Introduction
Idiopathic pulmonary fibrosis (IPF) is a progressive
disorder of unknown etiology, which has limited response to
currently available therapies and a mean survival expectancy of 3–5
years (1). Over the last few
years, there has been increasing evidence that IPF may result from
acute lung injury targeting alveolar epithelial cells and
consequent aberrant wound healing leading to the formation of
fibroblastic foci, which are considered to be the active sites of
fibrogenesis (2). Fibroblastic
foci are composed mainly of fibroblasts and myofibroblasts, which
promote excessive deposition of extracellular connective matrix in
the pulmonary interstitium during the pathogenesis and progression
of pulmonary fibrosis, which results in irreversible distortion of
the lung architecture (3–5).
The origin of fibroblasts and myofibroblasts in
these fibroblastic foci remain to be fully elucidated, although the
migration and proliferation of resident mesenchymal cells and
recruitment of fibrocytes may account for a fraction of them. In
2005, Willis et al identified commonly expressed epithelial
markers and α-smooth muscle actin (α-SMA) in the lung tissues of
patients with IPF, indicating that cells undergo phenotypic
transition in the IPF lung and describing, for the first time, the
possibility of epithelial-mesenchymal transition (EMT) in human
lung fibrosis (4). There has been
increasing evidence that lung fibroblasts and myofibroblasts may be
derived from epithelial cells through EMT (6–8). EMT
is crucial for germ layer formation and cell migration in the early
vertebrate embryo (9). Although
EMT is usually maintained in a silent state in adults, it may be
transiently activated for wound healing and tissue repair (9,10)
and there is increasing evidence that abnormal activation of EMT
programs are associated with tissue fibrosis (11). EMT is characterized by
morphological changes, including the change from a cuboidal cell
shape to an elongated or spindle-shaped form, acquisition of
fibroblast- or myofibroblast-specific markers vimentin, collagen
fiber I and α-SMA, loss of the characteristic epithelial marker
E-cadherin and epithelial cell polarity, abatement of adhesion
ability and cytoskeletal rearrangements (10,12).
The mechanisms of EMT, however, remain to be elucidated. Several
studies have demonstrated that multiple cytokines are effected in
EMT, including tumor growth factor-β, insulin-like growth factor-II
and fibroblast growth factor-2 (4,13–15).
Wnt signals are important in embryonic development
and organ morphogenesis. Previous studies have demonstrated that
abnormal activation of the Wnt/β-catenin signaling pathway occurs
in the lung tissue of patients with IPF and in models of
bleomycin-induced pulmonary fibrosis (16,17).
However, the precise mechanism through which this occurs remains
unclear. Wnt signaling cascades can be divided into at least three
distinct pathways, one of which is the Wnt/β-catenin signaling
pathway. This classical signaling pathway is initiated by
extracellular ligands, termed Wnts. β-catenin is the key member of
the Wnt signaling pathway in the regulation of transcriptional
activity (18). In the present
study, A549 cells were treated with different concentrations of
Wnt1 and transfected with a β-catenin plasmid, the results
indicated that Wnt/β-catenin led to activation of an EMT
transcriptome. Previously, a model has been proposed in which
injury to the epithelium initiates a proinflammatory and
profibrotic cascade resulting in fibroblast expansion and
progressive fibrosis reminiscent of abnormal wound healing
(19). The present study
investigated whether injured alveolar epithelia induce EMT and
activate the Wnt/β-catenin signal pathway. The A549 cells were
cultured with bronchoalveolar lavage fluid (BALF) from
bleomycin-treated mice in the presence or absence of a small
interfering (si)RNA designed to suppress the expression of
β-catenin.
Materials and methods
Cell culture
A549, human alveolar epithelial cells (American Type
Culture Collection, Manassas, VA, USA), were purchased from the
Institute of Biochemistry and Cell Biology (Shanghai Institute of
Biological Science, Chinese Academy of Sciences, Shanghai, China)
and maintained in Dulbecco’s modified Eagle’s medium (DMEM;
Sunshine Biotechnology Co., Ltd, Jiangsu, China) supplemented with
10% fetal bovine serum (FBS; Invitrogen Life Technologies,
Carlsbad, CA, USA) at 37°C in a humidified 5% CO2
atmosphere. The A549 cells were diluted with DMEM containing 10%
FBS to 1×105 cells/ml and seeded into six-well plates (2
ml/well; Corning Inc., Corning NY, USA). When cells reached 60–70%
confluence the culture medium was replaced with 2 ml serum-free
DMEM for 24 h prior to treatment.
BALF
In the present study, 6–8-week-old (18±2 g) Specific
pathogen-free female C57BL/6 mice (Laboratory Animal Center of
Jiangsu University, Jiangsu, China) were used. The animal
experiments were approved by the Animal Research Committee of
Jiangsu University School of Medicine (Jiangsu, China) and clean
food and water were provided ad libitum. The BALF procedure
was performed on day 7 following intra-tracheal injection of
bleomycin solution (5 mg/kg body weight; Nippon Kayaku Co., Ltd,
Tokyo, Japan), as previously described (20–22).
Briefly, on day 7 post-modeling, the mice were sacrificed by
cervical dislocation and, following excision of the trachea, a
plastic cannula was inserted into the trachea and 1.0 ml cold
sterile saline solution was injected gently with a syringe and
withdrawn. This procedure was repeated three times. The BALF was
then centrifuged for 5 min at 716 × g and the supernatants were
preserved at −70°C. Subsequently, the lung tissues were
harvested.
Hematoxylin and eosin (H&E)
staining
The mice were sacrificed and the lungs were rinsed
in phosphate-buffered saline (Huashun Biotechnology Co., Ltd,
Shanghai, China) fixed in 4% paraformaldehyde for 24 h, embedded in
paraffin and sectioned at 5 μM. H&E staining was performed for
cell alignment to evaluate the degrees of inflammation.
Cell transfection with plasmids
Plasmids expressing constitutively active β-catenin
(plasmid pcDNA DEST40) were obtained from Shanghai Integrated
Biotech Solutions Co, Ltd. (Shanghai, China). When the A549 cells
reached 60–70% confluence on six-well plates, they were transfected
using Lipofectamine 2000 (Invitrogen Life Technologies), according
to manufacturer’s instructions. Subsequently, 2 h prior to
transfection, the medium was replaced with serum-free DMEM and the
β-catenin plasmid and Lipofectamine were then diluted separately in
250 μl Opti-MEM (Invitrogen Life Technologies). Subsequently, 6 μg
plasmid DNA per well was complexed with 4 μl Lipofectamine. The
complexes were then incubated at room temperature for 20 min and
added to the cells in six-well plates. Following 4 h incubation,
the cell medium was replaced by fresh DMEM with 10% FBS and the
cells were incubated for a further for 48 h. Empty plasmids were
used as controls.
Transfection of lentiviral vectors with
shRNA for β-catenin
Stealth small interference (si)RNA sequences for
β-catenin were designed by Shanghai Integrated Biotech Solutions
Co, Ltd. The pLentilox3.7-GFP-shRNA-β-catenin lentiviral vectors
were synthesized using the following target shRNA sequence:
5′-CAGTTGTGGTTAAGCTCTT-3′. An unrelated shRNA sequence was used as
a negative control (shNC): 5′-TTCTCCGAACGTGTCACGT-3′. The
lentiviral vectors and lentiviral helper plasmids (VSVG, RSV-REV
and pMDLg/pRRE) were also cotransfected into the 293 T cells. At 48
and 72 h post-co-transfection, the culture media were colleted and
centrifuged for 20 min at 1,600 × g. The supernatants were filtered
through a Millex-HV polyvinylidene fluoride-0.45 μm filter
(Millipore, Billerica, MA, USA). The flow-through containing the
virus was stored at −70°C until further use as a viral stock. The
A549 cells were cultured to 40–50% confluence and then infected
with either the lentivirus expressing a shRNA to the human
β-catenin gene (sh β-catenin) or with the negative control plasmid
at a multiplicity of infection of 20. The number of green
fluorescent protein (GFP)-positive cells was determined using an
inverted fluorescent microscope (Axio Observer; magnification, ×20;
Carl Zeiss, Oberkochen, Germany) 4 days post-transduction to
evaluate the transfection efficiency. Validation of the shRNA
targeting sequence with the most efficient interference with
β-catenin was then performed by reverse transcription quantitative
polymerase chain reaction (RT-qPCR) and western blot analysis.
Western blotting
Cells were lysed in an ice-cold
radioimmunoprecipitation assay lysis buffer [50 mM Tris (pH 7.4),
150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate and 0.1%
sodium dodecyl sulfate (SDS)]. Equal quantities of protein (20
μg/lane) were resolved on a 12% SDS-polyacrylamide gel. The
proteins were then transferred onto polyvinylidene fluoride
membranes (Millipore, Billerica, MA, USA and Weiga Science and
Technology Co., Ltd, Guangzhou, China), inhibited with skimmed milk
and probed using mouse anti-human monoclonal antibodies against
α-SMA (sc-53015), vimentin (sc-373717) and β-actin (sc8432;
1:1,000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA),
rabbit anti-human polyclonal antibodies against collagen I
(sc-28657; 1:1,000; Santa Cruz Biotechnology, Inc.), E-cadherin
(BA0475; 1:200; Boster Biological Technology, Wuhan, China) or
β-catenin (9562; 1:1,000; Cell Signaling Technology, Inc., Boston,
MA, USA), followed by horseradish peroxidase-conjugated goat
anti-rabbit immunoglobulin G (sc-2004) and goat anti-mouse
antibodies (sc-2005; 1:5,000; Santa Cruz Biotechnology, Inc.).
Enhanced chemiluminescence detection reagents were used for
visualization (Amersham Pharmacia Biotech, Piscataway, NJ, USA) and
the band densities for each phenotype marker were quantified using
Lane 1D software (version 2.0; Beijing Sage Creation Science Co.,
Ltd., Beijing, China) following scanning with an ECL-PLUS
chemiluminescence system (Bio-Rad, Hercules, CA, USA). β-actin
staining served as an internal control and the ratio of band
density to total β-actin was determined.
RT-qPCR
Total RNA was isolated using TRIzol®
reagent (Invitrogen Life Technologies) according to the
manufacturer’s instructions and cDNAs were generated using a
PrimeScript RT reagent kit (Takara Bio, Inc., Dalian, China). qPCR
were performed using an Mx3000P system (Stratagene, La Jolla, CA,
USA) with SYBR Premix Ex Taq (Takara Bio, Inc.). The primers and
conditions for qPCR are detailed in Table I. The RT reaction mixture (1 μl)
was used for the qPCR reaction in a total volume of 20 μl. The
relative transcript abundance of a gene was presented as the ΔCt
values (ΔCt = Ctreference − Cttarget) and the
relative expression levels of the target genes, following
normalization to the endogenous sequence, were calculated using the
2−ΔΔCt method.
 | Table IRT-qPCR primers, conditions and
products. |
Table I
RT-qPCR primers, conditions and
products.
| RT-qPCR genes | S/AS | Primer sequence
(5′-3′) | Temperature (°C) | Product (bp) |
|---|
| α-SMA | S |
5′-TCAAATACCCCATTGAACACGG-3′ | 58 | 178 |
| AS |
5′-GGTGCTCTTCAGGTGCTACA-3′ | | |
| Vimentin | S |
5′-TGCGTGAAATGGAAGAGAACT-3′ | 58 | 240 |
| AS |
5′-TGCGTGAAATGGAAGAGAACT-3′ | | |
| Collagen I | S |
5′-TCTGACTGGAAGAGTGGAGAGTAC-3′ | 58 | 202 |
| AS |
5′-ATCCATCGGTCATGCTCTCG-3′ | | |
| E-cadherin | S |
5′-TTGCTACTGGAACAGGGACAC-3′ | 58 | 179 |
| AS |
5′-CCCGTGTGTTAGTTCTGCTGT-3′ | | |
| β-catenin | S |
5′-GCTACTCAAGCTGATTTGATGGA-3′ | 58 | 120 |
| AS |
5′-GGTAGTGGCACCAGAATGGATT-3′ | | |
| GAPDH | S |
5′-GGATTTGGTCGTATTGGG-3′ | 58 | 205 |
| AS |
5′-GGAAGATGGTGATGGGATT-3′ | | |
Statistical analysis
The results are expressed as the mean ± standard
deviation. Statistical comparisons between the groups were
performed using a two-tailed unpaired t-test or one-way analysis of
variance followed by a Student-Newman-Keuls-q test for analysis of
more than two groups. Correlation analysis adopted Pearson’s
correlation analysis using SPSS for Windows, version 16.0 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects on the mRNA and protein
expression levels of E-cadherin, SMA, vimentin and collagen I in
A549 cells stimulated by Wnt1
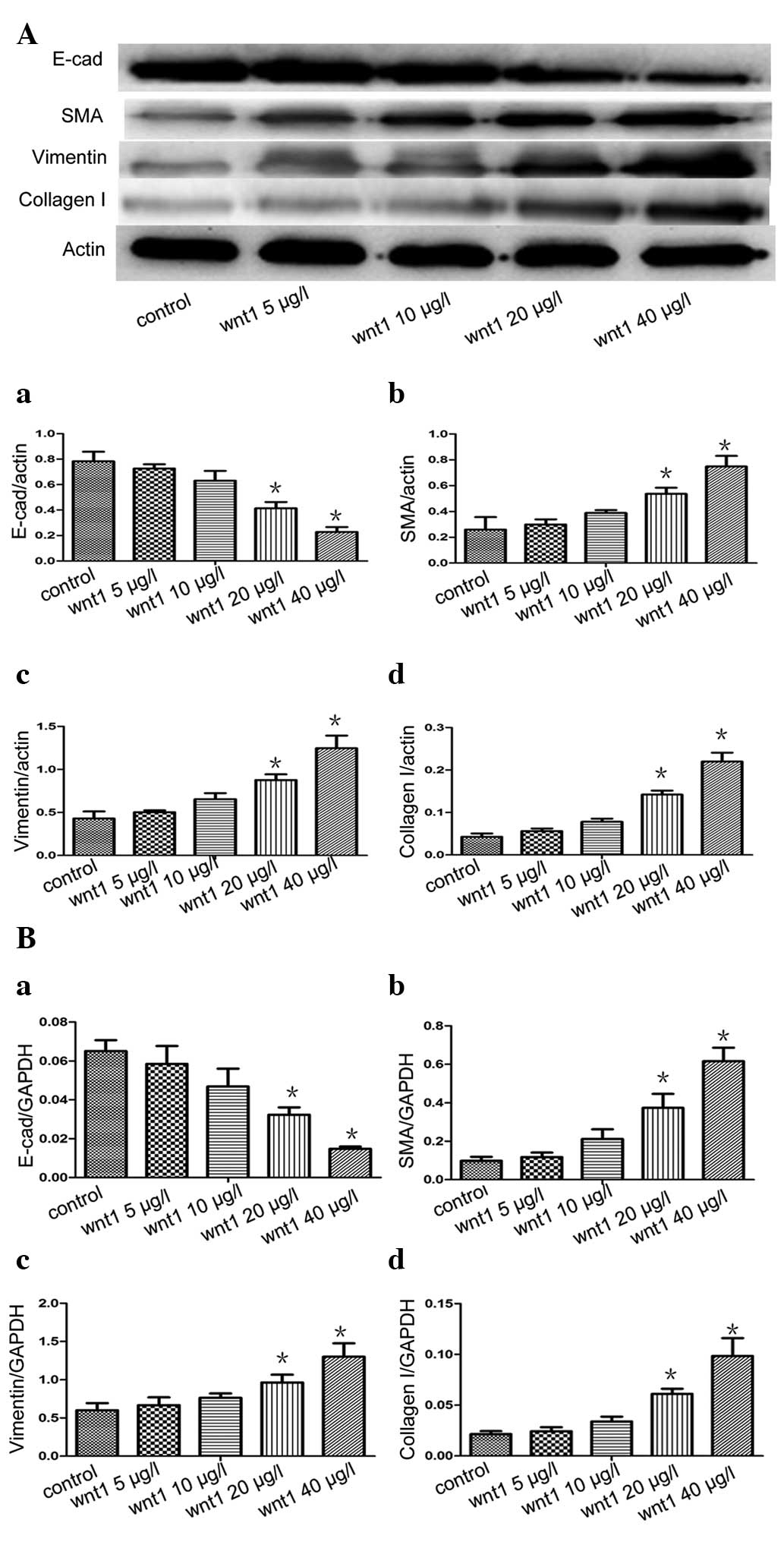
The A549 cells were stimulated with various
concentrations of Wnt1 (0, 5, 10, 20 and 40 μg/l) for 48 h. Western
blot analysis and RT-qPCR revealed that the mRNA and protein
expression of levels E-cadherin decreased and the mRNA and protein
expression levels of SMA, vimentin and collagen I increased in a
concentration-dependent manner, with Wnt1 concentration>20 μg/l
leading to a significant increase compared with the control group
(P<0.05) (Fig. 1).
A549 cell EMT by β-catenin
The main factor involved in the classical Wnt
signaling pathway is β-catenin. To examine the role of β-catenin in
the regulation of alveolar EMT, the present study used β-catenin
plasmid-transfected A549 cells. The morphology of the A549 cells
changed from a round, cube or polygon shape to a fibroblast-like,
stretched, spindle-shape on visualization with an inverted phase
contrast microscope (Fig. 2A). No
changes in morphology was observed in the A549 cells in the control
group, which maintained a typical epithelial morphology
(polygonal/cobblestone or round appearance; Fig. 2B). In the β-catenin plasmid group,
the relative gene and protein levels of the characteristic
epithelial phenotypic marker E-cadherin were significantly lower
(P<0.05; Fig. 2Ca and Da) and
the relative expression of the mesenchymal markers α-SMA, vimentin
and collagen I were significantly higher (P<0.05) compared with
the control group (Fig. 2Cb-d and
Db-d). However, no significant differences were observed in the
levels of E-cadherin, α-SMA, vimentin or collagen I levels between
the empty plasmid group and the control (P>0.05). Taken
together, these results demonstrated that A549 cells undergo EMT
in vitro when exposed to β-catenin stimuli.
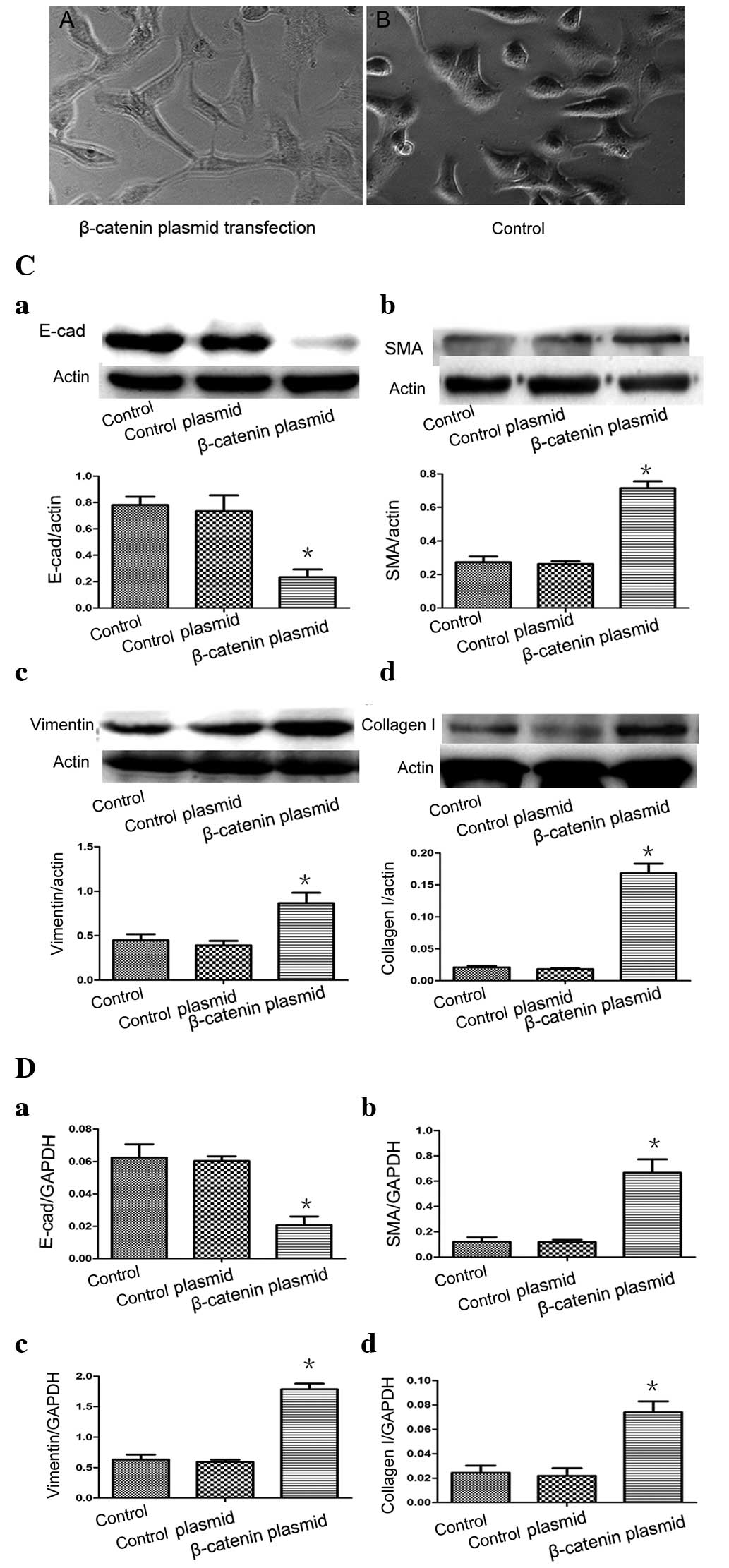 | Figure 2(A) A549 cells were transfected with
β-catenin plasmids and, after 48 h, the A549 cells assumed a
fibroblast-like morphology. (B) In the controls, epithelial cells
maintained their typical polygonal/cobblestone or round appearance
(original magnification, ×200). (C) Effects of β-catenin on the
protein expression of E-cad, α-SMA, vimentin and collagen I in the
A549 epithelial cells. (D) Reverse transcription-quantitative
polymerase chain reaction revealed the mRNA expression levels of
E-cad, α-SMA, vimentin and collagen I following β-catenin plasmid
transfection. (Control, medium-treated A549 cells; control plasmid,
A549 cells treated with empty plasmids). Each bar represents the
mean ± standard deviation. *P<0.05, compared with the
control. E-cad, E-cadherin; α-SMA, α-smooth muscle actin. |
BALF induces a significant reduction in
the expression of E-cadherin and significant increases in the
expression of α-SMA, vimentin and collagen I in A549 cells
At present, the most frequently used experimental
model of lung fibrosis is the bleomycin-induced model. In the
present study BALF and lung biopsies were obtained from
bleomycin-treated mice at day 7. In the H&E-stained sections,
inflammatory cells and erythrocytes were observed in the septum and
alveolus, which was associated with fibroblast proliferation
(Fig. 3B), indicating successful
construction of the bleomycin-induced model of pulmonary
fibrosis.
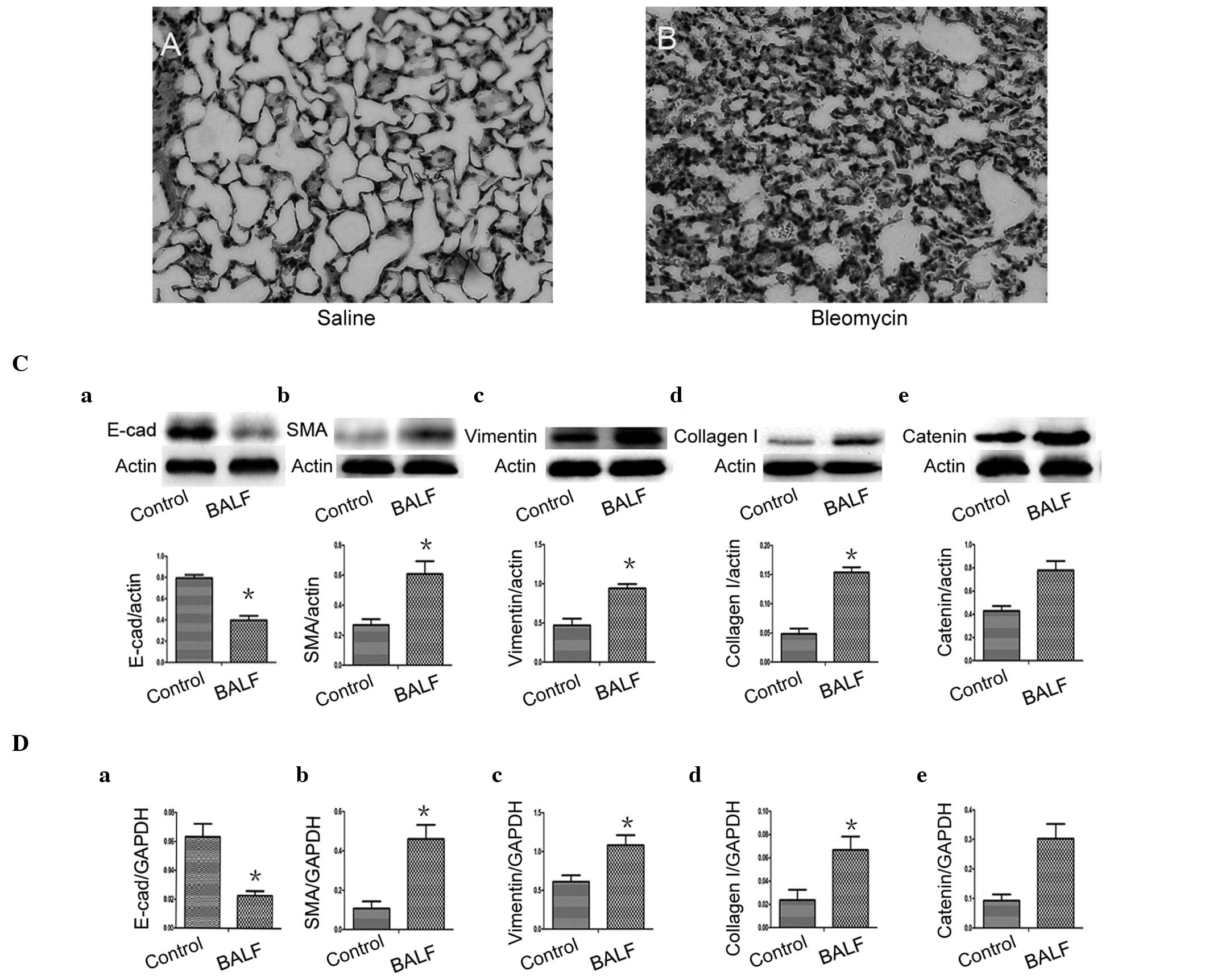 | Figure 3BALF induced a significant reduction
in the expression of E-cad and significant increases in the
expression levels of α-SMA, vimentin and collagen I in the A549
cells. Hematoxylin and eosin-stained lung sections from mice. (A)
Control group (saline); (B) BLM group (day 7 post-bleomycin
instillation). (C) Protein expression of E-cad, α-SMA, vimentin and
collagen I, in A549 epithelial cells cultured with BALF. (D)
Reverse transcription quantitative polymerase chain reaction of the
mRNA expression levels of E-cad, α-SMA, vimentin and collagen I in
the A549 epithelial cells cultured with BALF. (Ce and De) Changes
in the protein and mRNA expression of β-catenin in BALF-treated
A549 cells. (Control, medium-treated A549 cells). Each bar
represents means ± standard deviation; *P<0.05
compared with control. E-cad, E-cadherin; α-SMA, α-smooth muscle
actin; BALF, bronchoalveolar lavage fluid; BLM, bleomycin. |
To determine whether lung alveolar epithelial cell
injury induced the expansion of the fibroblast and myofibroblast
population through EMT, A549 cells were cultured with BALF and DMEM
(1:1) for 48 h and BALF was obtained from the bleomycin-treated
mice at day 7. In the A549 cells, expression of the epithelial
phenotypic marker E-cadherin was lost (Fig. 3Ca and Da) and overexpression of
α-SMA (Fig. 3Cb and Db), vimentin
(Fig. 3Cc and Dc) and collagen I
(Fig. 3Cd and Dd) were observed by
western blot analysis and RT-qPCR. These results indicated the
occurrence of a mesenchymal cell phenotype transition, which was
absent in the control group (*P<0.05). Notably, the
reduced levels of mRNA and protein expression of E-cadherin
correlated with levels of β-catenin (r=−0.817 and −0.831) and the
increased levels of mRNA and protein expression of α-SMA correlated
with levels of β-catenin (r=0.825 and 0.820). The mRNA and protein
expression levels of vimentin and collagen I also correlated with
β-catenin levels (r=0.815 and 0.816 and r=0.846 and 0.831,
repectively). Furthermore, the present study knocked down the
β-catenin gene by infecting the A549 cells with a lentivirus (sh
β-catenin) expressing β-catenin-specific siRNA and GFP. After 96 h,
the cells expressed GFP (Fig.
4Ab), indicating successful infection. Western blot analysis
and RT-qPCR revealed that the levels of β-catenin in the A549
siRNA-infected cells were significantly lower compared with the
cells infected with shNC (Fig. 4B and
C). These findings indicated that siRNA, directed towards A549,
was effective in specifically knocking down the β-catenin gene. The
A549 cells were then infected with the β-catenin-expressing shRNA
lentivirus prior to BALF treatment. As a negative control, a group
of A549 cells were infected by a lentivirus containing an unrelated
shRNA sequence. Notably, the siRNA restored the decreased
expression level of E-cadherin and increased expression levels of
α-SMA, vimentin and collagen I that were induced by BALF treatment
(Fig. 4D and E).
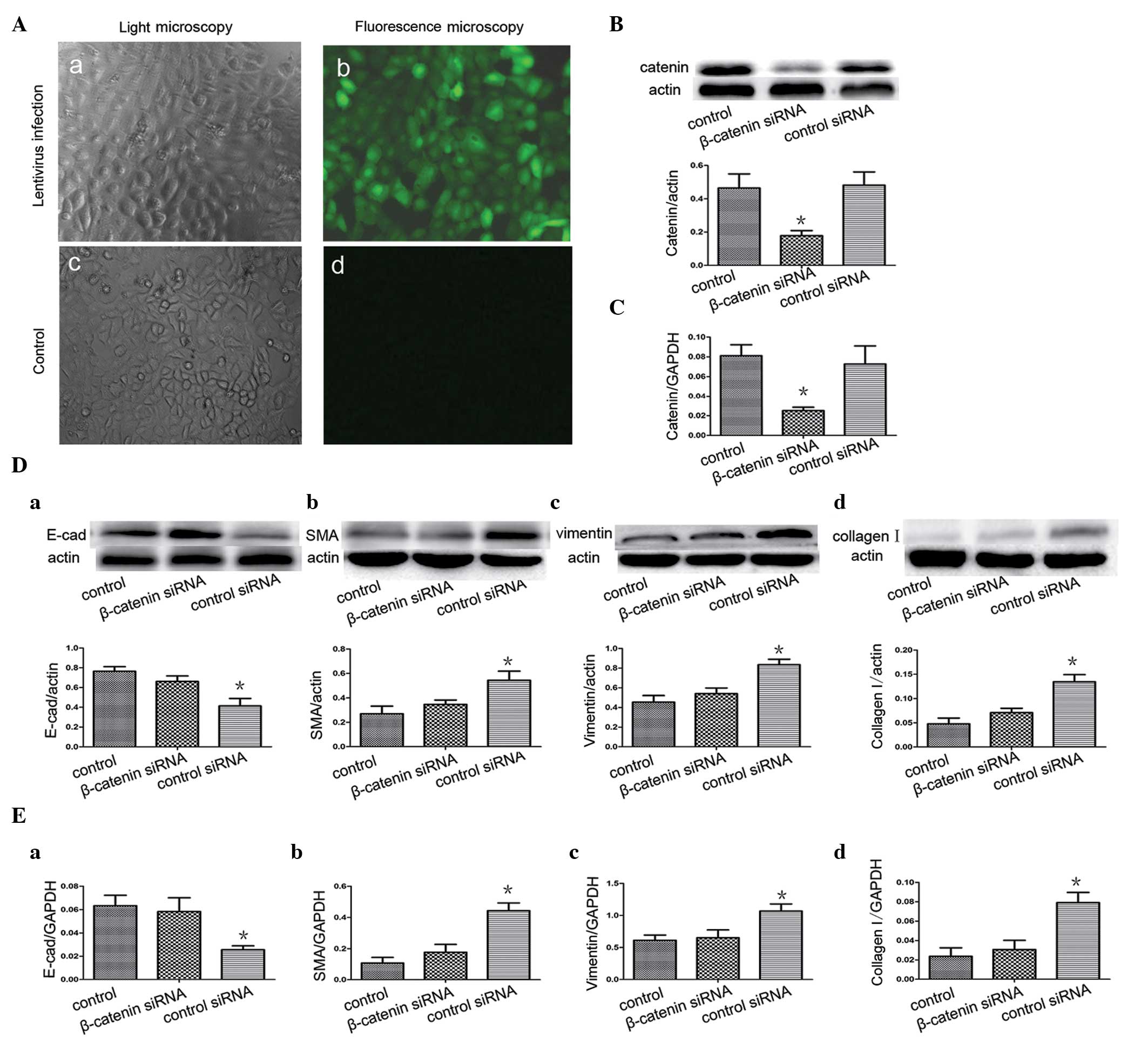 | Figure 4β-catenin siRNA inhibits the
expression of β-catenin and attenuates epithelial-mesenchymal
transition by BALF. (Aa) A549 cells were observed under light
microscope 96 h after infection with lentivirus (x200). (Ab) GFP
fluorescence (right panel) of A549 cells was observed using
fluorescence microscopy 96 h after infection with the lentivirus
containing pLL-sh catenin or shNC (x200). (Ac) A549 cells were
observed under a light microscope with no lentivirus infection.
(Ad) GFP fluorescence was not observed when the cells were not
infected with the lentivirus. Western blot analysis and RT-qPCR
assessment revealed that β-catenin siRNA suppresses (B) β-catenin
protein and (C) mRNA expression in the A549 cells. Following
infection of the A549 cells with the β-catenin-expressing shRNA
lentivirus and BALF treatment, (D) western blot analysis revealed
the protein expression of E-cad, α-SMA, vimentin and collagen I.
(E) mRNA expression of E-cad, α-SMA, vimentin and collagen I by
RT-qPCR. (Control, medium-treated A549 cells) Each bar represents
the mean ± standard deviation, *P<0.05. GFP, green
fluorescent protein; E-cad, E-cadherin; α-SMA, α-smooth muscle
actin; RT-qPCR, reverse transcription quantitative polymerase chain
reaction. |
Discussion
The Wnt/β-catenin signaling pathway is important in
the regulation of cell proliferation, differentiation and polarity.
Accumulating evidence from animal models and human diseases
indicate that Wnt signaling is enhanced in several fibrotic
diseases and in lung fibroblasts. Our previous studies demonstrated
high expression levels of β-catenin in bleomycin-induced pulmonary
fibrosis in mice and improvement in pulmonary fibrosis following
inhibition of the classical Wnt signaling pathway by SFRP4
antagonists.
In the classical Wnt/β-catenin pathway, a complex
between the Wnt ligands and the cell surface receptor frizzled
(FZD) binds low-density lipoprotein receptor-related protein (LRP),
which leads to activation of the dishevelled protein (Dvl),
inhibiting phosphorylation of GSK-3β and β-catenin decomposition.
This leads to subsequent β-catenin translocation into the nucleus,
which binds to the transcription factor (TCF)/lymphoid enhancer
factor (LEF) and activates target genes and the induction of
fibrosis (23). β-catenin has a
dual role. In normal cells, it is located on the cell membrane as a
structural protein in connection with E-cadherin that is important
in cellular adhesion junctions. On activation of Wnt signaling,
β-catenin, as an intermediary, is translocated into the nucleus
(24).
The formation of fibroblastic foci is considered to
be the main feature of IPF. Fibroblastic foci are composed mainly
of fibroblasts and myofibroblasts. EMT may be an important
mechanism in increasing the myofibroblast pool. E-cadherin is a
recognized phenotypic marker of epithelial cells, α-SMA and
collagen I are key markers of a myofibroblast phenotype and
vimentin is a cytoskeletal protein. In addition, α-SMA and vimentin
are often described as mesenchymal cell markers.
In the present study, different concentrations of
Wnt1-intervented A549 cells were used. The results demonstrated
downregulation in the epithelial phenotypic marker E-cadherin and
upregulation of the mesenchymal phenotypic marker and, when the
concentration of Wnt1 exceeded 20 μg/l, these changes were more
obvious. Furthermore, the A549 cells were transfected with a
β-catenin plasmid, which induced a decrease in the mRNA and protein
expression levels of E-cadherin and an increase in mRNA and protein
expression levels of α-SMA, vimentin and collagen I.
Intratracheal bleomycin instillation causes initial
alveolar epithelial cell injury and apoptosis (25). Bleomycin-induced injury is widely
used as a model of pulmonary fibrosis (26,27).
Lewis et al (28) compared
different mouse models of infection, allergy and lung injury and
found that regulation of the Wnt signaling pathway is specific to
the mouse model of bleomycin-induced lung fibrosis. Our previous
studies involving bleomycin-induced pulmonary fibrosis in mice
demonstrated that the expression of β-catenin increases on day 7
and peaks on day 14 and that alveolar epithelial injury is most
marked on day 7. In the present study, day 7 of BALF was selected
in a bleomycin mouse model and the results demonstrated that
increases in the mRNA and protein expression of vimentin, α-SMA and
collagen I were positively correlated with the expression of
β-catenin, however, decreases in the mRNA and protein expression of
E-cadherin were negatively correlated with the expression of
β-catenin. Furthermore, the present study also infected A549 cells
with a lentivirus containing β-catenin shRNA, which knocked down
the β-catenin gene, and the lung epithelial cells were then
cultured with the pulmonary lavage fluid. Following this, no
significant increases were observed in the mRNA and protein
expression levels of vimentin, α-SMA and collagen I and no decrease
was observed in the mRNA and protein expression levels of
E-cadherin. The expression of Wnt ligands and β-catenin in the
pulmonary lavage fluid from mice in the bleomycin model were not
measured in the present study, however, Levänen et al
(29) observed that the mRNA
expression levels of Wnt5A, Wnt7A and Wnt7B increased in BALF cells
in patients with sarcoidosis. A possible mechanism for this may be
that bleomycin-induced epithelial injury triggers an acute
inflammatory response and initiates lung repair mechanisms,
including activation of the Wnt signaling pathway. The Wnt family
proteins are released by the injured epithelial cells and
neighboring cells to the surrounding tissues and into the BALF. In
the present study, the use of BALF in the A549 cell culture, led to
β-catenin nuclear transcription, binding to TCF/LEF and activation
of downstream target genes, inducing cell EMT even in the absence
of initial injury factors. TGF-β is considered to be a key mediator
in the progression of fibrosis (30). Previous studies have demonstrated
that there are cross talks between the Wnt/β-catenin pathway and
TGF-β signaling (31–33). In bleomycin-induced mice, protein
levels of TGF-β in BALF are significantly increased (34,35).
In the present study, it was hypothesized that the BALF obtained
from the bleomycin-induced pulmonary fibrosis mouse model contained
TGF-β, which activated the Wnt signal through cross talk with the
Wnt/β-catenin pathway.
The BALF from pulmonary fibrosis mouse models
contains interleukin (IL)-1α, IL-6 and tumor necrosis factor
(TNF)-α (36). Whether the Wnt
signal is triggered by IL-1α, IL-6 or TNF-α requires further
investigation.
In conclusion, the present study demonstrated that
Wnt/β-catenin signaling increases the number of myofibroblasts in
pulmonary fibrosis through EMT. In addition, it revealed that
activation of the biological repair response at an injury site and
its persistence is important in the formation of pulmonary
fibrosis. In lung injury, the reactivation of aberrant
Wnt/β-catenin signaling is important in the formation of fibrotic
diseases and may provide a potential therapeutic strategy in the
future.
Acknowledgements
This study was supported by grants from the
Scientific Research Project of the Ministry of Public Health (no.
wkj2006-2-026) and the Shanghai Science and Technology Development
Fund (no. 10ZR1422600).
References
|
1
|
Kliment CR, Englert JM, Gochuico BR, et
al: Oxidative stress alters syndecan-1 distribution in lungs with
pulmonary fibrosis. J Biol Chem. 284:3537–3545. 2009. View Article : Google Scholar :
|
|
2
|
Selman M and Pardo A: The
epithelial/fibroblastic pathway in the pathogenesis of idiopathic
pulmonary fibrosis. Am J Resp Cell Mol Bio. 29:S93–S97. 2003.
|
|
3
|
Ramos C, Becerril C, Montaño M, et al:
FGF-1 reverts epithelial-mesenchymal transition induced by
TGF-{beta}1 through MAPK/ERK kinase pathway. Am J Physiol Lung Cell
Mol Physiol. 299:L222–L231. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Willis BC, Liebler JM, Luby-Phelps K, et
al: Induction of epithelial-mesenchymal transition in alveolar
epithelial cells by transforming growth factor-β1: potential role
in idiopathic pulmonary fibrosis. Am J Pathol. 166:1321–1332. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim KK, Kugler MC, Wolters PJ, et al:
Alveolar epithelial cell mesenchymal transition develops in vivo
during pulmonary fibrosis and is regulated by the extracellular
matrix. Proc Natl Acad Sci USA. 103:13180–13185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Willis BC and Borok Z: TGF-beta-induced
EMT: mechanisms and implications for fibrotic lung disease. AM J
Physiol Lung Cell Mol Physiol. 293:L525–L534. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tanjore H, Xu XC, Polosukhin VV, et al:
Contribution of epithelial-derived fibroblasts to bleomycin-induced
lung fibrosis. Am J Respir Crit Care Med. 180:657–665. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Selman M and Pardo A: Idiopathic pulmonary
fibrosis: misunderstandings between epithelial cells and
fibroblasts? Sarcoidosis Vasc Diffuse Lung Dis. 21:165–172.
2004.PubMed/NCBI
|
|
9
|
Acloque H, Adams MS, Fishwick K, et al:
Epithelial-mesenchymal transitions: the importance of changing cell
state in development and disease. J Clin Invest. 119:1438–1449.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kalluri R and Neilson EG:
Epithelial-mesenchymal transition and its implications for
fibrosis. J Clin Invest. 112:1776–1784. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zavadil J and Böttinger EP: TGF-beta and
epithelial-to-mesenchymal transitions. Oncogene. 24:5764–5774.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morali OG, Delmas V, Moore R, et al:
IGF-II induces rapid β-catenin relocation to the nucleus during
epithelium to mesenchyme transition. Oncogene. 20:4942–4950. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Strutz F, Zeisberg M, Ziyadeh FN, et al:
Role of basic fibroblast growth factor-2 in epithelial-mesenchymal
transformation. Kidney Int. 61:1714–1728. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kasai H, Allen JT, Mason RM, et al: TGF-β1
induces human alveolar epithelial to mesenchymal cell transition
(EMT). Respir Res. 6:562005. View Article : Google Scholar
|
|
16
|
Meuten T, Hickey A, Franklin K, et al:
WNT7B in fibroblastic foci of idiopathic pulmonary fibrosis. Respir
Res. 13:622012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo Y, Xiao L, Sun L and Liu F:
Wnt/beta-catenin signaling: a promising new target for fibrosis
diseases. Physiol Res. 61:337–346. 2012.PubMed/NCBI
|
|
18
|
Borok Z: Role for alpha3 integrin in EMT
and pulmonary fibrosis. J Clin Invest. 119:7–10. 2009.
|
|
19
|
Selman M and Pardo A: Role of epithelial
cells in idiopathic pulmonary fibrosis: from innocent targets to
serial killers. Proc Am Thorac Soc. 3:364–372. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lawson WE, Polosukhin VV, Stathopoulos GT,
et al: Increased and prolonged pulmonary fibrosis in surfactant
protein C-deficient mice following intratracheal bleomycin. Am J
Pathol. 167:1267–1277. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang D, Liang J, Campanella GS, et al:
Inhibition of pulmonary fibrosis in mice by CXCL10 requires
glycosaminoglycan binding and syndecan-4. J Clin Invest.
120:2049–2057. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang D, Liang J, Hodge J, et al:
Regulation of pulmonary fibrosis by chemokine receptor CXCR3. J
Clin Invest. 114:291–299. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moon RT, Kohn AD, De Ferrari GV and Kaykas
A: WNT and β-catenin signalling: diseases and therapies. Nat Rev
Genet. 5:691–701. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bao XL, Song H, Chen Z and Tang X: Wnt3a
promotes epithelial-mesenchymal transition, migration, and
proliferation of lens epithelial cells. Mol Vis. 18:1983–1990.
2012.PubMed/NCBI
|
|
25
|
Gauldie J, Bonniaud P, Sime P, et al:
TGF-beta, Smad3 and the process of progressive fibrosis. Biochem
Soc Trans. 35:661–664. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moeller A, Ask K, Warburton D, et al: The
bleomycin animal model: a useful tool to investigate treatment
options for idiopathic pulmonary fibrosis? Int J Biochem Cell Biol.
40:362–382. 2008. View Article : Google Scholar
|
|
27
|
Moore BB and Hogaboam CM: Murine models of
pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol.
294:L152–L160. 2008. View Article : Google Scholar
|
|
28
|
Lewis CC, Yang JY, Huang X, et al:
Disease-specific gene expression profiling in multiple models of
lung disease. Am J Respir Crit Care Med. 177:376–387. 2008.
View Article : Google Scholar
|
|
29
|
Levänen B, Wheelock AM, Eklund A, et al:
Increased pulmonary Wnt (wingless/integrated)-signaling in patients
with sarcoidosis. Respir Med. 105:282–291. 2011. View Article : Google Scholar
|
|
30
|
Biernacka A, Dobaczewski M and
Frangogiannis NG: TGF-β signaling in fibrosis. Growth Factors.
29:196–202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Carre AL, James AW, MacLeod L, et al:
Interaction of wingless protein (Wnt), transforming growth
factor-beta1, and hyaluronan production in fetal and postnatal
fibroblasts. Plast Reconstr Surg. 125:74–88. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sato M: Upregulation of the
Wnt/beta-catenin pathway induced by transforming growth factor-beta
in hypertrophic scars and keloids. Acta Derm Venereol. 86:300–307.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cheon SS, Nadesan P, Poon R and Alman BA:
Growth factors regulate beta-catenin-mediated TCF-dependent
transcriptional activation in fibroblasts during the proliferative
phase of wound healing. Exp Cell Res. 293:267–274. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Izumo T, Kondo M and Nagai A: Effects of a
leukotriene B4 receptor antagonist on bleomycin-induced pulmonary
fibrosis. Eur Respir J. 34:1444–1451. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Robb WB, Condron C, Moriarty M, et al:
Taurine attenuates radiation-induced lung fibrosis in C57/Bl6
fibrosis prone mice. Ir J Med Sci. 179:99–105. 2010. View Article : Google Scholar
|
|
36
|
Jiang C, Huang H, Liu J, et al: Fasudil, a
rho-kinase inhibitor, attenuates bleomycin-induced pulmonary
fibrosis in mice. Int J Mol Sci. 13:8293–8307. 2012. View Article : Google Scholar : PubMed/NCBI
|