Spandidos Publications style
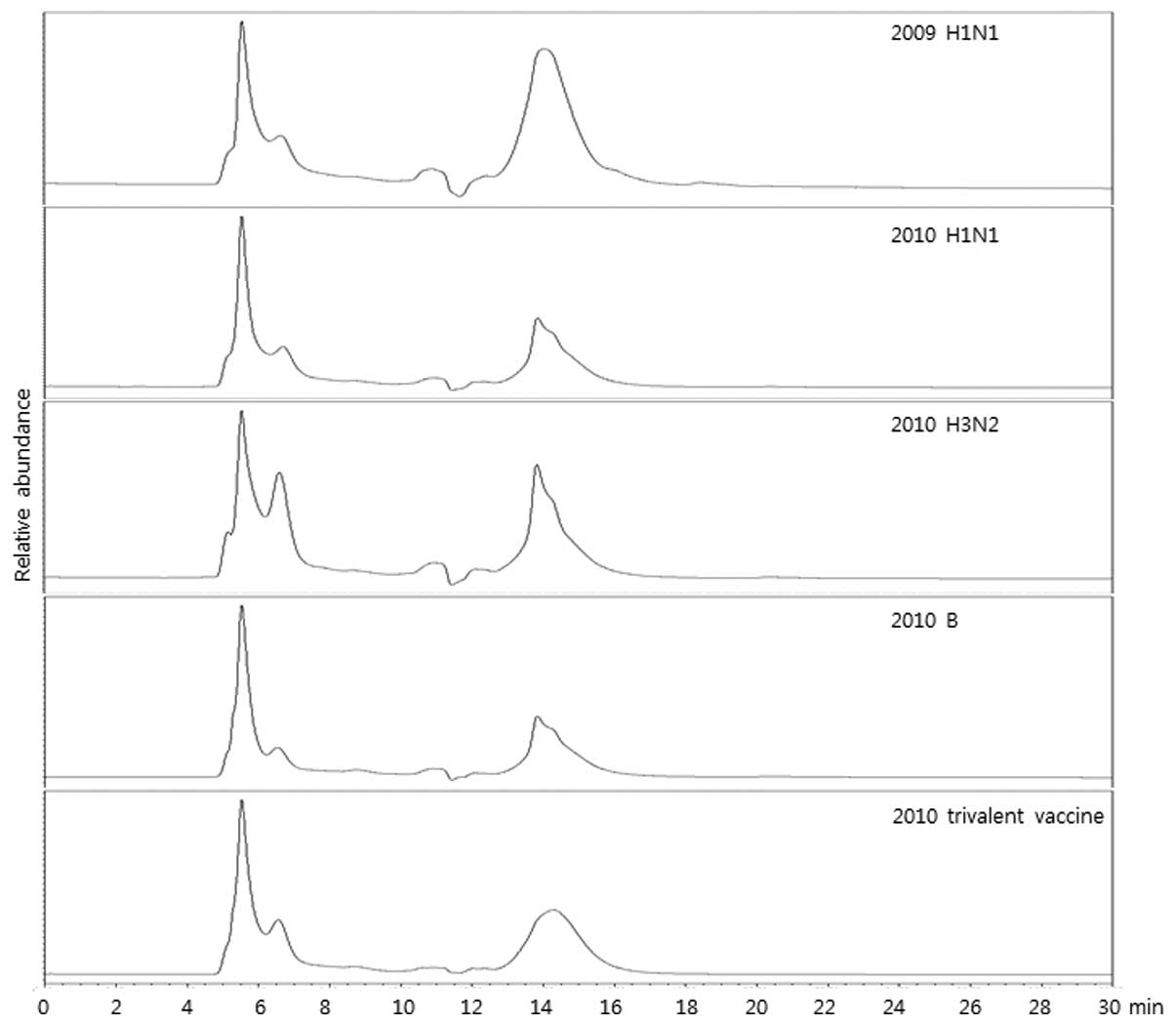
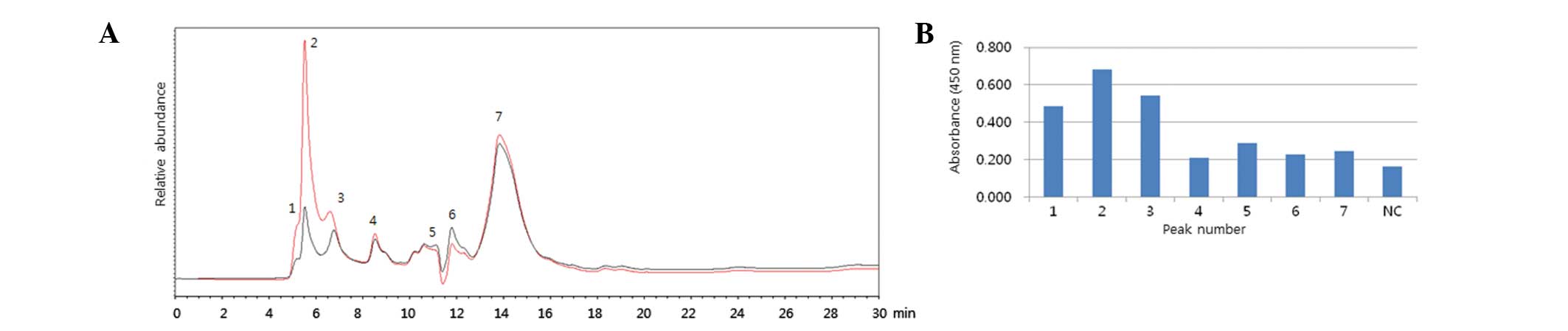
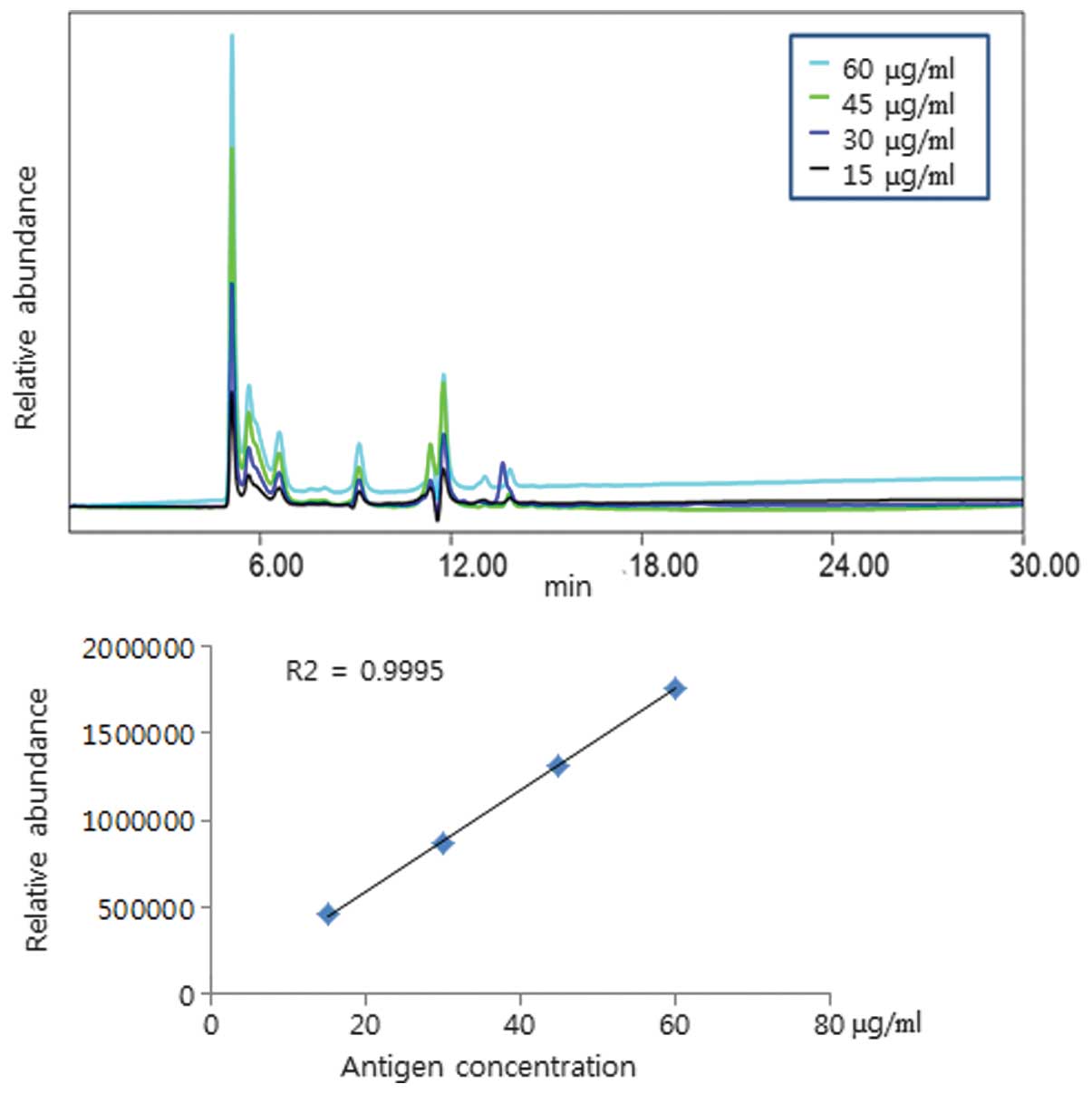
Roh HS, Song HM, Yun BR, Kang HK, Choi KS, Park YJ, Kim DS, Kim SH, Mo IP, An BS, An BS, et al: A hemagglutinin quantification method for development of an influenza pandemic vaccine using size exclusion high performance liquid chromatography. Mol Med Rep 11: 2819-2824, 2015.
APA
Roh, H.S., Song, H.M., Yun, B.R., Kang, H.K., Choi, K.S., Park, Y.J. ... Ahn, C.Y. (2015). A hemagglutinin quantification method for development of an influenza pandemic vaccine using size exclusion high performance liquid chromatography. Molecular Medicine Reports, 11, 2819-2824. https://doi.org/10.3892/mmr.2014.3049
MLA
Roh, H. S., Song, H. M., Yun, B. R., Kang, H. K., Choi, K. S., Park, Y. J., Kim, D. S., Kim, S. H., Mo, I. P., An, B., Ahn, C. Y."A hemagglutinin quantification method for development of an influenza pandemic vaccine using size exclusion high performance liquid chromatography". Molecular Medicine Reports 11.4 (2015): 2819-2824.
Chicago
Roh, H. S., Song, H. M., Yun, B. R., Kang, H. K., Choi, K. S., Park, Y. J., Kim, D. S., Kim, S. H., Mo, I. P., An, B., Ahn, C. Y."A hemagglutinin quantification method for development of an influenza pandemic vaccine using size exclusion high performance liquid chromatography". Molecular Medicine Reports 11, no. 4 (2015): 2819-2824. https://doi.org/10.3892/mmr.2014.3049