Introduction
Atrial fibrillation (AF) is the most commonly
observed form of clinical arrhythmia and its incidence increases
with age (1). AF may predispose
patients to thrombosis and myocardial ischemia induced by heart
failure, which in turn can trigger malignant arrhythmia events,
including ventricular tachycardia and ventricular fibrillation
(1). Therefore, investigating the
pathogenetic mechanisms underlying AF and developing effective
prevention and treatment is of paramount clinical significance.
Furthermore, there is currently no widely accepted treatment for
AF, highlighting the requirement for more robust and universally
applicable treatment strategies for AF.
In recent years, a number of studies have reported
that inflammation has a central and positive role in the etiology
of AF (2–7), which develops in 25–40% of patients
undergoing cardiac surgery (2).
The levels of pro-inflammatory interleukin (IL)-6 have been
observed to peak 6 h after surgery, whereas C-reactive protein
(CRP) and CRP-complement complexes peaked 2 and 3 days after
surgery, respectively (2). The AF
events were predominantly observed between 2 and 3 days after
surgery, a correlation that suggests inflammation may be involved
in AF in these patients. Consistent with this hypothesis, a
previous study observed that the levels of IL-6 were significantly
elevated in patients who developed AF following cardiac surgery
(3).
Previous reports have demonstrated that statins or
anti-inflammatory hormones inhibit inflammation and limit the
development of AF (8–12). Simvastatin inhibits atrial
electrical remodeling, which may be associated with its ability to
limit inflammatory responses (8).
In a canine sterile pericarditis model of AF, Kumagai et al
(9) demonstrated that atorvastatin
reduced the elevation of CRP caused by aseptic pericarditis and
reduced the inducibility of AF (9). Notably, anti-inflammatory therapy can
effectively prevent the occurrence of postoperative AF in patients
with cardiac surgery (10) and
these hormones can reduce CRP levels and prevent recurrence of AF
(11). Following electrical
cardioversion in cases of persistent AF, statins significantly
reduce the rate of recurrence of AF (12). Despite this correlative data,
however, it remains to be elucidated whether inflammation is
directly involved in the pathogenesis of AF. Therefore, improving
understanding is important for designing and implementing more
effective therapeutic strategies for patients with AF.
The prevalent aseptic pericarditis and goat rapid
atrial pacing models (13,14) for investigating AF have potential
limitations. In the aseptic pericarditis model, inflammation always
precludes AF, thus biasing against the observation of an
association between AF and inflammation (13). In the goat rapid atrial pacing
model, AF is induced through pacing-induced changes in cardiac
electrophysiological characteristics (14); however, this does not enable
assessment of the role of inflammation in the process. Thus, in the
present study, a goat aseptic pericarditis model that causes rapid
atrial excitement was established. A combination of aseptic
pericarditis with atrial excitement provides a physiological
context to investigate the contribution of the changes in
inflammatory cytokines and atrial electrophysiological properties
and provides additional insight into the role of statins in the
etiology of AF.
Materials and methods
Reagents and equipment
Sodium pentobarbital powder was purchased from Sino
Pharm Chemical Reagent Beijing Co., Ltd. (Beijing, China). Goat
serum hs-CRP, goat serum IL-6 and goat serum tumor necrosis factor
(TNF)-α enzyme-linked immunosorbent assay (ELISA) kits were
purchased from SunBio Biomedical Technology Co., Ltd. (Beijing,
China). Atorvastatin calcium tablets were purchased from Pfizer
(Dalian, China). An electrophysiological recording system (cat. no.
GY-6328) was purchased from HuaNan Medical Science and Technology
Co., Ltd. (Henan, China). A TECS II type program stimulator and
Siemens-SV 900C ventilator were purchased from Medico (Padua,
Italy) and Siemens (Erlangen, Germany), respectively. Ethicon
medical sutures were purchased from Johnson & Johnson (New
Brunswick, NJ, USA).
Animals
Healthy adult male goats (n=15) weighing 20–25 kg
were maintained in the PLA General Hospital Experimental Animal
Center (Haidian, China), with access to food and water ad
libitum. The animals were housed in separated large pens with
straw bedding and controlled temperature at 22°C. All animal
procedures were performed in compliance with the Institutional
Animal Care and Use Committee of the PLA General Hospital. The
present study was approved by the ethics committee of PLA General
Hospital.
Induction of aseptic pericarditis
The epicardial electrodes were prepared, as
described in a previous study (15). Briefly, 1 mm diameter silver wire
was used for preparation of the electrode tip, which was 1.5 mm in
diameter. The left and right atrial electrode sheets contained five
and two pairs of electrodes, respectively. The internal spacing and
the distance between the electrodes was 5 mm.
Following a 24 h fast, the goats were administered
with intravenous (i.v.) injection of 3% sodium pentobarbital (30
mg/kg) for anesthesia, with additional sodium pentobarbital (10
mg/kg) injected hourly. Ventilator-assisted breathing was provided
following right lateral endotracheal intubation. An incision was
made at the fourth intercostal space. The pericardium was opened
and the heart was exposed. Electrodes were sutured to the free wall
epicardium of both atria. Sterile talc powder (5–8 g; AppliChem,
Inc., Beijing, China) was used to cover the surface of the atria,
which was then covered with a layer of gauze. The control group
received electrode implantation only, without talc. The chest was
closed layer by layer and a drummed lung ventilator was used to
prevent pneumothorax. The electrode wiring was run to the neck skin
through a subcutaneous tunnel. Infection was prevented by a
twice-daily i.v. infusion of 4,800,000 units of penicillin sodium,
commencing 1 day prior to surgery and continuing for 3 days
thereafter. Following surgery, the goats were housed in their
original cages. Blood samples were harvested at baseline, 12, 24,
48 and 72 h, and 7, 14 and 21 days. Electrophysiological recordings
were performed at baseline, 24, 48 and 72 h, and 7, 14 and 21
days.
Experimental animal grouping and
parameter measurements
A total of 15 goats were randomly divided into the
following three groups, each containing five animals: No induction
of pericarditis (control group); pericarditis induction without
statin intervention (subsequently referred to as the pericarditis
group) and pericarditis induction with statin intervention (statin
group). The animals in the statin group were orally administered
with 60 mg atorvastatin calcium daily, commencing 1 week prior to
surgery until the experimental endpoint. All the animals underwent
thoracotomy, in which atrial epicardial electrodes were implanted.
Pericarditis was induced in the pericarditis and statin groups
only, as described above. The left and right atrial effective
refractory periods (ERP), rate of ERP adaptation, left and right
atrial conduction velocity (CV) and AF inducibility and duration
were measured in all the animals. The inflammatory markers were
assayed from jugular venous blood samples, which were obtained
prior to surgery and at the indicated time points following the
procedure for up to 21 days. The blood samples were centrifuged at
2,500 × g for 20 min at 4°C. The serum was collected and stored at
−80°C. The serum levels of hs-CRP, IL-6 and TNF-α were measured via
solid-phase ELISA.
Tissue specimen preparation and
staining
The animals were sacrificed by bleeding after
induction of general anesthesia. The left and right atrial free
wall tissues were harvested post-sacrifice and fixed in 10% neutral
formalin solution for 24 h. Tissue processing and hematoxylin and
eosin (H&E) staining was then performed according to standard
techniques.
Statistical analysis
All statistical analyses were performed using SPSS
11.0 software (SPSS, Inc., Chicago, IL, USA). The data are
presented as the mean ± standard deviation and were compared using
one-way analysis of variance (ANOVA). The inducibility of AF was
analyzed using a χ2 test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Atorvastatin reduces inflammatory markers
following pericarditis
In the preoperative baseline state, no statistically
significant difference was observed in the serum levels of hs-CRP,
IL-6 or TNF-α between the control, pericarditis and statin groups
(Tables I–III). The serum levels of hs-CRP in the
statin and pericarditis groups were significantly increased above
the baseline levels 12 h after surgery (P<0.05) and peaked 72 h
after surgery (1.542±0.114 and 1.287±0.091 ng/ml, respectively).
Furthermore, these numbers were significantly higher when compared
with the control group (P<0.05; Table I). The levels of hs-CRP began to
decline 7 days after surgery, however, they remained significantly
elevated compared with the preoperative state (P<0.05; Table I). Notably, the serum level of
hs-CRP in the atorvastatin group 48 h after surgery was
significantly lower compared with that of the pericarditis group
(P<0.05; Table I; Fig. 1A).
 | Table ISerum levels of high-sensitivity
C-reactive protein in different experimental groups. |
Table I
Serum levels of high-sensitivity
C-reactive protein in different experimental groups.
| Time period | Control group
(ng/ml) | Pericarditis group
(ng/ml) | Statin intervention
group (ng/ml) |
|---|
| Preoperative | 0.401±0.036 | 0.407±0.055 | 0.445±0.051 |
| Postoperative |
| 12 h | 0.588±0.022a | 0.766±0.063a,b | 0.724±0.053a,b |
| 24 h | 0.612±0.072a | 0.974±0.075a,b | 0.903±0.067a,b |
| 48 h | 0.647±0.051a | 1.323±0.107a,b | 1.172±0.084a,b,c |
| 72 h | 0.569±0.047a | 1.542±0.114a,b | 1.287±0.091a,b,c |
| 7 days | 0.504±0.049a | 1.294±0.105a,b | 1.113±0.075a,b,c |
| 14 days | 0.486±0.068 | 1.204±0.086a,b | 1.042±0.074a,b,c |
| 21 days | 0.471±0.072 | 1.127±0.096a,b | 1.007±0.063a,b,c |
 | Table IIISerum tumor necrosis factor-α levels
in different experimental groups. |
Table III
Serum tumor necrosis factor-α levels
in different experimental groups.
| Time period | Control group
(pg/ml) | Pericarditis group
(pg/ml) | Statin intervention
group (pg/ml) |
|---|
| Preoperative | 63.2±6.4 | 61.9±5.6 | 65.1±7.4 |
| Postoperative |
| 12 h | 68.9±3.5a | 92.4±5.7a,b | 85.5±6.1a,b |
| 24 h | 71.7±5.1a | 105.7±7.4a,b | 94.2±6.4a,b,c |
| 48 h | 73.4±7.6a | 114.8±8.3a,b | 102.1±6.7a,b,c |
| 72 h | 72.9±5.5a | 107.5±10.2a,b | 93.7±7.6a,b,c |
| 7 days | 66.3±7.2 | 96.2±8.9a,b | 86.1±5.8a,b,c |
| 14 days | 65.4±6.4 | 93.6±8.1a,b | 82.4±6.9a,b,c |
| 21 days | 65.7±7.3 | 92.4±7.2a,b | 81.6±7.4a,b,c |
In the atorvastatin and pericarditis groups, the
serum levels of IL-6 and TNF-α were significantly increased 12 h
after surgery (P<0.05), peaked 48 h after surgery and were
significantly higher compared with the levels in the control group
(P<0.05; Tables II and
III; Fig. 1B and C). As with the levels of
hs-CRP, the serum levels of IL-6 and TNF-α in the control group
began decrease significantly 7 days after surgery and continued to
decline until the end of the experiment. At the experiment
endpoint, IL-6 and TNF-α remained increased compared with the
preoperative baseline level, however, the difference was not
statistically significant. In the atorvastatin and pericarditis
groups, serum levels of IL-6 and TNF-α gradually decreased, but
remained higher than at the preoperative stage, even following the
21 day endpoint (P<0.05). The levels of both cytokines were
significantly lower in the atorvastatin group compared with the
pericarditis group 24 h after surgery (P<0.05; Tables II and III; Fig.
1B and C).
 | Table IISerum levels of interleukin-6 in
different experimental groups. |
Table II
Serum levels of interleukin-6 in
different experimental groups.
| Time period | Control group
(pg/ml) | Pericarditis group
(pg/ml) | Statin intervention
group (pg/ml) |
|---|
| Preoperative | 139.1±14.1 | 134.7±16.6 | 142.4±15.2 |
| Postoperative |
| 12 h | 160.2±13.9a | 198.9±15.7a,b | 187.4±13.3a,b |
| 24 h | 167.6±13.3a | 237.1±12.3a,b | 217.8±15.3a,b,c |
| 48 h | 165.2±16.4a | 254.2±11.5a,b | 225.5±17.2a,b,c |
| 72 h | 161.4±14.7a | 240.9±12.8a,b | 215.4±16.1a,b,c |
| 7 days | 144.8±15.7 | 215.0±15.2a,b | 193.1±15.8a,b,c |
| 14 days | 142.3±17.1 | 202.7±14.9a,b | 182.3±15.8a,b,c |
| 21 days | 143.4±15.5 | 199.2±13.5a,b | 180.2±13.2a,b,c |
Atorvastatin extends left atrial ERP
following pericarditis
As expected, the preoperative basic cycle lengths
(BCL) at 500, 400, 300 and 200 ms were not significantly different
between the three groups (Tables
IV–VII). In all groups, the
left atrial ERP was significantly lower immediately following
surgery compared with the preoperative baseline (P<0.05) and was
gradually decreased until 72 h after surgery (Tables IV–VII). The animals in each group
exhibited extended left atrial ERP 7 days after surgery, whereas no
significant differences in left atrial ERP were observed in the
control group at 14 and 21 days compared with the baseline ERP.
However, in the atorvastatin and pericarditis groups, the left
atrial ERP remained significantly lower than the baseline
(P<0.05; Tables IV–VII). Compared with the control group,
the left atrial ERP in the pericarditis group was significantly
shorter (P<0.05), while in the atorvastatin group, it was
significantly prolonged relative to the pericarditis group
(P<0.05). No differences were observed in right atrial ERP in
the preoperative state, however, between 24 and 72 h
postoperatively, the right atrial ERP in the pericarditis group was
significantly shorter compared with the control group (P<0.05).
The right atrial ERP in each group was significantly longer than
the left atrial ERP (P<0.05; Tables IV–VII).
 | Table IVComparison of atrial effective
refractory period in different experimental groups at a basic cycle
length of 500 ms. |
Table IV
Comparison of atrial effective
refractory period in different experimental groups at a basic cycle
length of 500 ms.
| Control group
(ms) | Pericarditis group
(ms) | Statin intervention
group (ms) |
|---|
|
|
|
|
|---|
| LA | RA | LA | RA | LA | RA |
|---|
| Preoperative | 182.7±19.3 | 228.8±16.0 | 188.9±21.4 | 216.9±18.6 | 184.7±14.1 | 211.3±12.7 |
| Postoperative |
| 24 h | 151.1±13.4a | 169.9±11.7a | 122.3±13.7a,b | 143.2±12.2a,b | 140.7±8.9a,c | 163.6±9.8a,c |
| 48 h | 149.9±9.6a | 172.2±11.9a | 123.6±8.7a,b | 141.4±13.2a,b | 135.4±7.3a,b | 160.3±8.0a,c |
| 72 h | 145.9±9.8a | 170.7±11.8a | 121.1±11.1a,b | 142.7±19.0a,b | 133.9±7.4a | 159.6±9.4a |
| 7 days | 157.4±14.8a | 190.5±9.3a | 134.4±17.5a,b | 158.0±27.5a | 160.7±7.4a,c | 172.6±7.8a |
| 14 days | 166.3±19.1 | 196.3±9.6 | 144.3±8.3a,b | 179.1±18.1a | 158.0±5.3a,c | 181.7±11.0a |
| 21 days | 175.1±20.4 | 219.7±8.5 | 147.2±12.1a,b | 186.0±15.5a,b | 170.6±7.6a,c | 190.1±13.8a,b |
 | Table VIIComparison of atrial effective
refractory period in different experimental groups at a basic cycle
length of 200 ms. |
Table VII
Comparison of atrial effective
refractory period in different experimental groups at a basic cycle
length of 200 ms.
| Control group
(ms) | Pericarditis group
(ms) | Statin intervention
group (ms) |
|---|
|
|
|
|
|---|
| LA | RA | LA | RA | LA | RA |
|---|
| Preoperative | 148.8±12.1 | 170.7±8.5 | 151.9±11.1 | 168.4±8.9 | 150.5±9.1 | 165.5±7.5 |
| Postoperative |
| 24 h | 135.3±11.9a | 154.8±10.2a | 103.3±9.2a,b | 129.1±9.1a,b | 118.4±6.6a,b,c | 141.8±10.1a |
| 48 h | 134.0±12.7a | 155.9±8.2a | 104.9±10.2a,b | 131.3±11.9a,b | 116.8±7.1a,b | 140.3±9.7a,c |
| 72 h | 131.4±13.8a | 160.3±11.9 | 105.8±10.6a,b | 131.0±17.0a,b | 119.1±8.4a | 140.3±8.1a,b |
| 7 days | 140.5±12.5 | 166.6±9.8 | 111.7±11.4a,b | 146.1±18.9a | 126.9±4.9a,c | 155.5±7.4a |
| 14 days | 142.1±10.8 | 168.3±7.5 | 117.1±11.7a,b | 162.7±13.0 | 132.6±6.5a,c | 163.5±6.7 |
| 21 days | 144.1±11.3 | 170.5±6.5 | 118.4±10.9a,b | 167.1±8.9 | 141.7±8.0c | 165.0±5.6 |
Atorvastatin assists in the recovery of
atrial ERP rate adaptation following pericarditis
The atrial ERP rate adaptation did not differ
significantly between the groups in the preoperative state
(Table VIII). However, the
atrial ERP rate adaptation 24 h after surgery was significantly
lower in each group (P<0.05). In the pericarditis group, the
left and right atrial ERP rate adaptation 72 h after surgery
declined to 0.00±0.02 and 0.01±0.02 ms, respectively, with complete
loss of atrial ERP rate adaptation. Following this time point, the
atrial ERP rate adaptation of each group gradually recovered. In
the control and atorvastatin groups, the left and right atrial ERP
rate adaptation had recovered to normal values by the end of the
experiment, however, the pericarditis group exhibited poor atrial
ERP rate adaptation, which remained significantly lower than
baseline levels (P<0.05; Table
VIII; Fig. 2A and B).
 | Table VIIIChanges of atrial effective
refractory period rate adaptation in the different experimental
groups. |
Table VIII
Changes of atrial effective
refractory period rate adaptation in the different experimental
groups.
| Control group
(ms) | Pericarditis group
(ms) | Statin intervention
group (ms) |
|---|
|
|
|
|
|---|
| LA | RA | LA | RA | LA | RA |
|---|
| Preoperative | 0.16±0.02 | 0.22±0.11 | 0.16±0.03 | 0.24±0.07 | 0.18±0.06 | 0.21±0.03 |
| Postoperative |
| 24 h | 0.06±0.02a | 0.05±0.04a | 0.03±0.01a | 0.03±0.03a | 0.06±0.04a | 0.05±0.03a |
| 48 h | 0.06±0.04a | 0.05±0.04a | 0.02±0.02a | 0.03±0.02a | 0.05±0.02a | 0.05±0.05a |
| 72 h | 0.05±0.01a | 0.05±0.04a | 0.00±0.02a,b | 0.01±0.02a | 0.04±0.04a,c | 0.04±0.03a |
| 7 days | 0.07±0.03a | 0.06±0.02a | 0.03±0.04a | 0.06±0.03a | 0.07±0.03a | 0.06±0.04a |
| 14 days | 0.11±0.06 | 0.12±0.04a | 0.05±0.02a | 0.07±0.02a | 0.08±0.05a | 0.06±0.03a |
| 21 days | 0.13±0.05 | 0.16±0.12 | 0.06±0.02a | 0.07±0.03a | 0.09±0.06a | 0.09±0.07a |
Atrial ERP rate adaptation is negatively
correlated with serum levels of hs-CRP
Linear regression analysis between the left and
right atrial ERP rate adaptations and levels of hs-CRP in the
pericarditis group revealed that the left (Fig. 2C) and right (Fig. 2D) atrial ERP rate adaptation and
the levels of hs-CRP were negatively correlated (multiple
correlation coefficients of 0.9202 and 0.8901).
The left and right atrial conduction velocity (CV)
did not vary significantly between the experimental groups. In the
preoperative state, no significant difference was observed in
atrial CV between the left and right atria in the groups. The
atrial CV in each group decelerated postoperatively, however, not
significantly compared with the baseline values.
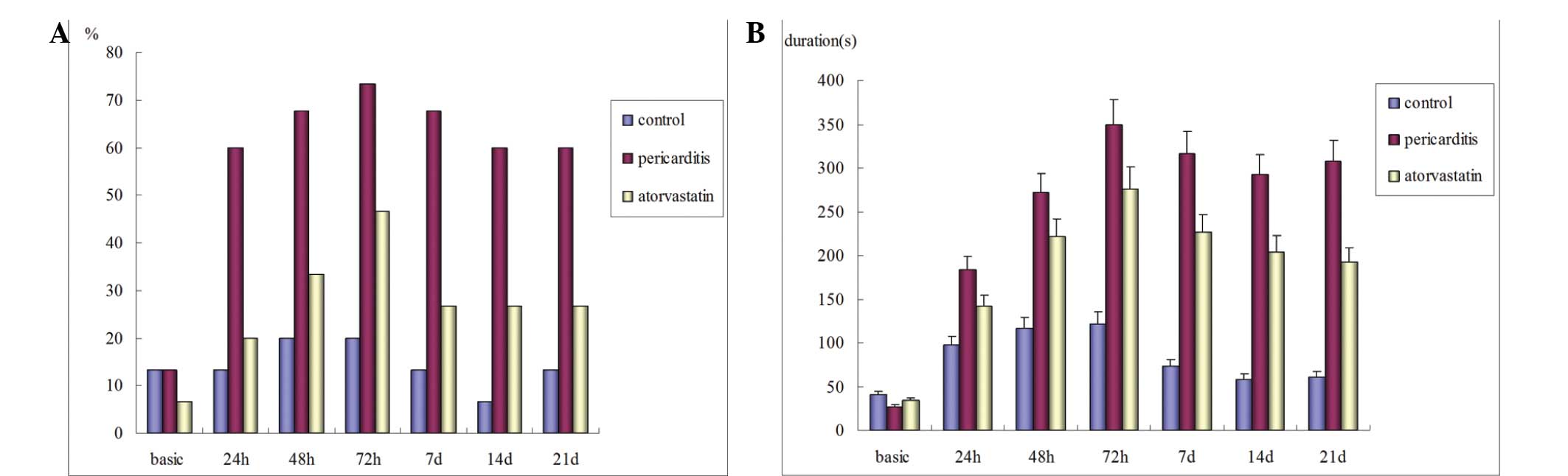
Atorvastatin reduces the duration, but
not the inducibility, of AF following pericarditis
Preoperatively, the inducibility of left AF in the
control, statin and pericarditis groups were 13.3, 13.3 and 6.7%,
respectively (P>0.05). No spontaneous postoperative AF was
observed in any of the groups. In the control group, the
postoperative left AF inducibility increased marginally compared
with the preoperative level, however, the difference was not
statistically significant. In the atorvastatin and pericarditis
groups, the left AF inducibility was significantly higher compared
with the baseline (P<0.05), peaking 72 h after surgery. However,
the difference between the two groups was not statistically
significant. Additionally, no significant difference was observed
in the postoperative right AF inducibility between the three groups
(P>0.05; Table IX; Fig. 3A). The durations of postoperative
AF in the atorvastatin and pericarditis groups were significantly
prolonged compared with the preoperative levels (P<0.05) and
were significantly longer compared with the control group
(P<0.05). The postoperative AF duration was significantly
shorter in the atorvastatin group compared with the pericarditis
group (P<0.05; Fig. 3C).
 | Table IXComparison between the RA and LA
percentages of fibrillation inducibility in the different
experimental groups. |
Table IX
Comparison between the RA and LA
percentages of fibrillation inducibility in the different
experimental groups.
| Control group
(%) | Pericarditis group
(%) | Statin intervention
group (%) |
|---|
|
|
|
|
|---|
| LA | RA | LA | RA | LA | RA |
|---|
| Preoperative | 13.3 | 6.7 | 13.3 | 0.0 | 6.7 | 0.0 |
| Postoperative |
| 24 h | 13.3 | 13.3 | 60.0a,b | 13.3 | 20.0a,c | 13.3 |
| 48 h | 20.0 | 13.3 | 66.7a,b | 20.0 | 33.3a | 13.3 |
| 72 h | 20.0 | 6.7 | 73.3a,b | 20.0 | 46.7a,b | 13.3 |
| 7 days | 13.3 | 6.7 | 66.7a,b | 13.3 | 26.7a,c | 6.7 |
| 14 days | 6.7 | 6.7 | 60.0a,b | 6.7 | 26.7a | 6.7 |
| 21 days | 13.3 | 0.0 | 60.0a,b | 6.7 | 26.7a | 6.7 |
Comparison of the pathological changes in
the different experimental groups
H&E staining revealed different levels of
visible atrial tissue inflammation in the three groups (Fig. 4). The atrial epicardium in the
control group sections exhibited a low level of inflammatory cell
infiltration, however the cardiomyocytes were generally normal
(Fig. 4A). The pericarditis group
tissue exhibited signs of epicardial thickening, infiltration of
lymphocytes, myocardial rupture and necrosis (Fig. 4B). The atorvastatin group also
exhibited epicardial thickening, however, only moderate levels of
lymphocyte infiltration and myocardial sarcoplasmic condensation
were observed (Fig. 4C).
Discussion
Since the initial suggestion of an association
between inflammation and AF in 1997 (2), a number of clinical studies have
examined the possible links between the two, however, an
association between inflammation and AF, and the role of statins in
treating AF remain to be elucidated.
At present, there are several animal models of AF,
including vagus nerve stimulation (16), sterile pericarditis (13), chronic mitral regurgitation
(17), rapid atrial/ventricular
pacing (14,18) and hyperthyroidism (19). In the sterile pericarditis model,
aseptic inflammation is caused by surgical procedures through AF
stimulation. In the present study, this model was selected to
induce inflammation and examine the association between
inflammation and AF.
Significant increases were observed in the serum
levels of hs-CRP, IL-6 and TNF-α 12 h after surgery in the
atorvastatin and pericarditis groups, suggestive of an inflammatory
response and demonstrating that the sterile pericarditis model in
goats induced a distinctive and desirable inflammatory response. In
the control group, the serum levels of inflammatory factors also
exhibited an initial significant increase, possibly due to the
thoracotomy wound. Importantly, however, these molecules decreased
to preoperative levels within 14–21 days after surgery.
In the present study, postoperative atrial ERP in
the pericarditis group was significantly shorter 2–3 days after
surgery, compounded with simultaneous increases of inflammatory
cytokines. Following this time point, with the reduction of
inflammation and decreased levels of inflammatory factors, atrial
ERP did not decrease further and was found to extend, however, it
remained significantly shorter compared with the control group.
This suggested that the inflammation associated with atrial ERP led
to shortened atrial ERP up to 7 days after surgery. In addition, as
with the aforementioned inflammatory response, the atrial ERP rate
of adaptation gradually declined. Furthermore, at the peak period
of the inflammatory response atrial, ERP rate adaptation was lost.
Through linear correlation analysis, a negative correlation was
identified between the atrial ERP rate adaptability and the serum
levels of hs-CRP. In addition, atrial CV did not change
significantly following aseptic pericarditis, indicating that
inflammation has no impact on CV. This finding is in contrast with
the results from a canine sterile pericarditis model reported by
Kumagai et al (9), although
the difference may be due to a lower density of electrodes in the
previous study, reducing the ability to accurately measure
intra-atrial conduction time.
In the goat aseptic pericarditis model used in the
present study, spontaneous AF was observed postoperatively in the
goats from each group. AF was further induced by programmed
stimulation or burst stimulation, suggesting that, while
inflammation caused certain electrophysiological changes in the
goat atria, additional predisposing factors were required to
consistently trigger AF. Therefore, inflammation had an effect on
atrial electrophysiological characteristics, shortened atrial ERP
and reduced ERP rate adaptation. Together, these data suggested
that the inflammatory processes can form an ‘AF matrix’, in which
AF may be more easily induced and maintained in the presence of the
appropriate predisposing factors.
Several studies have demonstrated that during the
inflammatory response involved in atrial structural remodeling in
patients with AF, widespread inflammatory infiltration, myocardial
necrosis and interstitial fibrosis are observed in the affected
atrial tissue (2–7). This suggests that inflammation can
lead to atrial structural remodeling, making it a factor in
development and maintenance of AF. A variety of mechanisms
underlying the affect of inflammation on atrial structural
remodeling have been reported (20). Inflammation can produce
TNF-α-induced expression of connective tissue growth factor,
thereby inducing myocardial interstitial fibrosis (6). This leads to atrial structural
remodeling with subsequent deposition of excess collagens and
fibronectin, ultimately resulting in separation among myocardial
cells and impaired cell conduction.
Hydroxymethyl glutaryl coenzyme A reductase
inhibitors (statins) inhibit cholesterol biosynthesis and are
associated with cardiovascular protective effects, which has led to
their wide use in clinical settings. In recent years, a growing
number of clinical studies have suggested that statins exert an
anti-AF effect (21–26). It has also been demonstrated that
statins can reduce the expression of inflammatory mediators,
including IL-6, TNF-α, CRP and cyclooxygenase (27,28).
In the present study, the levels of hs-CRP, IL-6 and TNF-α in the
atorvastatin group were significantly lower compared with those in
the postoperative group, indicating that atorvastatin significantly
reduced the serum levels of inflammatory cytokines in the goat
aseptic pericarditis model. H&E staining revealed that the
pericarditis group exhibited extensive atrial inflammatory
infiltration, whereas only a moderate level was observed in the
atorvastatin group. These finding suggested that atorvastatin
reduced the extent of atrial muscle inflammation. Compared with the
pericarditis group, the atorvastatin group exhibited a
significantly prolonged atrial ERP, increased AF inducibility and
shorter AF duration, suggesting that atorvastatin inhibited
postoperative atrial electrophysiological changes in the model
used. Together, these findings indicated that atorvastatin
inhibited the atrial electrophysiological changes and contingent
structural changes induced by inflammatory response, thereby
reducing the inducibility and duration of AF.
A potential limitation of the present study was the
lack of multi-site detection on the whole atrial epicardium and on
the pulmonary vein, meaning that the full picture of atrial
activation was not determined. This reduced the ability to further
analyze the role of the atria and the pulmonary vein in AF induced
by sterile pericarditis.
The present study demonstrated that inflammation is
important in the initiation and maintenance of AF. Inflammation may
promote AF by shortening atrial ERP and ERP rate adaptation.
Additionally, elevation in the levels of hs-CRP, IL-6, TNF-α
induced by atrial tachyrhythmia suggested that AF may promote the
inflammatory processes. It is possible that atorvastatin inhibited
the atrial electrophysiological changes and contingent structural
changes induced by the inflammatory response, thereby reducing the
inducibility and duration of AF. Together, these results suggested
a potential role for atorvastatin in reducing the incidence of AF
following cardiac surgery.
References
|
1
|
Benjamin EJ, Wolf PA, D’Aqostion RB,
Silbershatz H, Kannel WB and Levy D: Impact of atrial fibrillation
on the risk of death: The Framingham Heart Study. Circulation.
98:946–952. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bruins P, te Velthuis H, Yazdanbakhsh AP,
et al: Activation of the complement system during and after
cardiopulmonary bypass surgery: postsurgery activation involves
C-reactive protein and is associated with postoperative arrhythmia.
Circulation. 96:3542–3548. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gaudino M, Andreotti F, Zamparelli R, et
al: The-174 G/C interleukin-6 polymorphism influences postoperative
interleukin-6 levels and postoperative atrial fibrillation. Is
atrial fibrillation an inflammatory complication? Circulation. 108
Suppl 1:II195–II199. 2003. View Article : Google Scholar
|
|
4
|
Frustaci A, Chimenti C, Bellocci F,
Morgante E, Russo MA and Maseri A: Histological substrate of atrial
biopsies in patients with lone atrial fibrillation. Circulation.
96:1180–1184. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chung MK, Martin DO, Sprecher D, et al:
C-Reactive protein elevation in patients with atrial arrhythmias:
inflammatory mechanisms and persistence of atrial fibrillation.
Circulation. 104:2886–2891. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aviles RJ, Martin DO, Apperson-Hansen C,
et al: Inflammation as a risk factor for atrial fibrillation.
Circulation. 108:3006–3010. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sata N, Hamada N, Horinouchi T, Amitani S,
Yamashita T, Moriyama Y and Miyahara K: C-reactive protein and
atrial fibrillation. Is inflammation a consequence or a cause of
atrial fibrillation? Jpn Heart J. 45:441–445. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shiroshita-Takeshita A, Schram G, Lavoie J
and Nattel S: Effect of simvastatin and antioxidant vitamins on
atrial fibrillation promotion by atrial-tachycardia remodeling in
dogs. Circulation. 110:2313–2319. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kumagai K, Nakashima H and Saku K: The
HMG-CoA reductase inhibitor atorvastatin prevents atrial
fibrillation by inhibiting inflammation in a canine sterile
pericarditis model. Cardiovasc Res. 62:105–111. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yared JP, Starr NJ, Torres FK, et al:
Effects of single dose, post induction dexamethasone on recovery
after cardiac surgery. Ann Thorac Surg. 69:1420–1424. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dernellis J and Panaretsu M: Relationship
between C-reactive protein concentrations during glucocorticoid
therapy and recurrent atrial fibrillation. Eur Heart J.
25:1100–1107. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Siu CW, Lau CP and Tse HF: Prevention of
atrial fibrillation recurrence by statin therapy in patients with
lone atrial fibrillation after successful cardioversion. Am J
Cardiol. 92:1343–1345. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pagé PL, Plumb VJ, Okumura K and Waldo AL:
A new animal model of atrial flutter. J Am Coll Cardiol. 8:872–879.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wijffels MC, Kirchhof CJ, Dorland R, et
al: Atrial fibrillation begets atrial fibrillation: A study in
awake chronically instrumented goats. Circulation. 92:1954–1968.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shan ZL, Wang YT, Shi XM, et al: Effects
of atrial excitable period on the stability of atrial fibrillation
in goats. Zhonghua Xin Xue Guan Bing Za Zhi. 33:992–994. 2005.(In
Chinese).
|
|
16
|
Patterson E, Lu Z, Lin J, Scherlag BJ, Po
SS, Coscia D and Lazzara R: Antifibrillatory properties of
mivacurium in a canine model of atrial fibrillation. J Cardiovasc
Pharmacol. 51:293–303. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim KH, Kim YJ, Ohn JH, et al: Long-term
effects of sildenafil in a rat model of chronic mitral
regurgitation: benefits of ventricular remodeling and exercise
capacity. Circulation. 125:1390–1401. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Watanabe I, Okumura Y, Kogawa R, et al:
Linear catheter ablation of the right atrium for rapid atrial
pacing-induced sustained atrial fibrillation in dogs. Int Heart J.
53:375–382. 2012.PubMed/NCBI
|
|
19
|
Chen YC, Chen SA, Chen YJ, Chang MS, Chan
P and Lin Cl: Effects of thyroid hormone on the arrhythmogenic
activity of pulmonary vein cardiomyocytes. J Am Coll Cardiol.
39:366–372. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dernellis J and Panaretou M: Effects of
C-reactive protein and the third and fourth components of
complement (C3 and C4) on incidence of atrial fibrillation. Am J
Cardiol. 97:245–248. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dernellis J and Panaretou M: Effect of
C-reactive protein reduction on paroxysmal atrial fibrillation. Am
Heart J. 150:10642005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ozaydin M, Varol E, Aslan SM, Kucuktepe Z,
Dogan A, Ozturk M and Altinbas A: Effect of atorvastatin on the
recurrence rates of atria fibrillation after electrical
cardioversion. Am J Cardiol. 97:1299–1300. 2006.
|
|
23
|
Arribas-Leal JM, Pascual-Figal DA,
Tornel-Osorio PL, et al: Epidemiology and new predictors of atrial
fibrillation after coronary surgery. Rev Esp Cardiol. 60:841–847.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ozaydin M, Dogan A, Varol E, et al: Statin
use before by-pass surgery decreases the incidence and shortens the
duration of postoperative atrial fibrillation. Cardiology.
107:117–121. 2007. View Article : Google Scholar
|
|
25
|
Patti G, Chello M, Candura D, Pasceri V,
D’Ambrosio A, Covino E and Di Sciascio G: Randomized trial of
atorvastatin for reduction of postoperative atrial fibrillation in
patients undergoing cardiac surgery: results of the ARMYDA-3
(Atorvastatin for Reduction of Myocardial Dysrhythmia After cardiac
surgery) study. Circulation. 114:1455–1461. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Patel AA, White CM, Shah SA, Dale KM,
Kluger J and Coleman CI: The relationship between statin use and
atrial fibrillation. CurrMed ResOpin. 23:1177–1185. 2007.
View Article : Google Scholar
|
|
27
|
Schönbeck U and Libby P: Inflammation,
immunity and HMG-CoA reductase inhibitors: statins as
antiinflammatory agents? Circulation. 109(Suppl 1): II11–II18.
2004. View Article : Google Scholar
|
|
28
|
Mora S and Ridker PM: Justification for
the Use of Statins in Primary Prevention: an intervention trial
evaluating rosuvastatin (Jupiter) can C-reactive protein be used to
target statin therapy in primary prevention? Am J Cardiol.
97:33A–41A. 2006. View Article : Google Scholar
|