Introduction
Influenza is a respiratory disease, which is caused
by an infectious virus. It can be effectively prevented by
antibodies formed as a result of vaccination. An influenza vaccine
can be developed using hemagglutinin (HA), present on the viral
surface, which is a main antigen of the influenza virus. The HA
content of an influenza virus is currently measured using a single
radial immunodiffusion (SRID) assay, which is internationally
authorized by the European Medicines Agency, Food and Drug
Administration and World Health Organisation (1–5).
However, this assay has the disadvantages of requiring the
corresponding reference antigen and antiserum for the vaccine, and
these reference factors require a longer time to develop (6–11).
Due to these factors, vaccine development may be delayed, as
revealed in the 2009 influenza pandemic. To overcome this issue,
various methods have been investigated to develop a more rapid
measurement of the HA content of an influenza vaccine. For example,
Kapteyn et al (7,8) measured HA content using reverse
phase-high performance liquid chromatography. In addition, during
the 2009 influenza pandemic, Li et al (12), measured HA content using SDS-PAGE
and densitometry, which resulted in an 88–122% similarity with that
of the conventional SRID for four subtypes of influenza vaccine
(H1N1, H3N2, H5N1 and B type). Based on this study, the authors
manufactured the first vaccine for the 2009 influenza pandemic
(12). In the present study, size
exclusion high performance liquid chromatography (SE-HPLC) was
examined to develop a novel measurement method for HA content,
which can be used without the preparation of reference antigen and
antiserum.
Materials and methods
Influenza vaccine and reference virus
samples
The 2009 pandemic A/California/7/2009 (H1N1)v
NYMC-X179A (2009 H1N1; Green Cross Corp., Yongin, Korea) vaccine,
the A/California/7/2009 (H1N1) NYMC-X181 (2010 H1N1, Green Cross
Corp.) monovalent seasonal vaccine, the A/Victoria/210/2009 (H3N2)
NYMC-X187 (2010 H3N2, Green Cross Corp.) monovalent seasonal
vaccine, the B/Brisbane/60/2008 (2010 B, Green Cross Corp.)
monovalent seasonal vaccines and the 2010 trivalent vaccine
(combined 2010 H1N1, 2010 H3N2 and 2010 B monovalent vaccines,
Green Cross Corp.) were assessed in the present study. Reference
antigens for 2009 H1N1 [National Institute for Biological
References and Control (NIBSC) code: 09/146], 2010 H1N1 (NIBSC
code: 09/294), 2010 H3N2 (NIBSC code: 10/102), 2010 B (NIBSC code:
8/352) were provided by NIBSC (Potters Bar, UK). The assessed
vaccines, reference antigens and antiserums are listed in Table I.
 | Table IVaccines and reference reagents. |
Table I
Vaccines and reference reagents.
| Vaccine type | Vaccine strain | Antigen reference
code | Antiserum reference
code |
|---|
| 2009 | H1N1 NYMC-X179A
(A/California/7/2009) | NIBSC 09/146 | NIBSC 09/152 |
| 2010 | H1N1 NYMC-X181
(A/California/7/2009) | NIBSC 09/294 | NIBSC 09/152 |
| 2010 | H3N2 NYMC-X187
(A/Victoria/210/2009) | NIBSC 10/102 | NIBSC 09/270 |
| 2010 B |
B/Brisbane/60/2008 | NIBSC 08/352 | CBER B-Ab-0913 |
SRID
The SRID assay was conducted using reference
antigens and antiserums (Table I)
provided by the NIBSC, which were appropriate for the influenza
vaccines used in the present study, following the standard
operating procedure of the National Institute of Food and Drug
Safety evaluation and the Korea Food and Drug administration
(Cheongwon-gun, Korea) as previously reported by Wood and
Levandowski (13).
SE-HPLC
HPLC Alliance 2695 (Waters, Co., Milford, MA, USA),
Waters 2489 UV-Vis Detector (Waters, Co.) and TSK G3000SWxl 7.8 ×
300 mm pore size 250Å (Tosoh, Tokyo, Japan) were used for HPLC. For
the mobile phase, phosphate buffer (Sigma-Aldrich, St. Louis, MO,
USA) was used at a flow rate of 1.0 ml/min. The experimental
condition was as follows: Sample injection volume, 20 μl;
wavelength, 210 nm and temperature, 15°C.
HA chromatographic peak
Subsequent to the vaccines and the corresponding
reference antigens being assessed using HPLC, elutes of each peak
in the chromatogram were collected and then the accuracy of HA
level in each peak was examined using ELISA and
immunoprecipitation.
ELISA
Fractions of the peak from the HPLC results were
plated on a Nunc-Immuno MaxiSorp™ plate (Sigma-Aldrich) and
incubated for 2 h at 37°C. Following incubation of the fractions,
the supernatants were removed and the plates were treated with
anti-A/California/07/2009 antiserum (anti-H1N1 serum, NIBSC code:
09/152) (1:20,000 in phosphate-buffered saline (PBS)-Tween) for 2 h
at 37°C. The plates were then washed with PBS-Tween three times and
secondary antibody (rabbit polyclonal secondary antibody to sheep
immunoglobulin G-horseradish peroxidase; Abcam, Cambridge, UK) was
applied for 2 h at 37°C. SigmaFast™ o-phenylenediamine
(Sigma-Aldrich) was added and the intensity was measured at 450 nm
with an ELISA reader (Spectra Max 190, Molecular Devices,
Sunnyvale, CA, USA).
Immunoprecipitation
Protein GHP SpinTrapTM column (GE
Healthcare, Amersham, UK) was washed and equilibrated with binding
buffer (1X Tris-buffered saline) following removing the storage
solution and reacting with 200 μl of anti-H1N1 serum for 30 min.
The column was washed with 400 μl of binding buffer and 200 μl of
reference antigen or the influenza vaccine was applied for 1 h.
Following centrifugation at 150 × g for 1 min, the eluted solution
was subjected to HPLC to verify the HA peak intensity.
Analysis of the correlation of the HA
content between SRID and SE-HPLC
The SRID assessment was conducted to measure the HA
content of the influenza vaccines relative to the reference
antigens. In addition, the SE-HPLC chromatogram area for the HA
content of the reference antigens and influenza vaccines was
measured. The SE-HPLC chromatogram area/HA (1 μg) was calculated
for the reference antigens and influenza vaccines, respectively.
The SE-HPLC chromatogram area was calculated and compared with that
of the SRID.
Results
SE-HPLC analysis: HA peak examination by
SE-HPLC
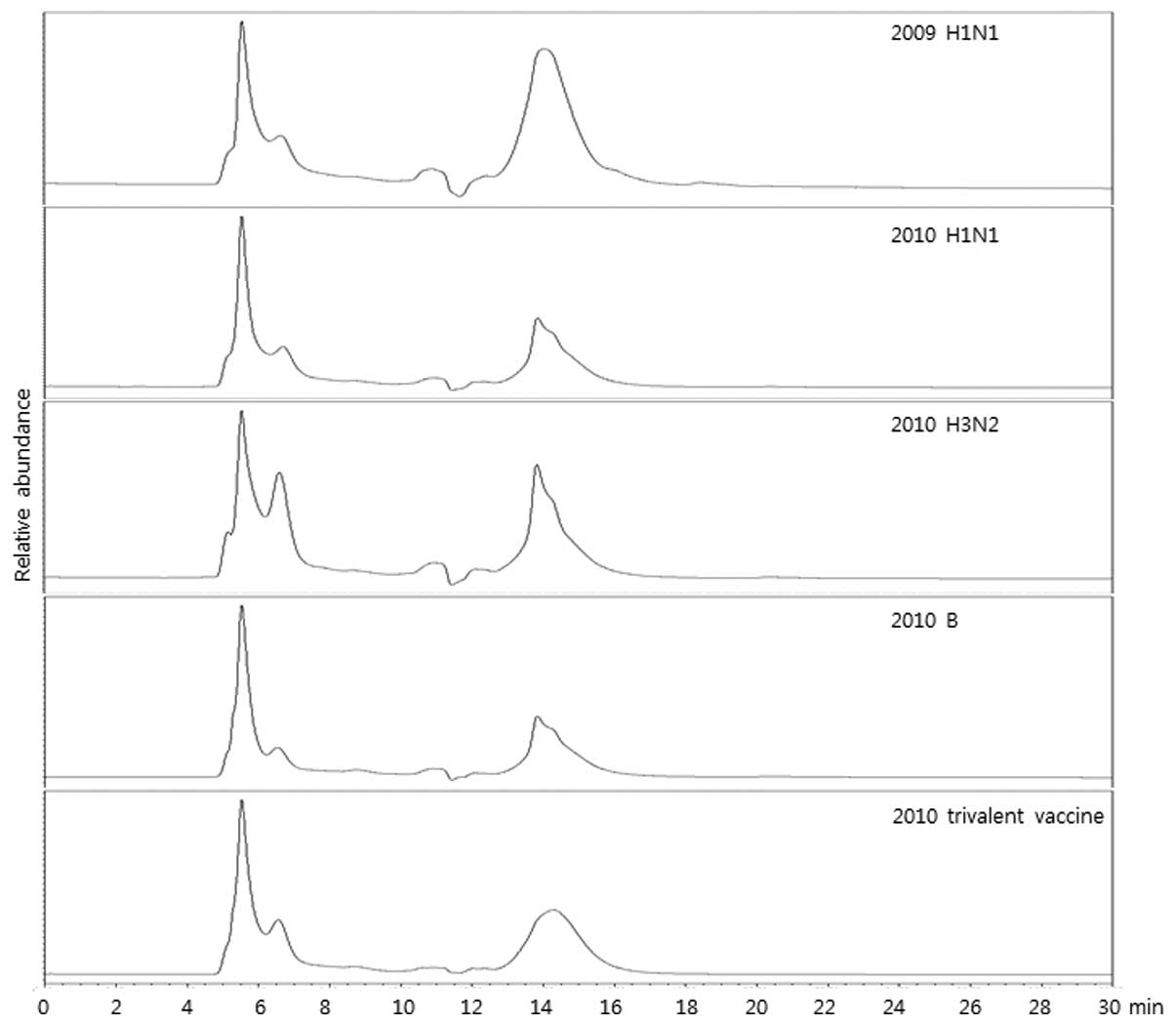
To compare the HA content, vaccine samples (2009
H1N1, 2010 H1N1, 2010 H3N2 and 2010 B monovalent vaccines and 2010
seasonal trivalent vaccines) were analyzed using SE-HPLC. The
result demonstrated that the patterns of chromatogram were similar
among the assessed vaccines, meaning that SE-HPLC analysis revealed
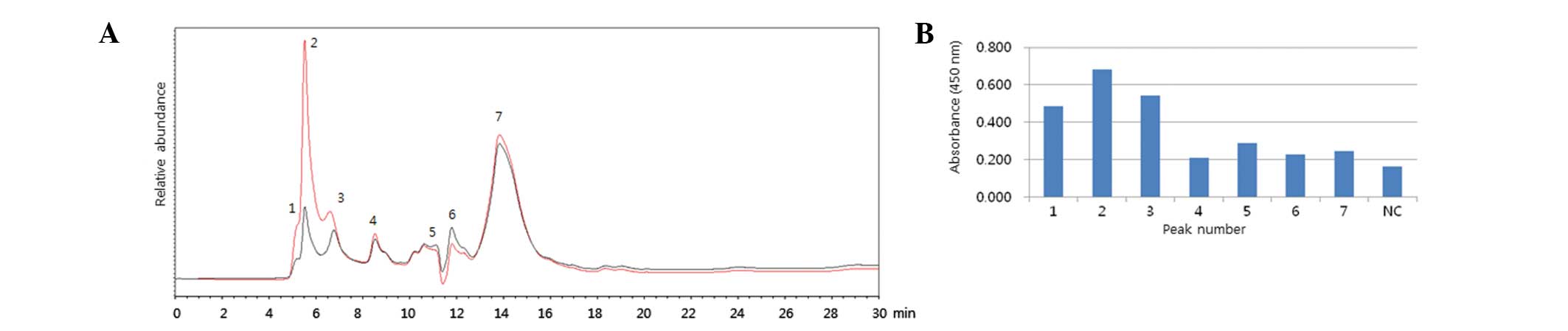
similar results although a different HA was applied (Fig. 1). To verify the specificity of
SE-HPLC, 2009 H1N1 reference antigen was immunoprecipitated with
antiserum for H1N1 and peaks for HA were compared prior to and
following immunoprecipitation (Fig.
2A). The results revealed that the peaks of SE-HPLC were
reduced following immunoprecipitation, suggesting that the peaks
from SE-HPLC were correlated with HA antigen. The fractions from
each peak were designated 1 to 7, harvested and subject to an ELISA
assay (Fig. 2B). When the
fractions were reacted with antiserum for H1N1, fractions between
1, 2 and 3 peaks exhibited higher absorbance than others. These
results suggested that major HA contents may be present in 1, 2 and
3 peaks. To confirm the specificity of SE-HPLC, an
immunoprecipitation and ELISA assay were also conducted with the
2009 H1N1 vaccine (Fig. 3A and B).
A total of 7 peaks were also designated and assessed and the
results were similar to those of Fig.
2.
Correlation of HA content with peak
area
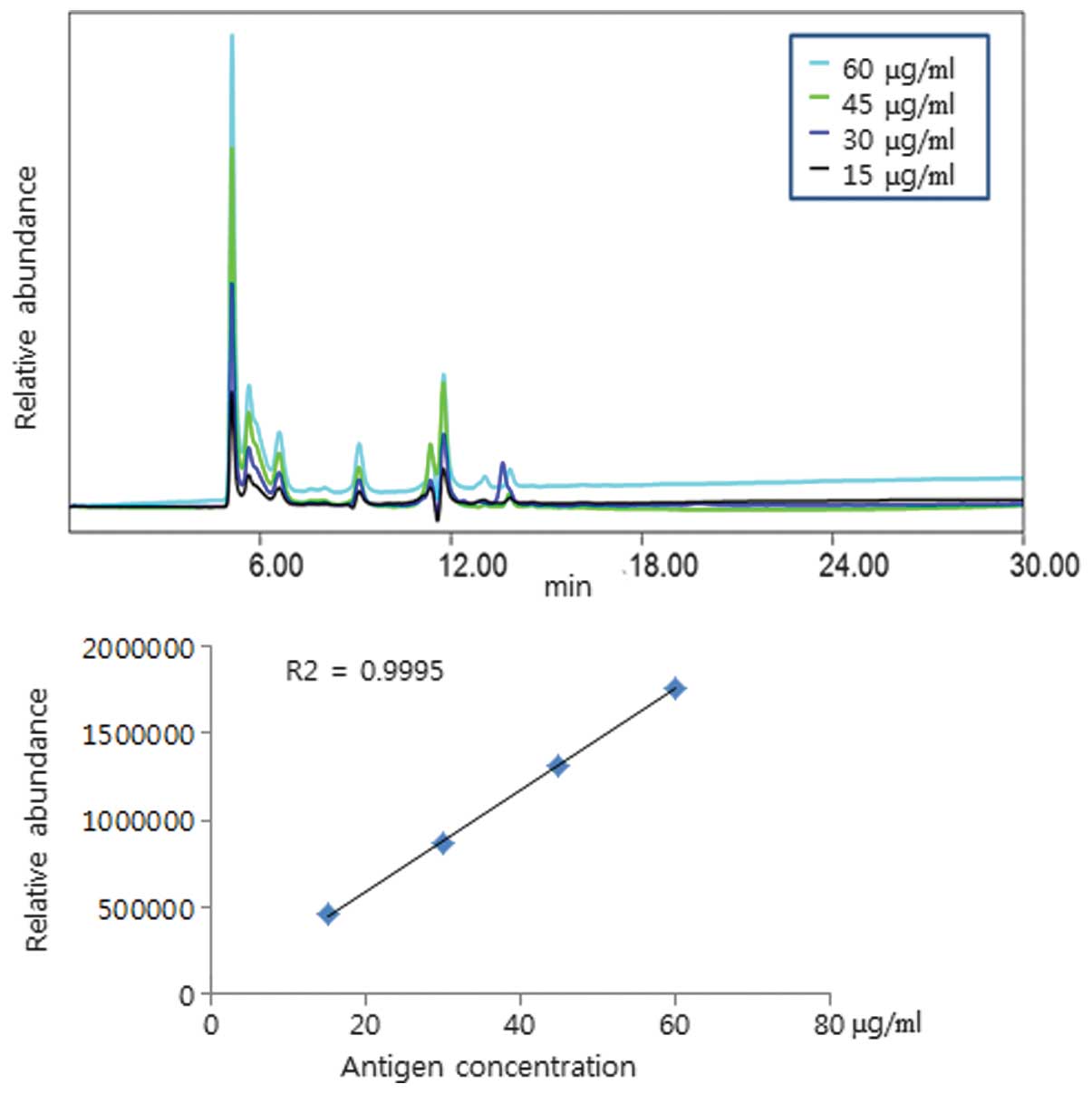
To examine the correlation of the HA content
according to the SE-HPLC chromatogram area, different
concentrations of 2009 H1N1 reference antigen were assessed. The
result revealed that the chromatogram area was enhanced
proportionately following the increased HA concentration from the
2009 H1N1 reference antigen (Fig.
4) and 2009 H1N1 vaccine (Fig.
5; R2>0.99). The present results revealed that
the quantitative read-out from the SE-HPLC is matched with the
content of HA.
Correlation of SRID with SE-HPLC analyses
in the measurement of HA content
To examine the accuracy and similarity of SE-HPLC
compared with SRID, 2009 H1N1 (Table
II) and 2010 H1N1 (Table
III) vaccines were assessed. Initially, 10 repeats of the 2009
H1N1 vaccine, together with 2009 H1N1 reference antigen were
examined and the content of HA, analyzed by SRID and SE-HPLC was
evaluated. To compare HA content from SRID or SE-HPLC, the peak
area from SE-HPLC was applied to the following equation: HA content
(μg/0.5 ml) = (vaccine SE-HPLC peak area/reference SE-HPLC peak
area) × reference HA content (μg) from SRID/average of normalized
HA peak area. The HA content of the 2009 H1N1 vaccines was measured
by SRID using the 2009 H1N1 reference antigen. In addition, the HA
peak area was measured by SE-HPLC using the same reference antigen
and then converted into the HA content using the above equation. In
the present results, the similarity of HA content analyzed by SRID
and SE-HPLC was 99.91% (SD, 5.94), meaning that evaluation of HA
content by SE-HPLC is as reliable as SRID in 99.91% of cases. The
HA content of the 2010 H1N1, 2010 H3N2 and 2010 B monovalent
vaccines was also measured by SE-HPLC and compared with those of
SRID using the 2009 H1N1 reference antigen. The result was similar
to that of the 2009 H1N1 vaccines with 100% (SD, 6.73)
similarity.
 | Table IIContent of HA in pandemic influenza
vaccine by SE-HPLC using the 2009 H1N1 reference antigen. |
Table II
Content of HA in pandemic influenza
vaccine by SE-HPLC using the 2009 H1N1 reference antigen.
| Reference and
vaccinesa | HA content from SRID
(μg/0.5 ml) | HA peak area from
SE-HPLC | HA peak
area/1μgb | Normalized HA peak
areac | HA content from
SE-HPLC (μg/0.5ml)d | Similarity
(%)e |
|---|
| 2009 H1N1
antigen | 15.00 | 831,671 | 55,445 | | | |
| Lot 1 | 12.05 | 728,850 | 60,485 | 1.09 | 12.29 | 101.95 |
| Lot 2 | 11.52 | 731,220 | 63,474 | 1.14 | 12.33 | 106.99 |
| Lot 3 | 12.72 | 725,524 | 57,038 | 1.03 | 12.23 | 96.14 |
| Lot 4 | 12.99 | 725,642 | 55,862 | 1.01 | 12.23 | 94.16 |
| Lot 5 | 13.07 | 723,723 | 55,373 | 1.00 | 12.20 | 93.34 |
| Lot 6 | 12.80 | 722,592 | 56,453 | 1.02 | 12.18 | 95.16 |
| Lot 7 | 10.87 | 721,421 | 66,368 | 1.20 | 12.16 | 111.87 |
| Lot 8 | 11.10 | 647,786 | 58,359 | 1.05 | 10.92 | 98.37 |
| Lot 9 | 10.85 | 657,194 | 60,571 | 1.09 | 11.08 | 102.10 |
| Lot 10 | 10.86 | 647,892 | 59,659 | 1.08 | 10.92 | 100.56 |
| Average/SD value | | | | 1.07/0.06 | | 99.91/5.94 |
 | Table IIIContent of HA in monovalent influenza
vaccines by SE-HPLC using 2009 H1N1 as a reference material. |
Table III
Content of HA in monovalent influenza
vaccines by SE-HPLC using 2009 H1N1 as a reference material.
| Reference and
vaccinesa | HA content from SRID
(μg/0.5 ml) | HA peak area from
SE-HPLC | HA peak area/1
μgb | Normalized HA peak
areac | HA content from
SE-HPLC (μg/0.5 ml)d | Similarity
(%)e |
|---|
| Reference
antigen | 15.00 | 650,164 | 43,344 | | | |
| 2010 H1N1 | 14.03 | 899,807 | 64,134 | 1.48 | 14.72 | 104.94 |
| 2010 H3N2 | 12.82 | 724,381 | 56,504 | 1.30 | 11.85 | 92.45 |
| 2010 B | 14.56 | 917,087 | 62,987 | 1.45 | 15.01 | 103.06 |
| Average (SD) | | | | 1.41 (0.09) | | 100.00(6.73) |
Discussion
The prevention of influenza through vaccination is
the most effective method to control an influenza pandemic
(14). This has been confirmed
once again during the 2009/2010 H1N1 influenza pandemic. In
addition, to maximize the preventive effect, vaccination should be
performed as soon as possible following the onset of the influenza
virus outbreak. For rapid vaccination, the shortening of the time
required for vaccine development, including isolation of the
pandemic virus, manufacturing of the vaccine virus with high yield,
measurement of antigen content and clinical assessment is required
(6,13).
At present, the internationally authorized
measurement of the antigen content of an influenza vaccine is using
SRID. This method measures HA content by comparing the areas of the
reference antigen with known HA content and the vaccine. Therefore,
for the measurement of the HA content, the reference antigen and
antibody are essentially required. It generally takes 2–3 months to
prepare the reference antigen and antibody, which is the most
significant obstacle in vaccine development.
The present study was conducted to develop a simple
method that measures the HA content without the reference antigen
and antibody. The present study revealed a relevant similarity in
data produced using SE-HPLC to that of SRID, meaning SE-HPLC may be
applied to any type of influenza virus in the situation of an
influenza pandemic. The results of SE-HPLC analysis on H1, H3 and B
type influenza vaccines revealed similar chromatogram patterns.
Furthermore, the retention time designating HA separation revealed
consistent results with the chromatogram. The SE-HPLC chromatogram
area was increased in proportion to HA concentration, which
demonstrated a significant correlation between the HA concentration
and peak area. Although the HA content, as examined using SE-HPLC
did not accurately represent HA antigenicity compared with that of
SRID, it exhibited a high similarity to that of SRID
(99.91–100.00%) with regards to HA concentration.
During the SE-HPLC process, protease treatment for
the specimens of the reference antigen and the vaccine was not
applied, which differs from the SRID method. As the protease step
was skipped, the results may not be as accurate as SRID. However,
an experimental protocol, which excludes protease treatment saves
time in the analysis of the HA content. Therefore, SE-HPLC is not a
method that replaces the SRID assay, but a supplementary assessment
to overcome delayed vaccine development, a disadvantage of the SRID
assay. If a clinical dose of HA content is prepared using SE-HPLC
prior to the development of the reference antigen and antibody for
a pandemic influenza virus, a shortening of the vaccination
development time against the pandemic influenza virus may be
achieved. A further study on the influenza pandemic virus type H5
is required for more accurate measurement.
Acknowledgements
The authors would like to thank Green Cross for
providing influenza vaccines and NIBSC for providing reference
materials. The present study was supported by a grant from the
Korean Food and Drug Administration (no. 10171KFDA307).
References
|
1
|
Schild GC, Wood JM and Newman RW: A
single-radial-immunodiffusion technique for the assay of influenza
haemagglutinin antigen. Proposals for an assay method for the
haemagglutinin content of influenza vaccines. Bull World Health
Organ. 52:223–231. 1975.PubMed/NCBI
|
|
2
|
Wood JM, Schild GC, Newman RW and
Seagroatt V: An improved single-radial-immunodiffusion technique
for the assay of influenza haemagglutinin antigen: application for
potency determinations of inactivated whole virus and subunit
vaccines. J Biol Stand. 5:237–247. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wood JM, Mumford J, Schild GC, Webster RG
and Nicholson KG: Single-radial-immunodiffusion potency tests of
inactivated influenza vaccines for use in man and animals. Dev Biol
Stand. 64:169–177. 1986.PubMed/NCBI
|
|
4
|
Williams MS: Single-radial-immunodiffusion
as an in vitro potency assay for human inactivated viral vaccines.
Vet Microbiol. 37:253–262. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu J, Liu DC and Wang TR: Clinical
application of the single radial immunodiffusion (SRID) technic to
detect antitubercle bacillus antibody. Zhonghua Jie He He Hu Xi Za
Zhi. 16:270–271. 318–279. 1993.(In Chinese).
|
|
6
|
Gerdil C: The annual production cycle for
influenza vaccine. Vaccine. 21:1776–1779. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kapteyn JC, Saidi MD, Dijkstra R, et al:
Haemagglutinin quantification and identification of influenza
A&B strains propagated in PER. C6 cells: a novel RP-HPLC
method. Vaccine. 24:3137–3144. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kapteyn JC, Porre AM, de Rond EJ, et al:
HPLC-based quantification of haemagglutinin in the production of
egg- and MDCK cell-derived influenza virus seasonal and pandemic
vaccines. Vaccine. 27:1468–1477. 2009. View Article : Google Scholar
|
|
9
|
Garcia-Canas V, Lorbetskie B, Bertrand D,
Cyr TD and Girard M: Selective and quantitative detection of
influenza virus proteins in commercial vaccines using
two-dimensional high-performance liquid chromatography and
fluorescence detection. Anal Chem. 79:3164–3172. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Williams TL, Luna L, Guo Z, et al:
Quantification of influenza virus hemagglutinins in complex
mixtures using isotope dilution tandem mass spectrometry. Vaccine.
26:2510–2520. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peyre M, Fusheng G, Desvaux S and Roger F:
Avian influenza vaccines: a practical review in relation to their
application in the field with a focus on the Asian experience.
Epidemiol Infect. 137:1–21. 2009. View Article : Google Scholar
|
|
12
|
Li C, Shao M, Cui X, et al: Application of
deglycosylation and electrophoresis to the quantification of
influenza viral hemagglutinins facilitating the production of 2009
pandemic influenza (H1N1) vaccines at multiple manufacturing sites
in China. Biologicals. 38:284–289. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wood JM and Levandowski RA: The influenza
vaccine licensing process. Vaccine. 21:1786–1788. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ferguson NM, Cummings DA, Fraser C, Cajka
JC, Cooley PC and Burke DS: Strategies for mitigating an influenza
pandemic. Nature. 442:448–452. 2006. View Article : Google Scholar : PubMed/NCBI
|