Introduction
Gastric cancer is one of the most common types of
cancer and is the second leading cause of cancer-induced mortality
(~800,000 mortalities annually) throughout the world (1). Detection of gastric cancer at an
early stage markedly reduces its mortality (2). Although certain diagnostic
modalities, including endoscopy, have been used to implement early
detection, less invasive and more accurate methods are required
(3).
Measurement of tumor biomarkers in serum is an
alternative screening method for the detection of gastric cancer.
To facilitate early detection, more specific and sensitive
biomarkers than the classic biomarkers, including carcinoembryonic
antigen (CEA), carbohydrate antigen 19-9 (CA19-9) and C-reactive
protein (CRP), are required (1,4-6).
ANGPTL2 is a secreted protein belonging to the
angiopoietin-like protein family, which regulates angiogenesis
(7,8). Angiogenic factors produced by tumor
cells are essential for tumor growth (9). A study by Endo et al (10) indicated that ANGPTL2 may be a
biomarker for predicting human lung and breast cancers. These
previous studies suggested that ANGPTL2 has potential as a
biomarker for a variety of cancers in different tissues. To the
best of our knowledge, the present study was the first to have
evaluated the potential of angiopoietin-like protein 2 (ANGPTL2) as
a novel biomarker for gastric cancer.
In order to evaluate the potential of ANGPTL2 as a
biomarker for gastric cancer, expression levels of ANGPTL2 in
undifferentiated and differentiated gastric cancer cell lines
(HGC-27 and MKN7, respectively) were investigated. Additionally,
ANGPTL2 levels in serum of gastric cancer patients were compared
with those of healthy individuals to investigate the possibility of
the protein as a predictive biomarker for gastric cancer.
Materials and methods
Cell lines
HGC-27, an undifferentiated human gastric cancer
cell line and MKN7, a differentiated human gastric cancer cell
line, were purchased from RIKEN Cell Bank (Tsukuba, Japan). HGC-27
and MKN7 cells were cultured in Eagle’s minimum essential medium
(Sigma-Aldrich, Gillingham, UK) and RPMI-1640 medium
(Sigma-Aldrich), respectively, supplemented with 10% fetal bovine
serum (Life Technologies, Carlsbad, CA, USA), 100 U/ml penicillin
and 100 μg/ml streptomycin (both Meiji Seika Pharma Co., Ltd.,
Tokyo, Japan) at 37°C in 5% CO2.
Cell culture
HGC-27 and MKN7 cell lines were seeded in six-well
plates (6×105 cells/well) and incubated at 37°C in 5%
CO2. Media in the wells were changed daily. The medium
samples were stored at -80°C. Cells were collected from the wells
daily by trypsinization (Wako Pure Chemical Industries, Ltd.,
Osaka, Japan) and the cell numbers were determined using a
hemocytometer.
Patient samples
The study design was approved by the Ethics
Committee of Nanpuh Hospital Kagoshima Kyosaikai, Public Interest
Corporation, Japan. Clinical examinations were performed according
to the principles in the Declaration of Helsinki. Serum samples
were obtained from 50 participants from January to September 2013
at Nanpuh Hospital (Kagoshima, Japan). The participants included 12
patients with gastric cancer [aged 66.9±11.9 years, mean ± standard
deviation (SD)] and 38 with normal mucosa (aged 47.3±9.9 years).
The histological subtype of the 12 patients with gastric cancer was
adenocarcinoma. Nine of the 12 patients with gastric cancer were
diagnosed with clinical stage I disease and three were diagnosed
with clinical stage II disease. The staging of gastric cancer was
based on a routine histopathological analysis and clinical
assessment, according to tumor-nodulus-metastases classification.
Tumors were classified by the 5th International Union Against
Cancer (11). The characteristics
of the subjects are summarized in Table I. Informed consent was obtained
from all participants.
 | Table ICharacteristics of experimental
subjects. |
Table I
Characteristics of experimental
subjects.
| Characteristic | Gastric cancer
patients (n=12) | Healthy controls
(n=38) | Total (n=50) |
|---|
| Age (years) |
| Mean ± SD | 66.9±11.9 | 47.3±9.9 | 52.0±13.3 |
| Range | 43–88 | 35–75 | 35–88 |
| Gender |
| Male | 11 | 22 | 33 |
| Female | 1 | 16 | 17 |
| BMI
(kg/m2) |
| Mean ± SD | 24.0±4.5 | 22.0±2.8 | 22.5±3.3 |
| Range | 17.9–31.9 | 17.4–29.5 | 17.4–31.9 |
| Tumor stage |
| I | 9 | - | 9 |
| II | 3 | - | 3 |
| Wall
infiltration |
| M, SM | 8 | - | 8 |
| MP | 3 | - | 3 |
| SS | 1 | - | 1 |
Measurement of biomarkers in cell culture
media and serum
The concentrations of ANGPTL2 in human serum and
cell culture medium samples (collected on the second day of cell
culture) were determined using an ANGPTL2 ELISA kit
(Immuno-Biological Laboratories Co., Ltd., Gunma, Japan). Serum
concentrations of CRP were determined by latex agglutination using
BM6050 (Kyowa-Medex Co., Ltd., Tokyo, Japan) according to the
manufacturer’s instructions. Concentrations of CEA and CA19-9 in
serum were determined using the electro-chemiluminescence
immunoassay using LUMIPULSE G1200® (Fujirebio, Inc.,
Tokyo, Japan) according to the manufacturer’s instructions. A
multiplex suspension array (Bio-Plex Human Angiogenesis 9-Plex
Panel; Bio-Rad Laboratories, Hercules, CA, USA) was used to measure
the concentrations of the following angiogenic factors in the cell
culture medium samples: Granulocyte colony stimulating factor
(G-CSF), platelet endothelial cell adhesion molecule-1 (PECAM-1),
hepatocyte growth factor (HGF), vascular endothelial growth factor
(VEGF), leptin, platelet-derived growth factor-BB (PDGF-BB),
angiopoietin-2 and follistatin.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
HGC-27 and MKN7 cells (5.85×105 cells)
were collected from cell culture dishes by trypsinization and the
total RNA was extracted from the cells using the High Pure RNA
Tissue kit (Roche Diagnostics, Penzberg, Germany) according to the
manufacturer’s instructions.
The reverse transcription reaction was performed
using random hexamer primers (Thermo Fisher Scientific, Waltham,
MA, USA) and ReverTra Ace® (Toyobo Co., Ltd., Osaka,
Japan) according to the manufacturer’s instructions, under the
condition that the amount of RNA used was fixed at 200 ng.
The amplification of TRAIL variants was performed
using the StepOnePlus™ Real-Time PCR System (Applied
Biosciences Life Technologies, Foster City, CA, USA) using the SYBR
Green qPCR Mix kit (Toyobo Co., Ltd.) according to the
manufacturer’s instructions. The specific primers for human ANGPTL2
were: Forward, 5′-GCCACCAAGTGTCAGCCTCA-3′ and reverse,
5′-TGGTTCTGAACTGCATTCTGCTG-3′ (Life Technologies). Human β-actin,
used as a control, was amplified using the following specific
primers: Forward, 5′-TGGCACCCAGCACAATGAA-3′ and reverse,
5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′ (Life Technologies, Carlsbad, CA,
USA) (12).
Statistical analysis
Data were analyzed using SPSS version 14.0J (SPSS,
Inc., Chicago, IL, USA) The correlation between the serum ANGPTL2
concentration and different variables was analyzed using Pearson’s
correlation analysis. The statistical difference between ANGPTL2
concentrations in serum of gastric cancer patients and healthy
individuals was analyzed using the rank nonparametric statistical
Mann-Whitney U test. The statistical difference between two groups
was analyzed using the Student’s t-test. Values are presented as
the mean ± SD. A receiver operating characteristic (ROC) curve was
established to evaluate the diagnostic value for differentiating
between gastric cancer patients and healthy individuals. P<0.05
was considered to indicate a statistically significant difference
between values.
Results
ANGPTL2 mRNA expression levels are higher
in HGC-27 cells than those in MKN7 cells
ANGPTL2 mRNA expression levels of the HGC-27
and MKN7 cells were compared using RT-qPCR. The ANGPTL2 expression
levels of HGC-27 were 13.2-fold greater than those of MKN7
(P<0.001; Fig. 1). These
results demonstrated that the expression of ANGPTL2 mRNA was
significantly higher in undifferentiated HGC-27 cells than in
differentiated MKN7 cells.
ANGPTL2 expression rate is greater in
HGC-27 cells than that in MKN7 cells
Whether there was a difference in the expression
rate of ANGPTL2 between HGC-27 and MKN7 cells was investigated
using ELISA. Fig. 2 exhibits the
ANGPTL2 expression rate of the cell lines at day two of cell
culture. The rate of HGC-27-cell ANGPTL2 production was
5.72×10−6±1.20×10−6 ng/cell/day. The rate of
ANGPTL2 production was significantly lower in MKN7 cells than that
of HGC-27 cells (P<0.05). These results demonstrated that the
production of ANGPTL2 was considerably higher in undifferentiated
HGC-27 cells than in differentiated MKN7 cells.
VEGF is expressed in HGC-27 and MKN7
cells
The expression of angiogenic factors that were
potential biomarkers for gastric cancer, including G-CSF, PECAM-1,
HGF, VEGF, leptin, PDGF-BB, angiopoietin-2 and follistatin was
investigated. Only VEGF expression was confirmed in both cell
lines, indicating that VEGF may be a biomarker for gastric cancer
(Fig. 3).
 | Figure 3Expression rates of G-CSF, PECAM-1,
HGF, VEGF, leptin, PDGF-BB, angiopoietin-2 and follistatin in
HGC-27 and MKN7 cells at day two of cell culture (n=3). Data are
presented as the mean ± standard deviation. G-CSF, granulocyte
colony stimulating factor; PECAM-1, platelet endothelial cell
adhesion molecule-1; HGF, hepatocyte growth factor; VEGF, vascular
endothelial growth factor; PDGF-BB, platelet derived growth
factor-BB. |
ANGPTL2 serum expression levels are
increased in patients with gastric cancer
As described above, increased expression levels of
ANGPTL2 were confirmed in the HGC-27 cell line in comparison with
those in the MKN7 cell line. It was therefore examined whether
ANGPTL2 expression was increased in patients with gastric cancers.
The concentration of ANGPTL2 in the serum of gastric cancer
patients (4.59±2.91 ng/ml) was significantly higher than that of
healthy individuals (2.68±0.60 ng/ml, P<0.01; Fig. 4). Table II exhibits correlations between
serum ANGPTL2 concentration and numerous variables. The ANGPTL2
concentration in the serum of gastric cancer patients demonstrated
no significant correlation with age (r=-0.323, P=0.307), BMI
(r=0.541, P=0.070), serum CRP concentration (r=0.178, P=0.579),
serum CEA concentration (r=-0.083, P=0.798) or CA19-9 concentration
(r=-0.312, P=0.324). The ANGPTL2 concentration in the serum of the
healthy controls also indicated no significant correlation with age
(r=0.302, P=0.065), BMI (r=0.233, P=0.160), serum CRP concentration
(r=0.123, P=0.463), serum CEA concentration (r=0.050, P=0.767) or
CA19-9 concentration (r=0.193, P=0.246). These data indicated that
ANGPTL2 levels in the serum were not significantly associated with
these factors and therefore depended solely on the existence of
gastric cancer.
 | Table IICorrelations between ANGPTL2
concentration in serum and numerous variables. |
Table II
Correlations between ANGPTL2
concentration in serum and numerous variables.
| Pearson’s correlation
coefficient | P-value |
|---|
| Gastric cancer
patients (n=12) |
| Age | −0.323 | 0.307 |
| BMI | 0.541 | 0.070 |
| CRP | 0.178 | 0.579 |
| CEA | −0.083 | 0.798 |
| CA19-9 | −0.312 | 0.324 |
| Healthy controls
(n=38) |
| Age | 0.302 | 0.065 |
| BMI | 0.233 | 0.160 |
| CRP | 0.123 | 0.463 |
| CEA | 0.050 | 0.767 |
| CA19-9 | 0.193 | 0.246 |
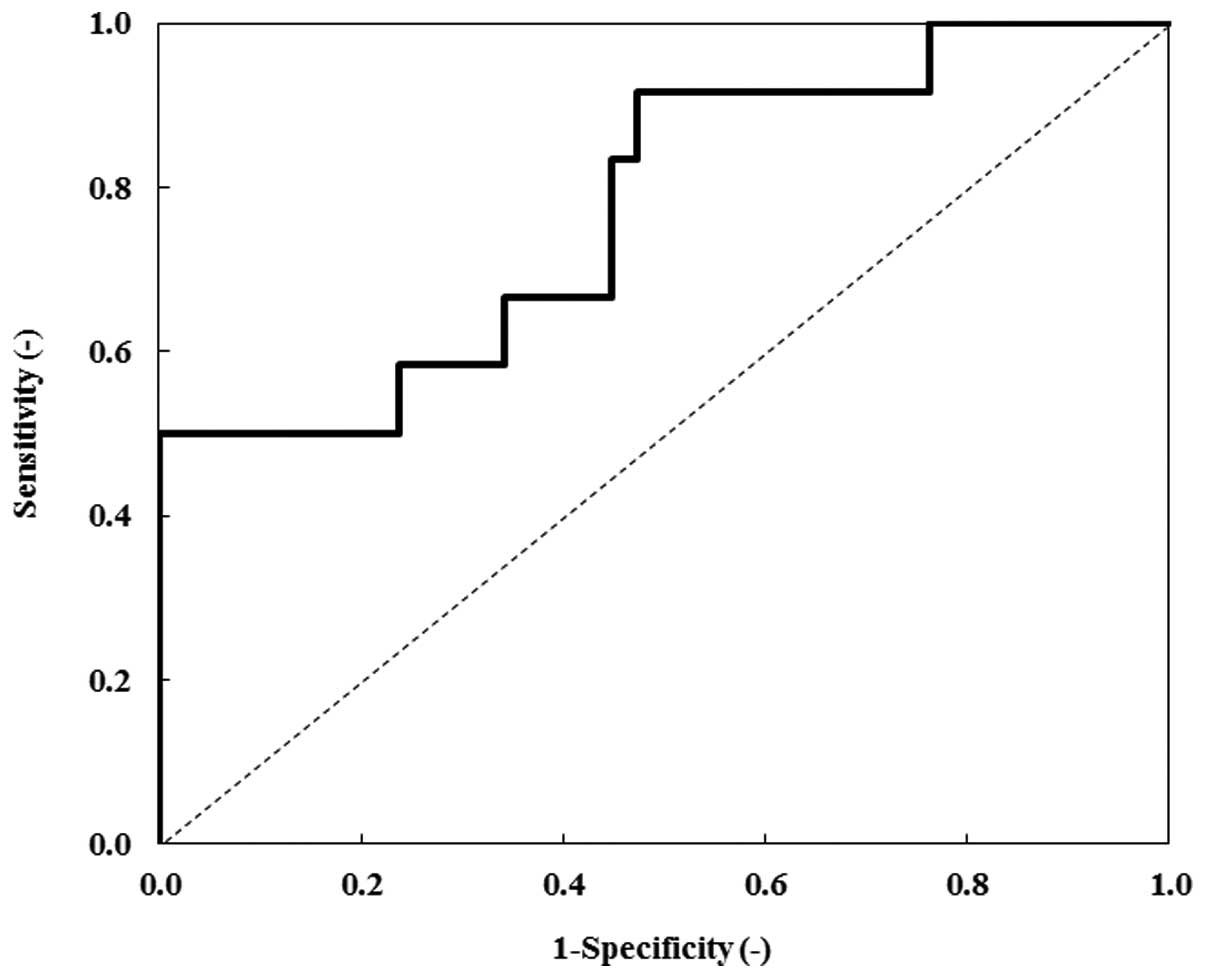
Fig. 5 exhibits the
ROC curve for ANGPTL2. Table III
indicates the AUC of the ROC curves, in order to evaluate the
diagnostic significance of serum ANGPTL2, CRP, CEA and CA19-9
levels of gastric cancer patients. In order to discriminate gastric
cancer patients from healthy individuals, the AUC for ANGPTL2 was
0.774 [P=0.005; 95% confidence interval (CI), 0.615–0.933], the AUC
for CRP was 0.669 (P=0.080; 95% CI, 0.484–0.853), the AUC for CEA
was 0.529 (P=0.768; 95% CI, 0.326–0.731) and the AUC for CA19-9 was
0.570 (P=0.467; 95% CI, 0.396–0.744). Thus, the AUC for ANGPTL2 was
higher than that of CRP, CEA and CA19-9. These data indicated that
ANGPTL2 was a potential biomarker for gastric cancer.
 | Table IIIAUC for ANGPTL2, CRP, CEA and CA19-9
(gastric cancer patients vs. healthy control). |
Table III
AUC for ANGPTL2, CRP, CEA and CA19-9
(gastric cancer patients vs. healthy control).
| Biomarker | AUC | Standard error | P-value | 95% confidence
interval |
|---|
| ANGPTL2 | 0.774 | 0.081 | 0.005a | 0.615–0.933 |
| CRP | 0.669 | 0.094 | 0.080 | 0.484–0.853 |
| CEA | 0.529 | 0.103 | 0.768 | 0.326–0.731 |
| CA19-9 | 0.570 | 0.089 | 0.467 | 0.396–0.744 |
Discussion
To the best of our knowledge, the present study was
the first to investigate ANGPTL2 expression in gastric cancer cell
lines. It was revealed that the mRNA and protein expression levels
of ANGPTL2 were higher in HGC-27 cells than those in MKN7 cells.
Furthermore, the expression levels of eight angiogenic factors
(G-CSF, PECAM-1, HGF, VEGF, leptin, PDGF-BB, angiopoietin-2 and
follistatin) that were potential biomarkers for gastric cancer were
investigated. Of these potential biomarkers, VEGF expression was
confirmed in both cell lines. VEGF protein mediates angiogenesis
associated with tumor growth and is a predictive biomarker for
tumor progression in certain types of cancer (13,14).
The results of the present study verified that VEGF was a biomarker
for gastric cancer. The results further indicated that ANGPTL2 may
also be a biomarker for gastric cancer.
The potential of ANGPTL2 as a novel biomarker for
gastric cancer was evaluated by measuring ANGPTL2 concentration in
the serum of gastric cancer patients. It was revealed that the
ANGPTL2 concentration in gastric cancer patients was higher than
that of healthy controls. According to the ROC analysis, ANGPTL2
demonstrated greater diagnostic ability than the classic biomarkers
(CRP, CEA and CA19-9).
It was previously reported that ANGPTL2 was closely
associated with adiposity and inflammation (15,16).
Therefore, the present study examined correlations between the
serum ANGPTL2 levels, serum CRP levels and the BMI. The serum
ANGPTL2 levels of the gastric cancer patients and healthy controls
were not correlated with the serum CRP concentration and the BMI
(Table II). These findings
suggested that ANGPTL2 was a specific factor for gastric
cancer.
In conclusion, these results demonstrated that
ANGPTL2 was upregulated in undifferentiated gastric cancer cells
and patients, which indicated that ANGPTL2 was a clinically
relevant biomarker for gastric cancer.
Acknowledgements
The present study was supported in part by the
Division of Gene Research, Kagoshima University and the Divisions
of Gastrointestinal Surgery and Clinical Laboratory, Nanpuh
Hospital (Kagoshima, Japan).
References
|
1
|
Loei H, Tan HT, Lim TK, Lim KH, So JB,
Yeoh KG and Chung MC: Mining the gastric cancer secretome:
identification of GRN as a potential diagnostic marker for early
gastric cancer. J Proteome Res. 11:1759–1172. 2012. View Article : Google Scholar
|
|
2
|
Yu X, Luo L, Wu Y, Yu X, Liu Y, Yu X, Zhao
X, Zhang X, Cui L, Ye G, et al: Gastric juice miR-129 as a
potential biomarker for screening gastric cancer. Med Oncol.
30:3652013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tan YK and Fielding JW: Early diagnosis of
early gastric cancer. Eur J Gastroenterol Hepatol. 18:821–829.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li C, Li JF, Cai Q, Qiu QQ, Yan M, Liu BY
and Zhu ZG: MiRNA-199a-3p: A potential circulating diagnostic
biomarker for early gastric cancer. J Surg Oncol. 108:89–92. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carpelan-Holmström M, Louhimo J, Stenman
UH, Alfthan H and Haglund C: CEA, CA 19-9 and CA 72-4 improve the
diagnostic accuracy in gastrointestinal cancers. Anticancer Res.
22:2311–2316. 2002.PubMed/NCBI
|
|
6
|
Lukaszewicz-Zając M, Mroczko B, Gryko M,
Kędra B and Szmitkowski M: Comparison between clinical significance
of serum proinflammatory proteins (IL-6 and CRP) and classic tumor
markers (CEA and CA 19-9) in gastric cancer. Clin Exp Med.
11:89–96. 2011. View Article : Google Scholar
|
|
7
|
Kikuchi R, Tsuda H, Kozaki K, Kanai Y,
Kasamatsu T, Sengoku K, Hirohashi S, Inazawa J and Imoto I:
Frequent inactivation of a putative tumor suppressor,
angiopoietin-like protein 2, in ovarian cancer. Cancer Res.
68:5067–5075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oike Y, Yasunaga K and Suda T:
Angiopoietin-related/angiopoietin-like proteins regulate
angiogenesis. Int J Hematol. 80:21–28. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sfiligoi C, de Luca A, Cascone I, Sorbello
V, Fuso L, Ponzone R, Biglia N, Audero E, Arisio R, Bussolino F, et
al: Angiopoietin-2 expression in breast cancer correlates with
lymph node invasion and short survival. Int J Cancer. 103:466–474.
2003. View Article : Google Scholar
|
|
10
|
Endo M, Nakano M, Kadomatsu T, Fukuhara S,
Kuroda H, Mikami S, Hato T, Aoi J, Horiguchi H, Miyata K, et al:
Tumor cell-derived angiopoietin-like protein ANGPTL2 is a critical
driver of metastasis. Cancer Res. 72:1784–1794. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jass JR and Sobin LH: Histological Typing
of Intestinal Tumours. 2nd edition. Springer-Verlag;
Berlin-Heidelberg-New York: 1989, View Article : Google Scholar
|
|
12
|
Okada T, Tsukano H, Endo M, Tabata M,
Miyata K, Kadomatsu T, Miyashita K, Semba K, Nakamura E, Tsukano M,
et al: Synoviocyte-derived angiopoietin-like protein 2 contributes
to synovial chronic inflammation in rheumatoid arthritis. Am J
Pathol. 176:2309–2319. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Presta LG, Chen H, O’Connor SJ, Chisholm
V, Meng YG, Krummen L, Winkler M and Ferrara N: Humanization of an
anti-vascular endothelial growth factor monoclonal antibody for the
therapy of solid tumors and other disorders. Cancer Res.
57:4593–4599. 1997.PubMed/NCBI
|
|
14
|
Wang Q, Diao X, Sun J and Chen Z: Stromal
cell-derived factor-1 and vascular endothelial growth factor as
biomarkers for lymph node metastasis and poor cancer-specific
survival in prostate cancer patients after radical prostatectomy.
Urol Oncol. 31:312–317. 2013. View Article : Google Scholar
|
|
15
|
Tabata M, Kadomatsu T, Fukuhara S, Miyata
K, Ito Y, Endo M, Urano T, Zhu HJ, Tsukano H, Tazume H, et al:
Angiopoietin-like protein 2 promotes chronic adipose tissue
inflammation and obesity-related systemic insulin resistance. Cell
Metab. 10:178–188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Doi Y, Ninomiya T, Hirakawa Y, Takahashi
O, Mukai N, Hata J, Iwase M, Kitazono T, Oike Y and Kiyohara Y:
Angiopoietin-like protein 2 and risk of type 2 diabetes in a
general Japanese population: the Hisayama study. Diabetes Care.
36:98–100. 2013. View Article : Google Scholar
|