as the mean ± standard
deviation. The significance of the differences between different
groups were determined by one-way analysis of variance, followed by
paired-samples t-test using SPSS software version 10.0 (SPSS, Inc.,
Chicago, IL, USA). A χ2 test was performed to compare
the ratio data. P<0.05 was considered to indicate a
statistically significant difference.
Results
Evaluation of liver injury by serum
analysis
The weight of the rats was reduced significantly in
the 20 Gy irradiation group compared with the untreated group
(Table I). This reduction in
weight started 2 weeks after irradiation and continued until the
end of the study at 12 weeks. The levels of AST, ALT and ALP in the
serum were increased significantly in the 20 Gy group from 2 weeks
after irradiation to the end of the study. However, no significant
changes in the levels of TB and DB (Table II) or the levels of HA, LN, PCIII
and IV-C (Table III) were
detected in the serum.
 | Table IWeight changes in the rats during
recovery following irradiation. |
Table I
Weight changes in the rats during
recovery following irradiation.
| Time period | 0 Gy group (g) | 20 Gy group
(g) | P-value |
|---|
| Before
irradiation | 223.00±4.795 | 222.00±8.794 | >0.05 |
| After
irradiation |
| 3 days | 237.18±6.461 | 216.91±26.27 | >0.05 |
| 1 week | 254.64±30.50 | 230.73±24.95 | >0.05 |
| 2 weeks | 274.64±23.95 | 240.73±24.03 | <0.05 |
| 4 weeks | 372.32±12.65 | 290.12±25.42 | <0.05 |
| 8 weeks | 421.78±11.98 | 332.75±24.08 | <0.05 |
| 12 weeks | 510.15±23.17 | 437.80±35.41 | <0.05 |
 | Table IIAnalysis of liver function in the
rats during recovery following irradiation. |
Table II
Analysis of liver function in the
rats during recovery following irradiation.
| Time period | AST (ng/l) | ALT (ng/l) | ALP (ng/l) | TB (ng/l) | DB (ng/l) |
|---|
| No radiation | 88.45±9.83 | 51.40±4.24 | 104.45±12.23 | 0.00±0.00 | 1.20±0.00 |
| After
irradiation |
| 3 days | 74.05±6.32 | 40.80±8.36 | 153.46±61.02 | 0.00±0.00 | 1.13±0.21 |
| 1 week | 73.60±0.28 | 38.25±3.32 | 241.95±41.65 | 0.00±0.00 | 0.85±1.20 |
| 2 weeks | 117.2±2.55 | 73.1±61.23 | 256.7±104.02 | 0.00±0.00 | 1.40±0.42 |
| 4 weeks | 127.98±3.27 | 75.28±17.43 | 223.56±17.22 | 0.00±0.00 | 1.48±0.29 |
| 8 weeks | 177.02±15.16 | 65.34±15.09 | 228.00±4.24 | 1.05±0.57 | 2.33±1.28 |
| 12 weeks | 245.78±2.36 | 98.32±15.36 | 278.12±3.85 | 2.24±1.56 | 2.79±2.35 |
| P-value | <0.05 | <0.05 | <0.05 | >0.05 | >0.05 |
 | Table IIIAssessment of liver fibrosis in the
rats during recovery following irradiation. |
Table III
Assessment of liver fibrosis in the
rats during recovery following irradiation.
| Time period | Hyaluronic acid
(ng/l) | Type III
procollagen (ng/l) | Laminin (ng/l) | Type IV collagen
(ng/l) |
|---|
| No irradiation | 0-110 | 0-130 | 1-130 | 0-84 |
| After
irradiation |
| 2 weeks | 157.65 | 7.18 | 53.54 | 83.21 |
| 4 weeks | 88.85 | 8.10 | 57.15 | 68.50 |
| 8 weeks | 111.60 | 8.57 | 62.32 | 75.16 |
| 12 weeks | 74.61 | 4.95 | 60.24 | 43.89 |
| P-value | >0.05 | >0.05 | >0.05 | >0.05 |
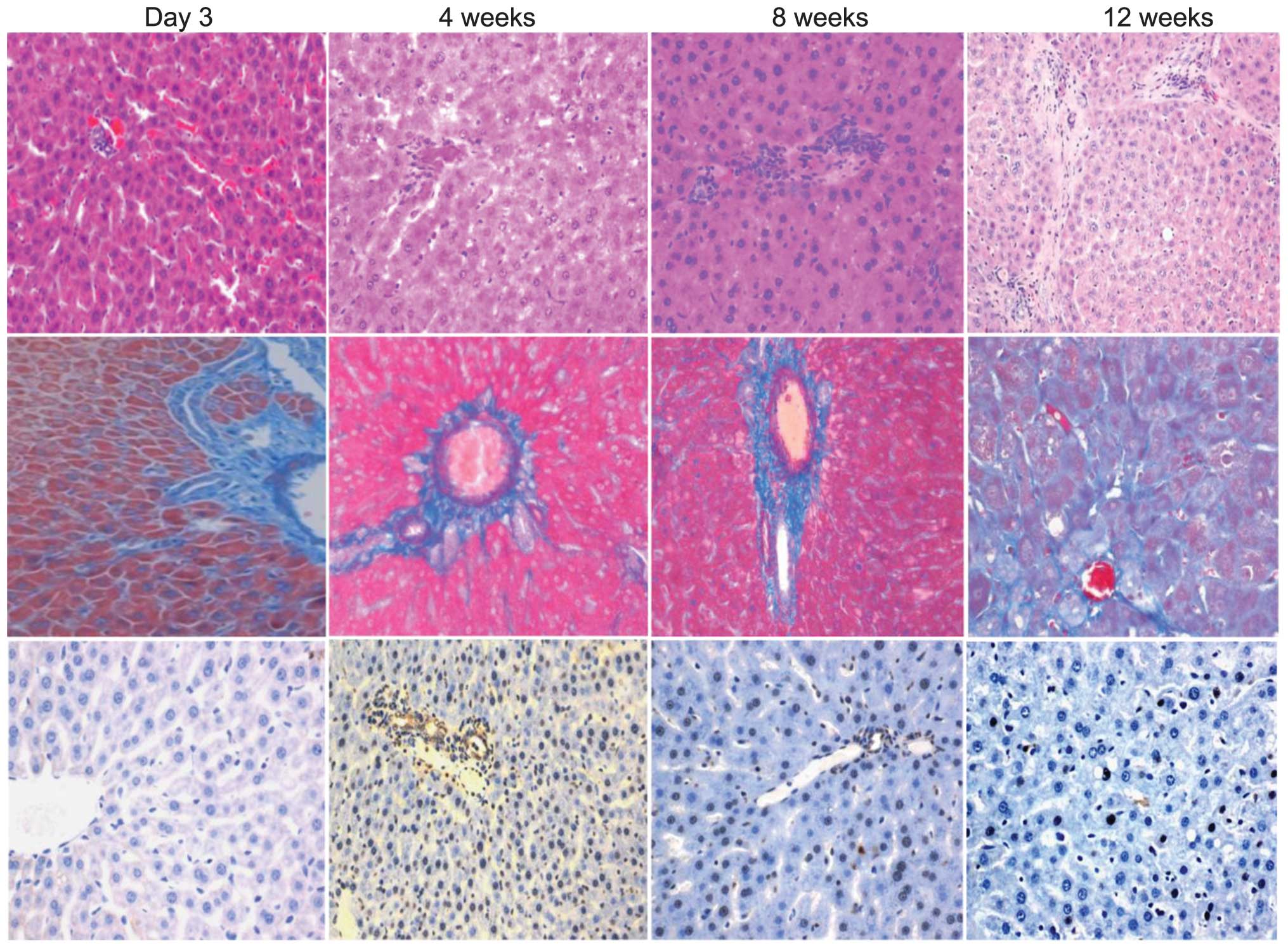
Liver histopathology and TUNEL
The images of the H&E staining demonstrated
fibrotic proliferation in the liver tissues. The apparent fibrosis
of the liver tissue was observed 12 weeks after irradiation
(Fig. 1, top). The MT images
revealed that the collagen levels were increased significantly 12
weeks after irradiation, located around the perivenous area and
between the hepatocytes (Fig. 1,
middle). The apoptotic cells, detected by TUNEL, were observed in
the perivenous area 2 and 4 weeks after irradiation (Fig. 1 bottom).
Immunohistology, RT-qPCR and western blot
analysis
The immunohistochemical, RT-qPCR and western blot
analysis all revealed the same alterations in the expression levels
of TGF-β1, NF-κB65, Smad4, Smad3, Smad7, TNF-α and CTGF (Fig. 2–4). The immunohistochemical analysis
demonstrated that the main area of positive staining was the
perivenous area. The positive staining of TGF-β1, Smad3, CTGF and
NF-κB65 increased between 3 days and 12 weeks after irradiation.
Positive staining of Smad4 and Smad7 only occurred 3 days and 1
week after irradiation and positive staining of TNF-α only occurred
between 1 and 4 weeks after irradiation (Fig. 2). The RT-qPCR and western blot
analysis revealed that the expression level of NF-κB65 was
increased 3 days after irradiation and remained at a high level to
the 12th week. The expression levels of CTGF and Smad3 were
significantly increased at 2 weeks and remained at a high level to
the 12th week after irradiation. The expression level of TGF-β1 was
significantly increased 1 week after irradiation and remained high
to the 12th week after irradiation. The expression level of TNF-α
was significantly increased 3 days after irradiation and remained
high to the 4th week after irradiation. The expression level of
Smad4 was significantly increased 3 days and 1 week after
irradiation. The expression level of Smad7 was significantly
increased 3 days after irradiation and was reduced significantly 1
and 2 weeks after irradiation, however the expression level
remained higher than the control. The expression level of Smad7
returned to the control level 4 weeks after irradiation (Figs. 3 and 4).
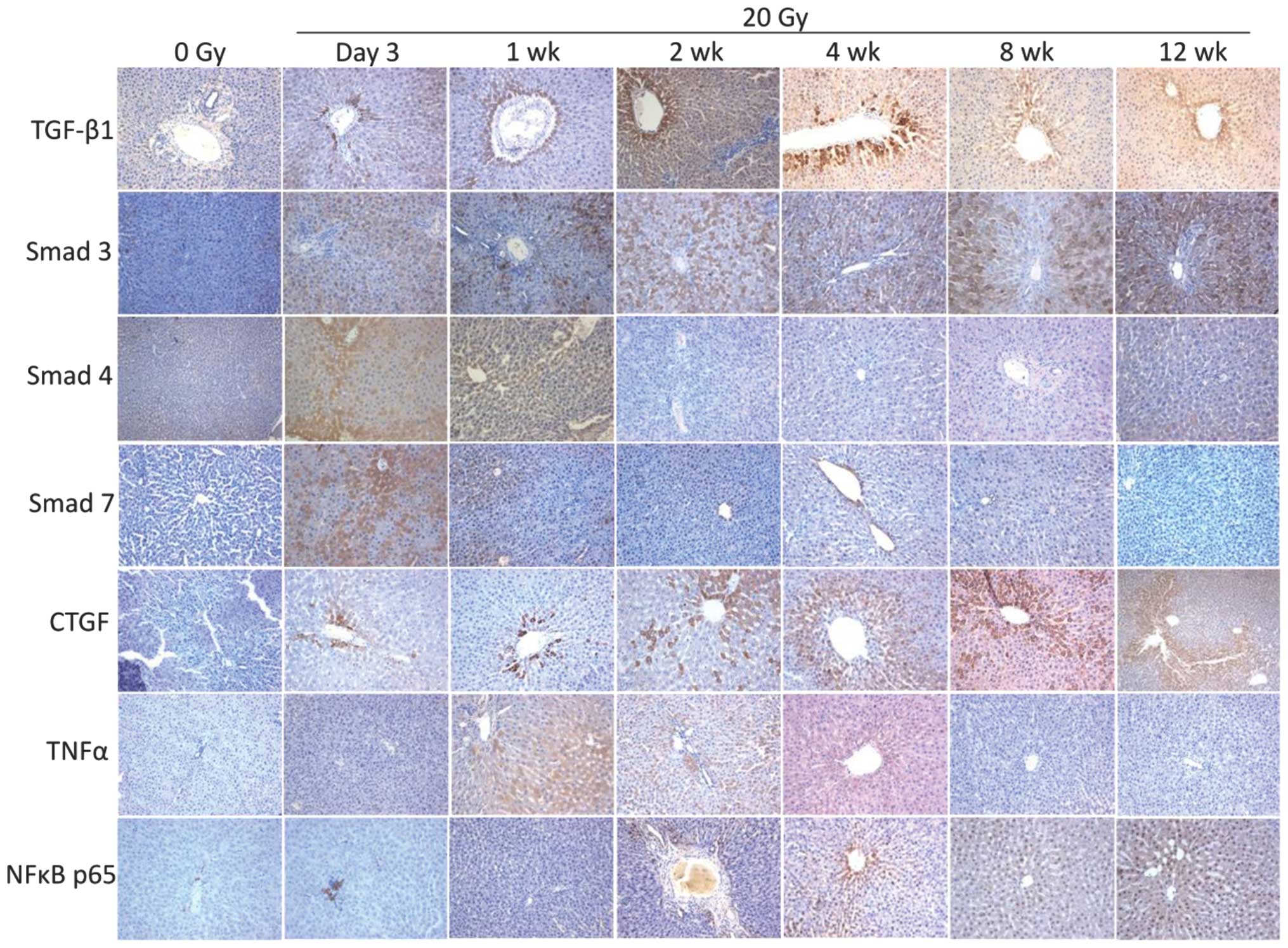 | Figure 2Immunohistochemical staining of liver
sections 3 days and 1, 2, 4, 8 and 12 weeks after 20 Gy
irradiation. Brown color denotes positivity. The perivenous area
was the major area of positive staining. TGF-β1, Smad3, CTGF and
NF-κB p65 staining was positive between 3 days and 12 weeks after
irradiation. Smad4 and Smad7 staining was positive between 3 days
and 1 week after irradiation. TNF-α staining was positive between 1
and 4 weeks after irradiation. (Magnification, ×200). Gy, grays;
TGF-β1, transforming growth factor-β1; CTGF, connective tissue
growth factor; TNFα, tumor necrosis factor-α, NF, nuclear
factor. |
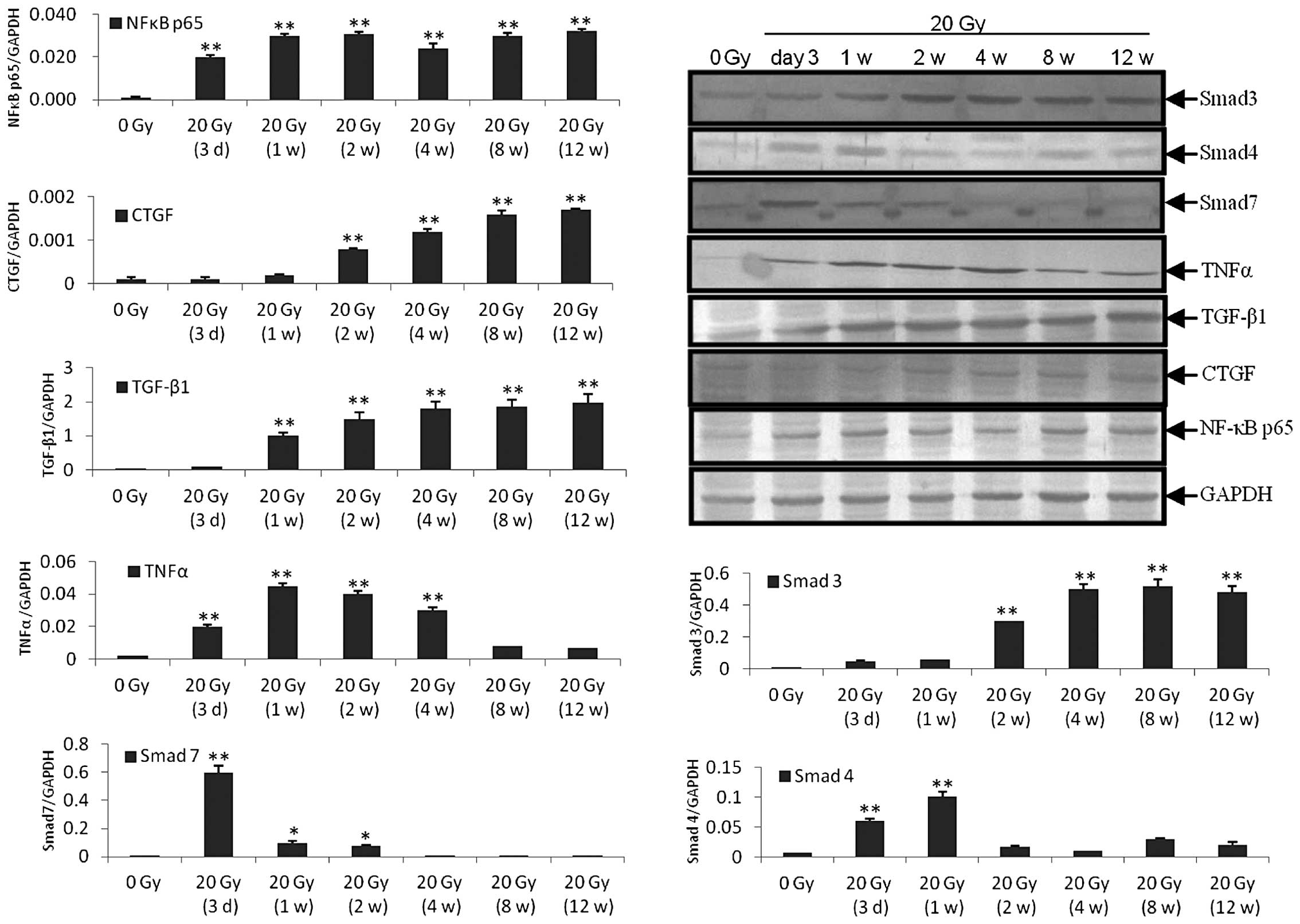 | Figure 4Western blot analysis (ratio of the
molecules investigated, vs. GAPDH). Similar to the results obtained
using the reverse transcription quantitative polymerase chain
reaction, the protein expression levels were as follows: NF-κB p65
was increased between 3 days and 12 weeks after irradiation. CTGF
and Smad3 were significantly increased between 2 and 12 weeks after
irradiation. TGF-β1 was significantly increased between 1 and 12
weeks after irradiation and TNF-α was significantly increased
between 3 days and 4 weeks after irradiation. Smad4 was
significantly increased between 3 days and 1 week after
irradiation. Smad7 was significantly increased 3 days after
irradiation and reduced significantly 1 and 2 weeks after
irradiation, however, it remained higher than that in the control.
From 4 weeks post-irradiation, the protein expression of Smad7
returned to the control level. *P<0.05,
**P<0.001 compared with the control group (0 Gy). Gy,
grays; CTGF, connective tissue growth factor; TGF-β1, transforming
growth factor-β1; TNFα, tumor necrosis factor-α; Smad, mothers
against decapentaplegic; NF, nuclear factor. |
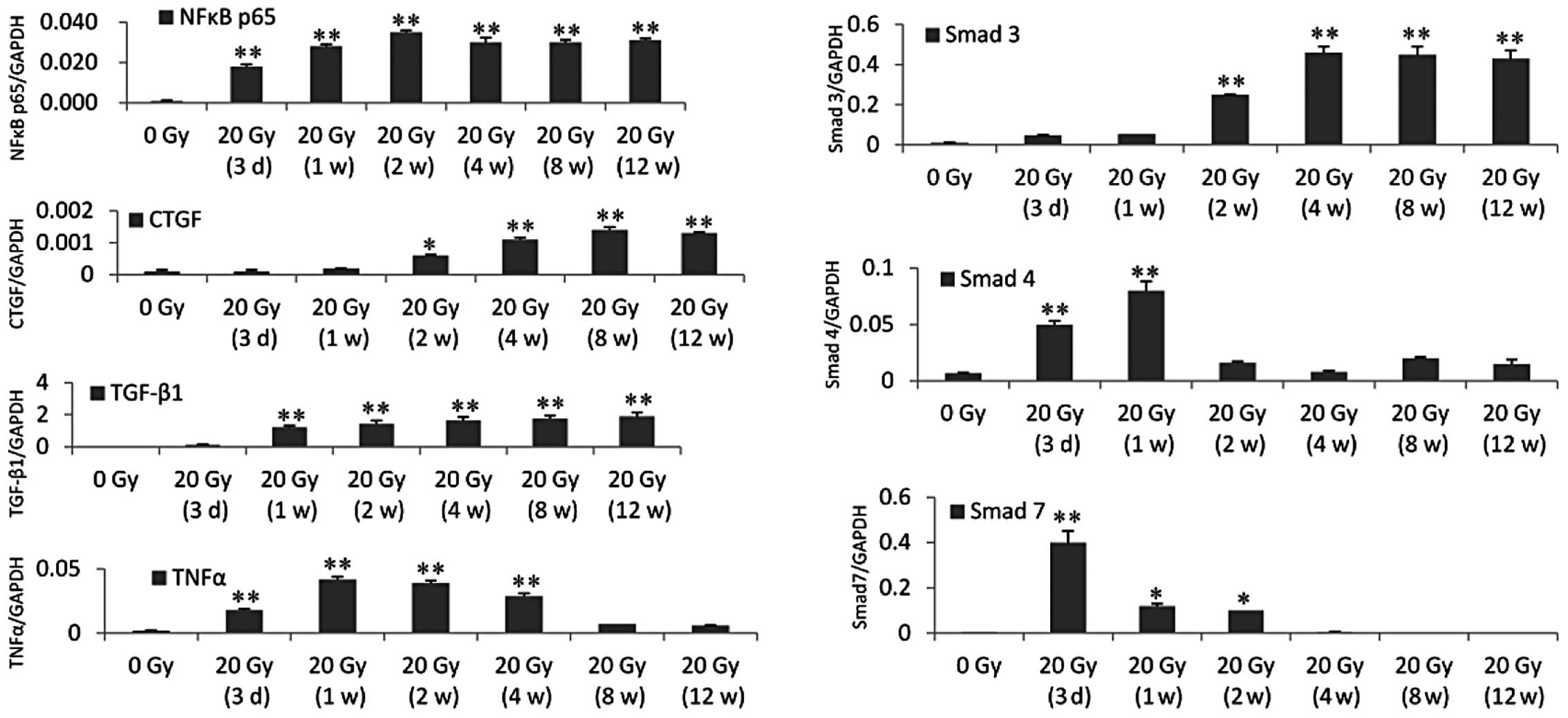 | Figure 3mRNA expression levels were
determined using reverse transcription quantitative polymerase
chain reaction (ratio of the molecules investigated, vs. GAPDH).
The mRNA expression of NF-κB p65 was upregulated between 3 days and
12 weeks after irradiation. The mRNA expression levels were as
follows: CTGF and Smad3 were significantly upregulated between 2
and 12 weeks after irradiation. TGF-β1 was significantly
upregulated between 1 and 12 weeks after irradiation. TNF-α was
significantly upregulated between 3 days and 4 weeks after
irradiation. Smad4 was significantly upregulated 3 days and 1 week
after irradiation. Smad7 was significantly upregulated 3 days after
irradiation and reduced significantly 1–2 weeks after irradiation,
however, the expression levels remained higher than that in the
control. From 4 weeks post-irradiation, the mRNA expression of
Smad7 returned to the control level. *P<0.05,
**P<0.001, compared with the control group (0 Gy).
CTGF, connective tissue growth factor; TGF-β1, transforming growth
factor-β1; TNFα, tumor necrosis factor-α, Smad, mothers against
decapentaplegic; NF, nuclear factor. |
Discussion
RT has become valuable in treating patients with
liver cancer, who are unsuitable for surgery or exhibit recurrence
following surgery. However, radiation induces hepatic toxicity,
which can be fatal (2). RILD is
characterized by anicteric ascites and hepatomegaly, isolated
elevation in the levels of ALP and/or markedly elevated serum
transaminases (1,6,7). In
the present study, the levels of AST, ALT and ALP were elevated
until 2 weeks after irradiation, which mimicked the clinical
situation. The tolerance of normal liver tissues to RT limits the
level of RT that can be administered to a patient undergoing cancer
treatment (2). Although RILD is a
dose-limiting complication, reduced doses may not be sufficient to
control tumor growth. Consequently, it may not be possible to cure
cancer as a result of the limitations imposed by normal tissue
tolerance (8,9).
The tolerance-dose of the whole liver is low and
liver damage is observed in 5–10% of patients treated with
fractionated doses of 30–35 Gy irradiation (10). Previous studies using RILD rat
models have observed different doses causing RILD, between 4 and 60
Gy (11–15). Our preliminary study used 5, 10 and
20 Gy irradiation to establish a RILD rat model. The results
revealed that 5 Gy irratiation caused no apparent liver damage and,
although RILD wasinduced at 10 Gy, the results were less stable
those observed at 20 Gy irradiation, which was used in the present
study. This suggested that ≥20 Gy is required to establish a
successful RILD model in SD rats. Veno-occlusive disease (VOD) is
the most frequently reported histopathological change following
human whole liver irradiation (16) and is characterized by perivenous
fibrosis, intimal proliferation with concentric endothelial
thickening and luminal narrowing by either edematous reticular or
collagen fibers (17). Collagen
proliferates along the hepatic sinusoids and produces mild
congestion in periportal areas. In the present study, H&E and
MT staining revealed collagen proliferation and perivenous fibrosis
12 weeks after irradiation, indicating that the VOD in the RILD rat
model occurred the at late phase of injury. Collagen proliferation
and perivenous fibrosis are responses of the liver to irradiation
stimulation (17). The growth
factors and cytokines involved in inflammation and immunity may be
important in this process. In RILD patients, hepatic stellate cells
are activated (18). Stellate
cells have multiple functions are involved in the regeneration of
hepatocytes and secretion of lipoproteins, growth factors and
cytokines, which are important in modulating inflammation and
fibrosis (19,20). Of these cytokines, TGF-β has been
implicated in subendothelial and hepatic fibrosis in RILD (21,22).
TGF-β is produced by numerous inflammatory, mesenchymal and
epithelial cells and converts fibroblasts and other cell types into
matrix-producing myofibroblasts (23–25).
Despite its role in normal wound healing, increased expression of
TGF-β1 has been demonstrated in a number of conditions
characterized by excessive fibrosis, including chronic hepatitis
and glomerulosclerosis (26–30).
In addition, effective treatments for these conditions have been
observed to reduce the development of fibrosis in the affected
organ, with a corresponding decrease in the expression of TGF-β1
(31,32). The present study demonstrated that
irradiation of the liver tissue activated TGF-β1 from 1 week after
irradiation to the end of the study (12 weeks after irradiation).
This indicated that certain inflammatory, stellate, mesenchymal and
epithelial cells may be involved in the complex process of
radiation-induced liver fibrosis by acting as cellular sources of
active TGF-β1. It has been suggested that the irradiation-induced
activation of TGF-β1 is rapid (33–35)
and prolonged exposure to TGF-β1 stimulates fibrosis and activates
the TGFβ1 signal transduction pathway (36,37).
The active form of TGF-β1 can then signal through either the
Smad-dependent or Smad-independent pathways. The TGF-β1 interactome
is highly complex, with several proteins interacting with its
transmembrane receptors and signaling proteins (Smads) within the
cytoplasm and the nucleus, affecting signaling crosstalk and
protein transcription (38,39).
There is substantial evidence supporting the importance of TGF-β1
in the development of excessive fibrosis following exposure to
radiation in animals and humans (40,41).
In addition, certain forms of radiation injury may develop via
Smad-independent TGF-β1 signaling (42,43).
Following exposure to radiation, reactive oxygen species are
produced, which are capable of activating latent TGF-β1 (44,45).
The upregulated levels of CTGF observed in the present study
suggested that it may be involved in mediating TGF-β1-induced
fibroblast collagen synthesis, as described previously (46). In the present study, the mRNA and
protein expression levels of TGF-β1 were elevated at the early
phase of recovery and were sustained at a high level until the late
phase of recovery. Similar changes were observed in the expression
levels of Smad3 and CTGF. However, the levels of Smad4 and Smad7
were only elevated in the early phase of recovery. The different
time windows observed in the molecular alteration of the
TGF-β1/Smads pathway indicate different potential strategies for
RILD intervention.
In the present study, the levels of NF-κB, an active
transcription factor in the radiation-induced adaptive response
(47), were upregulated. A cluster
of NF-κB regulated cytokines, including TNF-α, are induced by
radiation and contribute to the sensitivity of cells to radiation
(48). TNF-α activates NF-κB via
receptor activation (49) and
regulates the expression of numerous immune and inflammatory
response genes (50). TNF-α, which
normally induces an acute phase response in hepatocytes, becomes an
apoptotic agent (51). In the
present study, apoptosis was observed in the liver tissues
following irradiation, which was most severe between 2 and 4 weeks
after irradiation. However, the mRNA and protein expression levels
of TNF-α were increased as early as 3 days after irradiation and
were sustained at a high level until the 4th weeks after
irradiation. These results are in accordance with a previous report
that radiation induces the upregulation of TNF-α and causes
hepatocytes to become susceptible to TNF-α mediated apoptosis
(52). Previous animal studies
have observed that the initial inflammatory reaction following
irradiation is not followed by a recovery phase and complete
restitution. Instead, progressive liver fibrosis and cirrhosis is
regularly observed (53,54). TNF-α is involved in disparate
processes, including apoptosis, cell survival, inflammation and
immunity and may be an important initial step towards RILD and
liver fibrosis (53,54).
In conclusion, numerous cells, mediators and
signaling pathways are involved in the initiation and progression
of RILD, suggesting multiple complex potential mechanisms are
involved in the prevention and treatment of this disease. The
present study examined the potential targets of these pathways. The
alterations observed in the levels of the molecules and their
different time windows provided valuable information for future
studies. The results suggested that the mechanisms may involve the
early phase inflammation and immunity following irradiation and the
late phase of the recovery with fibrosis formation. The
NF-κB-mediated radiation response in the early phase requires
further investigation and TNF-α may activate NF-κB following
irradiation. The TGF-β1/Smads pathway is important for liver
fibrosis, with levels of CTGF being upregulated at the late phase
of irradiation. In addition, the specific roles of Smad3, Smad4 and
Smad require identification. Further studies on these pathways and
on the pharmaceutical intervention of these potential targets in
the pathway may be valuable for eliminating RILD.
Acknowledgements
This study was supported by the Natural Science
Foundation of Xinjiang Uygur Autonomous Region, China (no.
2012211A077).
References
|
1
|
Liang SX, Zhu XD, Xu ZY, Zhu J, Zhao JD,
Lu HJ, et al: Radiation-induced liver disease in three-dimensional
conformal radiation therapy for primary liver carcinoma: The risk
factors and hepatic radiation tolerance. Int J Radiat Oncol Biol
Phys. 65:426–434. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gil-Alzugaray B, Chopitea A, Iñarrairaegui
M, Bilbao JI, Rodriguez-Fraile M, Rodriguez J, et al: Prognostic
factors and prevention of radioembolization-induced liver disease.
Hepatology. 57:1078–1087. 2013. View Article : Google Scholar
|
|
3
|
Guha C and Kavanagh BD: Hepatic radiation
toxicity: Avoidance and amelioration. Semin Radiat Oncol.
21:256–263. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shi SR, Key ME and Kalra KL: Antigen
retrieval in formalin-fixed, paraffin-embedded tissues: an
enhancement method for immunohistochemical staining based on
microwave oven heating of tissue sections. J Histochem Cytochem.
39:741–748. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu ZY, Liang SX, Zhu J, Zhu XD, Zhao JD,
Lu HJ, et al: Prediction of radiation-induced liver disease by
Lyman normal-tissue complication probability model in
three-dimensional conformal radiation therapy for primary liver
carcinoma. Int J Radiat Oncol Biol Phys. 65:189–195. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng JC, Wu JK, Huang CM, Liu HS, Huang
DY, Cheng SH, et al: Radiation-induced liver disease after
three-dimensional conformal radiotherapy for patients with
hepatocellular carcinoma: Dosimetric analysis and implication. Int
J Radiat Oncol Biol Phys. 54:156–162. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Emami B, Lyman J, Brown A, et al:
Tolerance of normal tissue to therapeutic irradiation. Int J Radiat
Oncol Biol Phys. 21:109–122. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Milano MT, Constine LS and Okunieff P:
Normal tissue tolerance dose metrics for radiation therapy of major
organs. Semin Radiat Oncol. 17:131–140. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dancygier H and Schirmacher P:
Radiation-induced liver damage. Clinical Hepatology. Dancygier H:
Springer; New York, NY, USA: pp. 2032010
|
|
11
|
Rave-Frank M, Malik IA, Christiansen H,
Naz N, Sultan S, Amanzada A, et al: Rat model of fractionated (2
Gy/day) 60 Gy irradiation of the liver: long-term effects. Radiat
Environ Biophys. 52:321–338. 2013. View Article : Google Scholar
|
|
12
|
Erbil Y, Oztezcan S, Giris M, Barbaros U,
Olgac V, Bilge H, et al: The effect of glutamine on
radiation-induced organ damage. Life Sci. 4:376–382. 2005.
View Article : Google Scholar
|
|
13
|
Adaramoye O, Ogungbenro B, Anyaegbu O and
Fafunso M: Protective effects of extracts of vernonia amygdalina,
hibiscus sabdariffa and vitamin C against radiation-induced liver
damage in rats. J Radiat Res. 49:123–131. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chi K, Liao C, Chand C, et al: Angiogenic
blockade and radiotherapy in hepatocellular carcinoma. Int J Radiat
Oncol Biol Phys. 1:188–193. 2010. View Article : Google Scholar
|
|
15
|
Gencel O, Naziroqlu M, Celik O, Yalman K,
Bayram D, et al: Selenium and vitamin E modulates radiation-induced
liver toxicity in pregnant and nonpregnant rat: effects of
colemanite and hematite shielding. Biol Trace Elem Res. 135:253–63.
2010. View Article : Google Scholar
|
|
16
|
Ingold JA, Reed GB, Kaplan HS and Bagshaw
MA: Radiation Hepatitis. Am J Roentgenol Radium Ther Nucl Med.
93:200–208. 1965.PubMed/NCBI
|
|
17
|
Reed GB Jr and Cox AJ Jr: The human liver
after radiation injury. A form of veno-occlusive disease. Am J
Pathol. 48:597–611. 1966.PubMed/NCBI
|
|
18
|
Friedman SL: Mechanisms of hepatic
fibrogenesis. Gastroenterology. 134:1655–1669. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Taimr P, Higuchi H, Kocova E, et al:
Activated stellate cells express the TRAIL receptor-2/death
receptor-5 and undergo TRAIL-mediated apoptosis. Hepatology.
37:87–95. 2003. View Article : Google Scholar
|
|
20
|
Wright M, Issa R, Smart D, et al:
Gliotoxin stimulates the apoptosis of human and rat hepatic
stellate cells and enhances the resolution of liver fibrosis in
rats. Gastroenterology. 121:685–698. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Anscher MS, Chen L, Rabbani Z, et al:
Recent progress in defining mechanisms and potential targets for
prevention of normal tissue injury after radiation therapy. Int J
Radiat Oncol Biol Phys. 62:255–259. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Anscher MS, Crocker IR and Jirtle RL:
Transforming growth factor-beta 1 expression in irradiated liver.
Radiat Res. 122:77–85. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kong FM, Anscher M, Xiong Z, et al:
Elevated circulating transforming growth factor-1 levels decreased
after radiotherapy in patients with lung cancer, cervical cancer
and Hodgkin’s disease: A possible tumor marker. Int J Radiat Oncol
Biol Phys. 32:2391995. View Article : Google Scholar
|
|
24
|
Novakov-Jiresova A, Van Gameren MM, Coppes
RP, et al: Transforming growth factor-beta plasma dynamics and
post-irradiation lung injury in lung cancer patients. Radiother
Oncol. 71:183–189. 2004. View Article : Google Scholar
|
|
25
|
Hakenjos L, Bamberg M and Rodemann H:
TGF-beta1-mediated alterations of rat lung fibroblast
differentiation resulting in the radiation-induced fibrotic
phenotype. Int J Radiat Biol. 76:503–509. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Border WA, Brees D and Noble NA:
Transforming growth factor-beta and extracellular matrix deposition
in the kidney. Contrib Nephrol. 107:140–145. 1994.PubMed/NCBI
|
|
27
|
Peters H, Border WA and Noble NA:
Targeting TGF-beta overexpression in renal disease: Maximizing the
antifibrotic action of angiotensin II blockade. Kidney Int.
54:1570–1580. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Border WA and Ruoslahti E: Transforming
growth factor-β in disease: The dark side of tissue repair. J Clin
Invest. 90:1–7. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bartram U and Speer CP: The role of
transforming growth factor beta in lung development and disease.
Chest. 125:754–765. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Anscher MS, Kong FM and Jirtle RL: The
relevance of transforming growth factor beta 1 in pulmonary injury
after radiation therapy. Lung Cancer. 19:109–120. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rabbani ZN, Anscher MS, Zhang X, et al:
Soluble TGFbeta type II receptor gene therapy ameliorates acute
radiation-induced pulmonary injury in rats. Int J Radiat Oncol Biol
Phys. 57:563–572. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rabbani ZN, Anscher MS, Golson ML, et al:
Overexpression of extracellular superoxide dismutase reduces
severity of radiation-induced lung toxicity through downregulation
of the TGF-β1 signal transduction pathway. Int J Radiat Oncol Biol
Phys. 57:S158–S159. 2003. View Article : Google Scholar
|
|
33
|
Anscher MS, Kong FM, Andrews K, et al:
Plasma transforming growth factor β1 as a predictor of radiation
pneumonitis. Int J Radiat Oncol Biol Phys. 41:1029–1035. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Anscher MS, Kong FM, Marks LB, et al:
Changes in plasma transforming growth factor beta during
radiotherapy and the risk of symptomatic radiation- induced
pneumonitis. Int J Radiat Oncol Biol Phys. 37:253–258. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Anscher MS, Murase T, Prescott DM, et al:
Changes in plasma TGF beta levels during pulmonary radiotherapy as
a predictor of the risk of developing radiation pneumonitis. Int J
Radiat Oncol Biol Phys. 30:671–676. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Franko AJ, Sharplin J, Ghahary A, et al:
Immunohistochemical localization of transforming growth factor beta
and tumor necrosis factor alpha in the lungs of fibrosis-prone and
‘non-fibrosing’ mice during the latent period and early phase after
irradiation. Radiat Res. 147:245–256. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liguang C, Larrier N, Rabbani ZN, et al:
Assessment of the protective effect of keratinocyte growth factor
on radiation-induced pulmonary toxicity in rats. Int J Radiat Oncol
Biol Phys. 57:S1622003. View Article : Google Scholar
|
|
38
|
Taylor IW and Wrana JL: SnapShot: The
TGFbeta pathway interactome. Cell. 133:3782008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bierie B and Moses HL: Tumour
microenvironment: TGF beta: The molecular Jekyll and Hyde of
cancer. Nat Rev Cancer. 6:506–520. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Flanders KC, Sullivan CD, Fujii M, et al:
Mice lacking Smad3 are protected against cutaneous injury induced
by ionizing radiation. Am J Pathol. 160:1057–1068. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Peters CA, Stock RG, Cesaretti JA, et al:
TGFβ1 single nucleotide polymorphisms are associated with adverse
quality of life in prostate cancer patients treated with
radiotherapy. Int J Radiat Oncol Biol Phys. 70:752–759. 2008.
View Article : Google Scholar
|
|
42
|
Haydont V, Mathe D, Bourgier C, et al:
Induction of CTGF by TGF-β1 in normal and radiation enteritis human
smooth muscle cells: Smad/Rho balance and therapeutic perspectives.
Radiother Oncol. 76:219–225. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Haydont V, Riser BL, Aigueperse J, et al:
Specific signals involved in the long-term maintenance of
radiation-induced fibrogenic differentiation: A role for CCN2 and
low concentration of TGF-beta1. Am J Physiol Cell Physiol.
294:C1332–C1341. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Riley PA: Free radicals in biology:
Oxidative stress and the effects of ionizing radiation. Int J
Radiat Biol. 65:27–33. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Barcellos-Hoff MH and Dix TA:
Redox-mediated activation of latent transforming growth
factor-beta1. Mol Endocrinol. 10:1077–1083. 1996.PubMed/NCBI
|
|
46
|
Duncan MR, Frazier KS, Abramson S,
Williams S, Klapper H, et al: Connective tissue growth factor
mediates transforming growth factor beta-induced collagen
synthesis: downregulation by cAMP. FASEB J. 13:1774–1786.
1999.PubMed/NCBI
|
|
47
|
Ahmed KM and Li JJ: NF-kappa B-mediated
adaptive resistance to ionizing radiation. Free Radic Biol Med.
44:1–13. 2008. View Article : Google Scholar :
|
|
48
|
Hallahan DE, Spriggs DR, Beckett MA, Kufe
DW and Weichselbaum RR: Increased tumor necrosis factor alpha mRNA
after cellular exposure to ionizing radiation. Proc Natl Acad Sci
USA. 86:10104–10107. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Blonska M, You Y, Geleziunas R and Lin X:
Restoration of NFkappaB activation by tumor necrosis factor alpha
receptor complex-targeted MEKK3 in receptor-interacting
protein-deficient cells. Mol Cell Biol. 24:10757–10765. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Baeuerle PA and Henkel T: Function and
activation of NF-kappa B in the immune system. Annu Rev Immunol.
12:141–179. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Majno PE, Morel P and Mentha G:
Mini-review: tumor necrosis factor (TNF) and TNF soluble receptors
(TNF-sR) in liver disease and liver transplantation. Swiss Surg.
4:182–185. 1995.PubMed/NCBI
|
|
52
|
Tello K, Christiansen H, Gürleyen H, et
al: Irradiation leads to apoptosis of Kupffer cells by a
Hsp27-dependant pathway followed by release of TNF-alpha. Radiat
Environ Biophys. 47:389–397. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Franko AJ, Sharplin J, Ghahary A and
Barcellos-Hof MH: Immunohistochemical localization of transforming
growth factor beta and tumor necrosis factor alpha in the lungs of
fibrosis-prone and non-fibrosing mice during the latent period and
early phase after irradiation. Radiat Res. 147:245–256. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Geraci JP, Mariano MS and Jackson KL:
Radiation hepatology of the rat: time-dependent recovery. Radiat
Res. 136:214–221. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Iwamoto KS and McBride WH: Production of
13-hydroxyoctadecadienoic acid and tumor necrosis factor-alpha by
murine peritoneal macrophages in response to irradiation. Radiat
Res. 139:103–108. 1994. View Article : Google Scholar : PubMed/NCBI
|