Introduction
Prostate cancer (PCa) is an aggressive invasive
tumor, which is one of the most prevalent malignancies in males and
the second most common cause of male cancer-related mortality
(1). PCa is a clinically
heterogeneous-multifocal disease and its incidence is increasing
(2). The mechanisms influencing
the progression and prognosis of PCa involve a multi-step process
(3). Novel effective diagnostic
and prognostic biomarkers for PCa are required to prevent the
overtreatment of indolent tumors and to ensure early detection of
aggressive PCa, which requires early treatment intervention
(4).
Nin one binding protein (NOB1), encoded by the NOB1
gene, is a subunit of the 26S proteasome and has a key role in the
protein degradation pathway (5).
The NOB1 protein is composed of a PIN domain and a C terminal zinc
ribbon domain (6), which has been
reported to function as a transcriptional regulator. NOB1 may have
a role in cell cycle progression, drug resistance and oncogenesis
(7,8). A previous study showed that knockdown
of NOB1 expression inhibits cell proliferation and migration in
human gliomas, suggesting that NOB1 promotes glioma cell growth and
migration, and may be a candidate for molecular targeting (9). These findings indicate that NOB1 may
be involved in various types of tumors and may serve as an
oncogenic factor. However, the expression of NOB1 and its
significance in PCa remains unclear. The present study aimed to
investigate NOB1 expression to assess its prognostic significance
in patients with PCa with long-term follow-up data and its
association with clinicopathological features.
Materials and methods
Patient and sample tissues
Fresh PCa samples and paired adjacent noncancerous
tissues (n=32) were obtained from Changzheng Hospital (Shanghai,
China) for quantitative polymerase chain reaction (qPCR) and
western blot analysis of NOB1 gene and protein expression,
respectively. Furthermore, the surgical prostate cancer database
(http://ef.inbi.ras.ru) was used to
retrospectively analyze 456 patients with PCa. All of the patients
used in the present study had undergone prostatectomy, transrectal
prostate biopsy under ultrasound guidance or transurethral
resection of the prostate between December 2003 and December 2010.
All samples were fixed with formalin and embedded in paraffin.
Clinical data, including the Gleason score, baseline
prostate-specific antigen (PSA) level, PSA nadir, level of distant
metastasis and follow-up status, were retrospectively obtained from
the Changzheng Hospital medical records. Paraffin-embedded prostate
tissues were obtained from the Department of Urology, Affiliated
Changzheng Hospital of the Second Military Medical University
(Shanghai, China). Serum PSA-based biochemical recurrence and
PCa-specific death were used as the end-points (10).
The present study was approved by the Review Board
of the Second Military Medical University (Shanghai, China) and was
performed in accordance with the Declaration of Helsinki. Signed
informed consent was obtained from all patients prior to
surgery.
qPCR analysis
Total RNA was extracted from fresh PCa samples using
an RNA extraction kit according to the manufacturer’s instructions
and reverse transcribed using a Reverse Transcription kit (Qiagen,
Valencia, CA, USA). Complementary (c)DNA (6 ng) SYBR®
Green (Invitrogen Life Technologies, Carlsbad, CA, USA) qPCR
analysis was then performed. The primers used were as follows:
Forward: 5′-AAGTGAGGAGGAGGAGGAG-3′ and reverse:
5′-ACTTTCTTCAGGGTCTTGTTC-3′ for NOB1; and forward:
5′-GTGGACATCCGCAAAGAC-3′ and reverse: 5′-AAAGGGTGTAACGCAACTA-3′ for
β-actin. Data were analyzed using the ΔΔCt method and were
normalized using β-actin mRNA expression.
Western blot analysis
Fresh PCa samples were lysed in cell lysis buffer
(Cell Signaling Technology, Inc., Beverly, MA, USA) and quantified
using a Bicinchoninic Acid Assay kit (Sigma-Aldrich, St Louis, MO,
USA). Proteins were separated using 10% SDS-PAGE, then
electroblotted onto polyvinylidene fluoride membranes (Millipore
Corporation, Billerica, MA, USA). Membranes were immunoblotted
overnight at 4°C with primary antibodies against human NOB1
(dilution, 1:2,000; LifeSpan Biosciences Inc., Seattle, WA, USA)
and β-actin (dilution, 1:5,000; LifeSpan Biosciences Inc.).
Following washing with Tris-buffered saline containing Tween-20
three times, membranes were incubated with horseradish peroxidase
(HRP)-conjugated secondary antibody immunoglobulin G (dilution,
1:2,000; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) for 2
h. Immunoreactive bands were detected using an enhanced
chemiluminescence detection reagent (Pierce Biotechnology, Inc.,
Rockford, IL, USA). Images were analyzed using Quantity
One® software (Bio-Rad, Hercules, CA, USA). All
experiments were performed in triplicate.
Immunohistochemical analysis
All slides were assessed by two blinded pathologists
and a consensus was reached. Sections were deparaffinized, hydrated
and immersed in peroxidase-blocking solution (Qiagen) to inhibit
any endogenous peroxidase activity. For antigen retrieval,
microwave pretreatment was performed using 0.01 mol/l citrate
buffer (pH 6.0) for 30 min. Sections were incubated overnight at
4°C with mouse monoclonal antibodies against NOB1 (dilution, 1:20;
LifeSpan Biosciences Inc.). Sections were then incubated with a HRP
rabbit/mouse detection reagent (LifeSpan Biosciences Inc.) for 20
min at room temperature. Staining was visualized using the
diaminobenzidine color substrate. Slides were then counterstained
with Mayer’s hematoxylin. The absence of primary antibody was used
as a negative control.
Immunohistochemical scoring
In order to assess the immunostaining, only nuclear
staining was scored. The scoring method was based on the intensity
and proportion of positively stained cells. The proportion of
positive tumor cells was determined semi-quantitatively and each
sample was scored using the following scale: 0, <1%; 1, 1–25%;
2, 26–50%; 3, 51–75%; and 4, 76–100%. The staining intensity was
scored as follows: 0, negative; 1, weak; 2, moderate; and 3,
strong. The immunoreactive score of each tumor was calculated as
the sum of these two parameters. The immunohistochemical results
were finally scored as negative (total score, 0–2) or positive
(total score, 3–7). All stained sections were assessed by two
independent pathologists without knowledge of the
clinicopathological features and any differences in interpretation
were resolved by consensus.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). The χ2 test was
used to investigate the significance of the association between
NOB1 and the clinicopathological variables. The association between
NOB1 expression and prognosis was estimated using univariate and
multivariate analyses. Overall survival (OS) and recurrence-free
survival (RFS) were analyzed using the Kaplan Meier method followed
by log-rank tests. Multivariate analysis was performed using a Cox
regression model. P<0.01 was considered to indicate a
statistically significant difference.
Results
NOB1 mRNA expression is increased in PCa
tissues
NOB1 mRNA expression was determined using qPCR
analysis in 32 fresh PCa samples and paired adjacent non-cancerous
tissues. NOB1 gene expression was found to be significantly
increased in the PCa tissues compared with the adjacent non-PCa
tissues (P<0.001; Fig. 1).
NOB1 protein expression is increased in
PCa tissues
Western blot analysis was performed in the 32 fresh
PCa samples and paired adjacent non-cancerous tissues. The protein
expression of NOB1 was semiquantified using densitometry.
Consistent with the qPCR analysis results, NOB1 protein expression
was observed to be significantly increased in the PCa tissues
compared with the matched adjacent non-PCa tissues (P<0.001;
Fig. 2).
Correlation between NOB1 expression and
clinicopathological parameters in patients with PCa
The mean age of the patients included in the present
study was 64.8±7.9 years (range, 38–86 years). All patient data and
characteristics are summarized in Table I. The immunohistochemical results
were graded as negative or positive (Fig. 3). The positive expression rate of
NOB1 in PCa samples was 53% (242/456). By contrast, only 6.1%
(28/456) of the paired adjacent non-PCa tissues showed positive
NOB1 expression. As shown in Table
I, positive NOB1 expression was significantly correlated with
the Gleason score (P<0.001) and distant metastasis
(P<0.001).
 | Figure 3Immunohistochemical detection of NOB1
in PCa. Paraffin-embedded sections of PCa tissue were scored for
nuclear NOB1 expression on a scale of 0–3 as follows: 0, negative;
1, weak; 2, moderate; and 3, strong. (A and B) negative staining
(magnification ×200 and ×400, respectively), (C and D) weak
staining (magnification, ×200 and ×400, respectively), (E and F)
moderate staining (magnification, ×200 and ×400, respectively) and
(G and H) strong staining (magnification, ×200 and ×400,
respectively). PCa, prostate cancer; NOB, nin one binding
protein. |
 | Table ICorrelation between
clinicopathological parameters and NOB1 expression in patients with
prostate cancer. |
Table I
Correlation between
clinicopathological parameters and NOB1 expression in patients with
prostate cancer.
| | NOB1 status | |
|---|
| |
| |
|---|
| Parameter | No. patients (%) | Negative (n=214) | Positive (n=24) | P-value |
|---|
| Age | | | | 0.861 |
| <65 | 220 (48) | 104 | 116 | |
| ≥65 | 236 (52) | 110 | 126 | |
| Gleason score | | | | <0.001 |
| 2–6 | 204 (45) | 112 | 92 | |
| 7 | 228 (50) | 99 | 129 | |
| 8–10 | 24 (5) | 3 | 21 | |
| Distant
metastasis | | | | <0.001 |
| Absent | 362 (79) | 204 | 158 | |
| Present | 94 (21) | 13 | 81 | |
| PSA (ng/ml) | | | | 0.684 |
| <4 | 26 (6) | 16 10 | | |
| 4–10 | 192 (42) | 58 | 134 | |
| >10 | 238 (52) | 140 | 98 | |
| pTNM system | | | | 0.495 |
| pT2a,b,c | 300 (66) | 136 | 164 | |
| pT3a,b | 156 (34) | 80 | 76 | |
Correlation between positive NOB1
expression and clinical outcome
The mean follow-up period was 39.6 months (range,
6–92 months). Among the 456 patients with PCa, 12 (2.6%) were lost
during the follow-up period and 68 (14.9%) succumbed to their
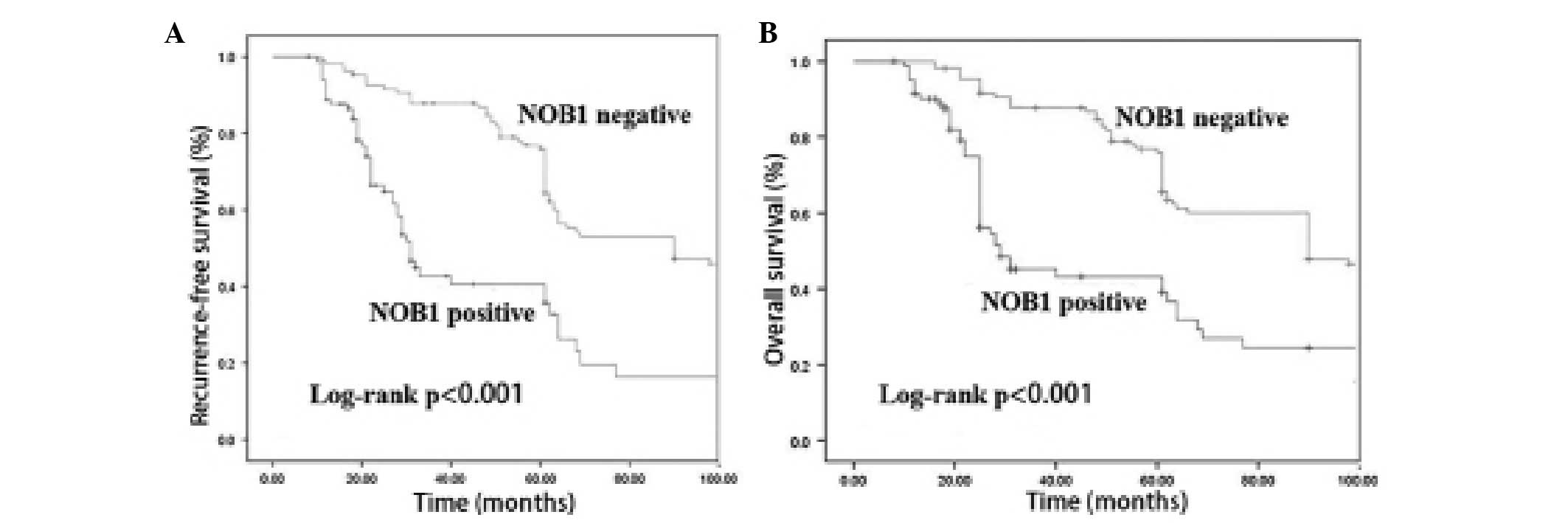
disease. During the follow-up period, the RFS of the patients with
positive NOB1 expression was observed to be significantly
decreased, as compared with that of the patients with negative NOB1
expression (P<0.001). The OS rate of the patients with positive
NOB1 expression was also significantly lower compared with that of
the patients with negative NOB1 expression (P<0.001; Fig. 4).
Univariate analysis (Table II) revealed that higher Gleason
score, higher prostate-specific antigen nadir, positive distant
metastasis and positive NOB1 expression were correlated with lower
RFS. Compared with the patients with positive NOB1 expression, the
patients with negative NOB1 expression had significantly enhanced
clinical outcomes in terms of RFS (P<0.001). Multivariate
analysis revealed that NOB1 expression was a significant predictor
of RFS, independent of other clinicopathological variables (hazard
ratio, 3.45; 95% confidence interval, 1.41–6.35; P=0.006).
 | Table IIUnivariate and multivariate analyses
of recurrence-free survival in patients with prostate cancer. |
Table II
Univariate and multivariate analyses
of recurrence-free survival in patients with prostate cancer.
| Univariate | Multivariate |
|---|
|
|
|
|---|
| Parameter | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age (≤60 vs. >60
years) | 0.86 | 0.50–1.47 | 0.269 | 0.83 | 0.46–1.58 | 0.592 |
| Gleason score (2–6
vs. 7 or 8–10) | 4.33 | 2.74–5.91 | <0.001 | 5.04 | 3.61–6.18 | <0.001 |
| Distant metastasis
(yes vs. no) | 5.48 | 3.67–7.02 | <0.001 | 5.34 | 3.39–7.21 | <0.001 |
| Baseline PSA (≤10 vs.
>10 ng/ml) | 3.74 | 1.89–4.51 | <0.001 | 3.03 | 1.92–5.83 | <0.001 |
| PSA nadir (≤1 vs. 1
ng/ml) | 4.21 | 2.97–5.84 | <0.001 | 4.76 | 3.23–6.46 | <0.001 |
| NOB1 (+ vs. −) | 3.45 | 2.34–5.76 | 0.0012 | 3.54 | 1.41–6.35 | 0.006 |
Discussion
Identification of PCa-specific biomarkers is
important for diagnosis, therapy and prognostic prediction. The
present study investigated the expression of NOB1 in 456 patients
with PCa and its association with patient survival and
clinicopathological characteristics. The findings suggest that
positive NOB1 expression was present in 53% of PCa cases.
Furthermore, significant correlation was observed between positive
NOB1 expression and poor prognosis, independent of other patient
characteristics. These results indicate that NOB1 expression may be
a novel prognostic marker for PCa.
The NOB1 gene was identified to be an oncogene
responsible for the high proliferation rates found in cancer cells
(11). Several studies have
reported that the repression of NOB1 gene expression inhibits the
growth of ovarian cancer and glioma (11,12).
Downregulation of NOB1 has been found to inhibit the growth of
human hepatocellular carcinoma cells (13). Furthermore, small interfering
RNA-mediated silencing of NOB1 inhibits the proliferation and
growth of breast cancer cells (14). Previous studies have also
associated the expression of NOB1 with clinical outcome in
papillary thyroid carcinoma and breast infiltrating ductal
carcinoma (15,16). Thus, the present study investigated
NOB1 mRNA expression in PCa samples using qPCR analysis and NOB1
protein expression in primary PCa tissues using western blot
analysis. Results revealed that NOB1 mRNA and protein levels were
significantly increased in tumor tissue samples compared with those
in the adjacent non-tumor tissue samples. These results suggest
that NOB1 may have a role in PCa.
In the present study, NOB1 protein expression was
analyzed in tumor tissue in order to assess its prognostic
significance for PCa. Immunohistochemical analysis demonstrated
high NOB1 expression in 53% (242/456) of the patients with PCa.
Moreover, in a relatively large population of patients with PCa
(n=456), high expression of NOB1 was found to be significantly
correlated with the Gleason score and distant metastasis of PCa,
suggesting that increased NOB1 expression may promote tumor growth
and invasion. These results suggest that NOB1 may have an important
role in the tumorigenesis and progression of PCa.
Kaplan-Meier survival analysis revealed that
patients with positive NOB1 expression had a significantly reduced
OS and RFS compared with the patients with negative NOB1
expression. Univariate analyses showed that increased NOB1
expression in PCa tissues was significantly correlated with RFS.
Furthermore, Cox hazard ratio regression analyses demonstrated that
NOB1 expression was an independent prognostic factor of disease
free survival in patients with PCa. These results suggest that NOB1
may be an important prognostic marker for patients with PCa.
In conclusion, the present study has demonstrated
that NOB1 expression in PCa is correlated with a more malignant
phenotype and worse clinical outcome in a relatively large number
of PCa samples. These findings suggest that NOB1 may function as a
potential prognostic indicator for PCa. Translational and
prospective studies of NOB1 as a therapeutic target in PCa are
required.
Acknowledgements
The authors would like to thank the Innovation
Program of the Shanghai Municipal Education Commission for grant
funding awarded to Dr Xingang Cui (grant no.14zz084).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim WT and Kim WJ: MicroRNAs in prostate
cancer. Prostate Int. 1:3–9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Madan RA and Arlen PM: Recent advances
revolutionize treatment of metastatic prostate cancer. Future
Oncol. 9:1133–1144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Facompre N and El-Bayoumy K: Potential
stages for prostate cancer prevention with selenium: implications
for cancer survivors. Cancer Res. 69:2699–2703. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Y, Ni J, Zhou G, Yuan J, Ren W, Shan
Y, Tang W, Yu L and Zhao S: Cloning, expression and
characterization of the human NOB1 gene. Mol Biol Rep. 32:185–189.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lamanna AC and Karbstein K: Nob1 binds the
single-stranded cleavage site D at the 3′-end of 18S rRNA with its
PIN domain. Proc Natl Acad Sci USA. 106:14259–14264. 2009.
View Article : Google Scholar
|
|
7
|
Hong L, Piao Y, Han Y, Wang J, Zhang X, Du
Y, Cao S, Qiao T, Chen Z and Fan D: Zinc ribbon domain-containing 1
(ZNRD1) mediates multidrug resistance of leukemia cells through
regulation of P-glycoprotein and Bcl-2. Mol Cancer Ther.
4:1936–1942. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Granneman S, Nandineni MR and Baserga SJ:
The putative NTPase Fap7 mediates cytoplasmic 20S pre-rRNA
processing through a direct interaction with Rps14. Mol Cell Biol.
25:10352–10364. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang H, Li P and Zhao B: Knockdown of NOB1
expression by RNAi inhibits cellular proliferation and migration in
human gliomas. Gene. 528:146–153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
D’Amico AV, Moul JW, Carroll PR, Sun L,
Lubeck D and Chen MH: Surrogate end point for prostate
cancer-specific mortality after radical prostatectomy or radiation
therapy. J Natl Cancer Inst. 95:1376–1383. 2003. View Article : Google Scholar
|
|
11
|
Lin Y, Peng S, Yu H, Teng H and Cui M:
RNAi-mediated downregulation of NOB1 suppresses the growth and
colony-formation ability of human ovarian cancer cells. Med Oncol.
29:311–317. 2012. View Article : Google Scholar
|
|
12
|
Zhou J, Xu T, Yan Y, Qin R, Wang H, Zhang
X, Huang Y, Wang Y, Lu Y, Fu D and Chen J: MicroRNA-326 functions
as a tumor suppressor in glioma by targeting the Nin one binding
protein (NOB1). PLoS One. 8:e684692013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu Z, Guo Q, Shi A, Xie F and Lu Q:
Downregulation of NIN/RPN12 binding protein inhibit the growth of
human hepatocellular carcinoma cells. Mol Biol Rep. 39:501–507.
2012. View Article : Google Scholar
|
|
14
|
Huang WY, Chen DH, Ning L and Wang LW:
siRNA mediated silencing of NIN1/RPN12 binding protein 1 homolog
inhibits proliferation and growth of breast cancer cells. Asian Pac
J Cancer Prev. 13:1823–1827. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin S, Meng W, Zhang W, Liu J, Wang P, Xue
S and Chen G: Expression of the NOB1 gene and its clinical
significance in papillary thyroid carcinoma. J Int Med Res.
41:568–572. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li XY, Luo QF, Li J, Wei CK, Kong XJ,
Zhang JF and Fang L: Clinical significance of NOB1 expression in
breast infiltrating ductal carcinoma. Int J Clin Exp Pathol.
6:2137–2144. 2013.PubMed/NCBI
|