Introduction
Nasopharyngeal carcinoma (NPC) is a squamous-cell
carcinoma derived from the epithelial lining of the nasopharynx
(1). Its incidence is rare in the
United States and Western Europe, however it is common in endemic
areas, including South China, North Africa and Alaska (2). South China has the highest incidence
rate of NPC, with 25–30 per 100,000 people affected annually, and
it is particularly common among people of Cantonese ancestry
(3). Since NPC is sensitive to
irradiation, radiotherapy is the main therapeutic strategy used to
treat NPC. With the improvement of radiotherapy techniques and
chemotherapy, the locoregional control of NPC has improved
(4), with a cure rate of ~70%
(5). However, overall survival
remains low. Therefore, the development of multidisciplinary
therapeutic strategies that improve locoregional control and
eradicate micrometastases is required.
Telomeres are repetitive nucleotides that reside at
the ends of human chromosomes, and telomere length is mainly
maintained through the activity of telomerase (6). Telomerase is activated in 80–95% of
cancers, and is present in very low to undetectable levels in
normal cells (7). In normal human
cells telomeres are shortened with cell division, however they are
continuously elongated in tumor cells. Due to the pivotal role of
telomerase in cancer cells, it is considered to be an attractive
target for anticancer therapy. Inhibition of telomerase may lead to
decreased telomere length, resulting in cell apoptosis in
telomerase-positive tumors (8).
Potential telomerase inhibitors, including small molecules,
antisense oligonucleotides and ribozymes, have previously been
developed (9). Preclinical studies
have demonstrated that antisense oligonucleotides targeting human
telomerase RNA (hTR ASODN) are promising agents for the treatment
of various human malignancies (10). In addition, the therapeutic effects
of chemotherapy (11,12) and radiation (13) were shown to be enhanced when
combined with hTR ASODN. However, the influence of hTR ASODN on the
anti-tumor effects of radiation in NPC remain to be elucidated.
The present study aimed to investigate the influence
of hTR ASODN on the proliferation and radiosensitivity of NPC
cells, and to further explore the underlying mechanisms.
Materials and methods
Cell culture
CNE-2 human undifferentiated NPC cells were cultured
in RPMI-1640 medium (Gibco Life Technologies, Carlsbad, CA, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco Life
Technologies) at 37°C, in a humidified incubator containing 5%
CO2. Cells at the logarithmic growth phase were used in
the following experiments.
Cell transfection
hTR ASODN was synthesized by Guangzhou Geneseed
Biotechnology Co., Ltd. (Guangzhou, China), the sequence of which
was: 5′-TAGGGTTAGACAA-3′. The CNE-2 human NPC cells were
transiently transfected with hTR ASODN using
Lipofectamine® 2000 Transfection reagent (Invitrogen
Life Technologies, Carlsbad, CA, USA). The CNE-2 cells
(5×106 cells/ml) were transfected with 0.5, 1.0, 1.5,
2.0, 2.5 or 3.0 μmol DNA/106 cells
(Lipofectamine®:DNA, 3:1). The transfection efficiency
was evaluated by flow cytometry 24 h post-transfection. Cells
transfected with Lipofectamine® 2000 only were used as
the control group.
Flow-fluorescent in situ hybridization
(FISH) assay
The CNE-2 cells were transfected with control or hTR
ASODN (2.5 μmol) for 12 h. Irradiation was then conducted at room
temperature using an RS 2000 X-ray Biological Irradiator (Rad
Source Technologies, Inc., Suwanee, GA, USA), at a dose rate of 1
Gy/min through a 0.2 mm copper filter. The cells were cultured for
a further 36 h in RPMI-1640 medium without FBS at 37°C in a
humidified incubator containing 5% CO2. A total of
1×106 cells from each sample were washed in 2 ml
phosphate-buffered saline (PBS; 135 mM NaCl, 1.3 mM KCl, 3.2 mM
Na2HPO4, 0.5 mM KH2PO4)
supplemented with 0.1% bovine serum albumin (BSA; Guangzhou
Chemistry Reagent Factory, Guangzhou, China). Each sample was
divided into two replicate tubes; one pellet was resuspended in 80
μl hybridization buffer (10 mM NaHPO4, pH 7.4; 10 mM
NaCl; 20 mM Tris, pH 7.5; 70% formamide), and another in
hybridization buffer without fluorescein isothiocyanate
(FITC)-labeled telomeric peptide nucleic acid probe, which was used
as a negative control. The samples were then denatured for 15 min
at 87°C with continuous agitation and hybridized for 2 h in the
dark at room temperature. The cells were subsequently washed twice
in washing solution (70% deionized formamide, 10 mM Tris, 0.1% BSA
and 0.1% Tween® 20 in distilled H2O; pH 7.2).
The cells were then centrifuged at 956 × g for 10 min, resuspended
in 500 μl of propidium iodide (PI) solution (Kaiji Biotechnology,
Nanjing, China), incubated for 2 h at room temperature and stored
at 4°C, prior to flow cytometric analysis.
Cell proliferation assay
Cell viability following transfection with hTR ASODN
was measured by an MTT assay (Sigma-Aldrich, St. Louis, MO, USA),
as described previously (14).
Cells in early log phase were trypsinized with 0.25% trypsin (Gibco
Life Technologies, Rockville, MD, USA) and seeded in 96-well plates
(2×103 cells/well). Following a 36 h incubation in
RPMI-1640 medium with 10% fetal bovine serum at 37°C, the medium
was refreshed. Cell density was measured using MTT, according to
the manufacturer’s instructions. The absorbance of the converted
dye was measured at a wavelength of 450 nm using a plate reader
(Multiskan MK3; Thermo Labsystems, Waltham, MA, USA), and the
absorbance was considered directly proportional to cell viability.
This experiment was repeated ≥three times.
Colony formation assay
The clonogenic potential of the cells treated with
hTR ASODN and/or ionizing radiation was assessed using a colony
formation assay. Briefly, the cells were transfected with the
control or hTR ASODN (2.5 μmol) for 12 h. Cells were trypsinized
and plated in six-well plates at 200, 500, 3,000, 5,000, and 10,000
cells per well and cultured overnight to allow for cell attachment.
Irradiation was then performed at room temperature using an RS 2000
X-ray Biological Irradiator, at doses corresponding to 0, 2, 4, 6,
and 8 Gy, through a 0.2 mm copper filter. The plates were then
incubated for 10 days at 37°C, and the growth medium was replaced
every three days. The plates were then stained with 0.1% crystal
violet (Sigma-Aldrich), and colonies containing ≥50 cells were
counted under an inverted microscope (IMT-2; Olympus Corp., Tokyo,
Japan). The cell surviving fraction was determined relative to the
survival of non-irradiated cells transfected with
Lipofectamine® 2000 only.
Apoptosis assay
The percentage of apoptotic cells was determined by
flow cytometry using the Annexin V-FITC Apoptosis Detection kit
(Kaiji Biotechnology Co., Nanjing, China), as described previously
(14). Briefly, the cells were
collected, washed three times with PBS and fixed with 1 ml ethanol
(70%) for 2 h at 4°C. The cells were washed again with PBS, and the
supernatants were removed following centrifugation at 956 × g for
10 min. The cells were then treated with 500 μl Annexin binding
buffer, 5 μl Annexin V-FITC and 5 μl PI, and incubated at room
temperature in the dark for 15 min. The rate of cell apoptosis was
determined by flow cytometry (BD Biosciences, Franklin Lakes, NJ,
USA).
Western blot analysis
The CNE-2 cells were transfected with the control or
hTR ASODN (2.5 μmol) and/or radiation at a total dose of 6 Gy 12 h
post-transfection. At 36 h post-transfection, whole cell lysates
were collected and protein concentrations were determined using the
Bio-Rad Protein Assay kit (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). Whole cell extracts were separated using 14% SDS-PAGE and
transferred onto PVDF membranes (Bio-Rad Laboratories, Inc.).
Following incubation with 5% nonfat milk in TBST (10 mM Tris, pH
8.0, 150 mM NaCl, 0.5% Tween 20) for 1 h, the membranes were washed
once with TBST and incubated with a polyclonal rabbit anti-Caspase
9 antibody (9502; 1:1,000; Cell signaling technology, Inc.,
Danvers, MA, USA) at 4°C overnight. Membranes were then washed
three times with TBST for 10 min and incubated with horseradish
peroxidase-conjugated anti-rabbit antibodies (1:2,000; A0208;
Beyotime Institute of Biotechnology, Shanghai, China) at 4°C for 2
h. Blots were washed with TBST three times and visualized using
Super Enhanced Chemiluminescence Plus Detection Reagent (Applygen
Technologies, Inc., Beijing, China) according manufacturer’s
instructions. The same membrane was stripped and re-blotted with an
anti-β-actin antibody (Sigma-Aldrich) for normalization.
Statistical analysis
Statistical analyses were performed using SPSS
version 17.0 software (SPSS, Inc., Chicago, IL, USA). The data are
expressed as the mean ± standard deviation and statistical
significance was analyzed by analysis of variance. P<0.05 was
considered to indicate a statistically significant difference.
Results
Transient transfection of hTR ASODN into
CNE-2 cells
To evaluate the efficacy of hTR ASODN transfection
by Lipofectamine® 2000, transfections using various
concentrations of DNA were performed. The CNE-2 cells
(5×106 cells/ml) were transfected with 0.5, 1.0, 1.5,
2.0, 2.5 or 3.0 μmol hTR ASODN (Lipofectamine®:DNA,
3:1). The transfection efficiency of the various concentrations of
DNA were 2.6±0.43, 5.2±0.79, 35.4±0.51, 69±0.57, 82.6±0.42 and
69.2±0.49% when transfected with 0.5, 1.0, 1.5, 2.0, 2.5 and 3.0
μmol DNA, respectively. The best transfection efficiency was
observed with a DNA concentration of 2.5 μmol DNA/5×106
cells, 48 h post-transfection (Fig.
1).
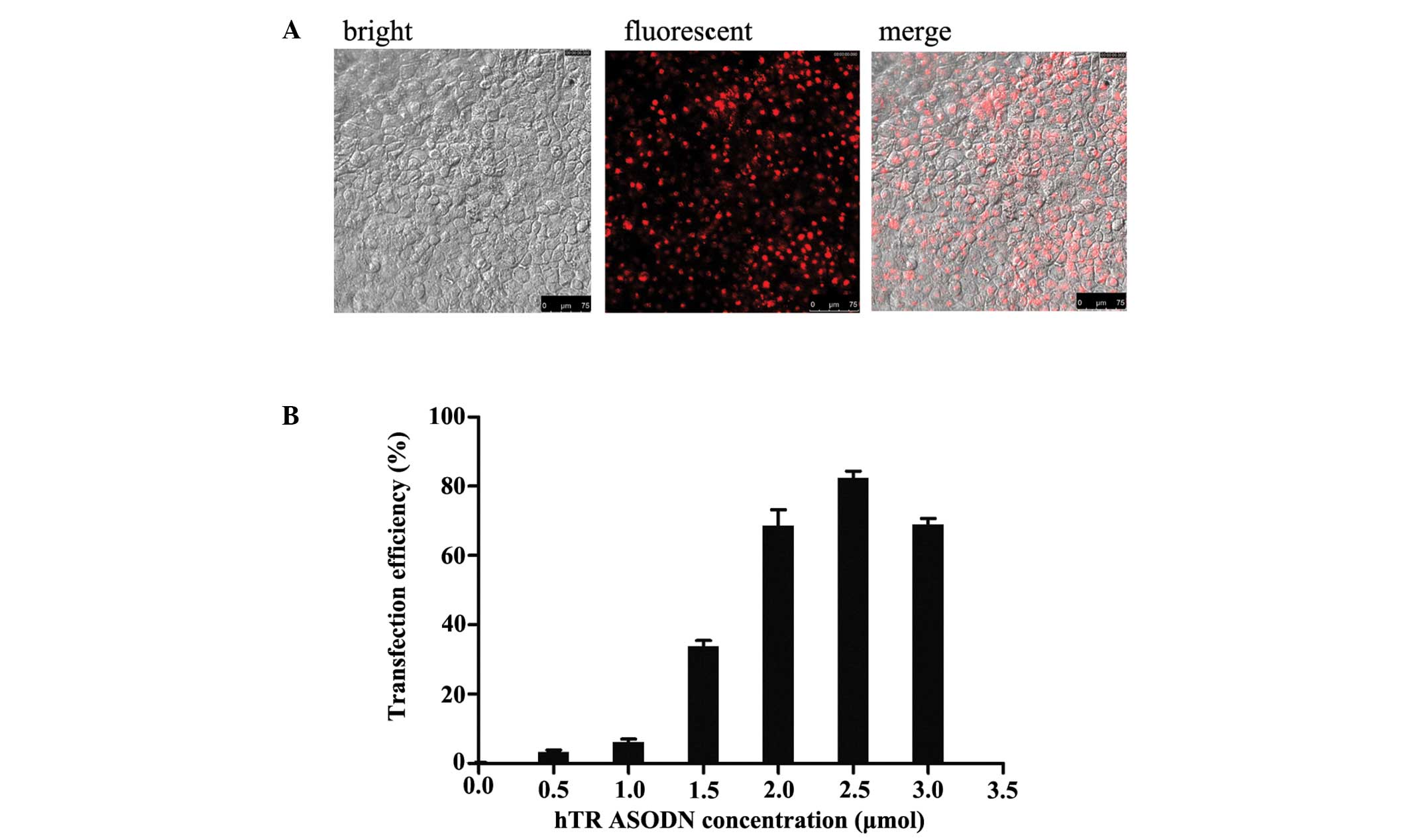 | Figure 1Transfection of antisense
oligonucleotides targeting human telomerase RNA (hTR ASODN) into
CNE-2 human nasopharyngeal carcinoma cells. CNE-2 cells were
transfected with hTR ASODN using Lipofectamine® 2000
reagent at various concentrations, as indicated. (A) At 12 h
post-transfection, transfection efficacy was evaluated by detecting
the fluorescent intensity using fluorescence correlation
microscopy. The images represent the hTR ASODN fluorescence from
the transfected cells. (B) Transfection efficiency of the various
concentrations of DNA were 2.6±0.43%, 5.2±0.79%, 35.4±0.51%,
69±0.57%, 82.6±0.42% and 69.2±0.49%, when transfected with 0.5,
1.0, 1.5, 2.0, 2.5 and 3.0 μmol DNA, respectively. The best
transfection efficiency was observed with a DNA concentration of
2.5 μmol DNA/5×106 cells, 48 h post-transfection. |
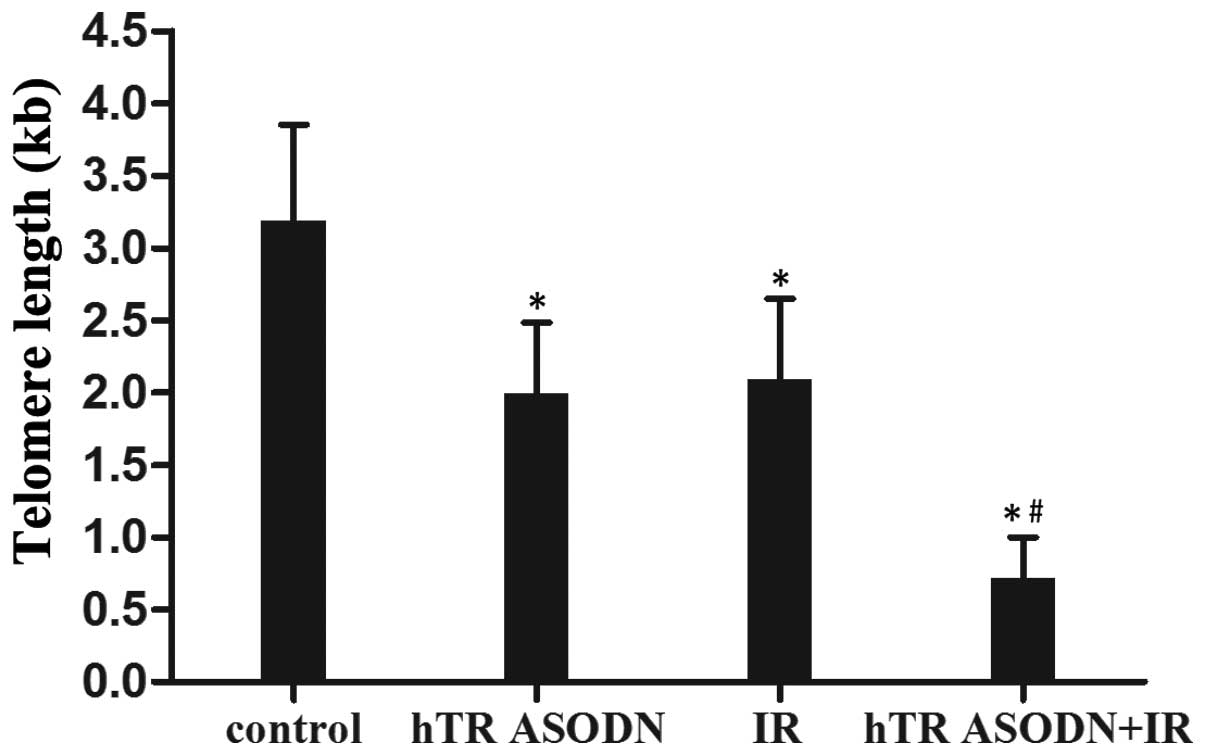
Transfection with hTR ASODN decreases
telomere length in CNE-2 cells
Flow-FISH techniques were used to determine the
effects of hTR ASODN and radiation on telomere length in CNE-2
cells. The telomere length of the untreated control cells and those
treated with irradiation, hTR ASODN and hTR ASODN combined with
irradiation were 3193±659 bases, 2093±555 bases, 1993±491 bases and
717±284 bases, respectively. In addition, combined treatment with
hTR ASODN and irradiation led to an increased rate of apoptosis of
the CNE-2 cells (Fig. 2).
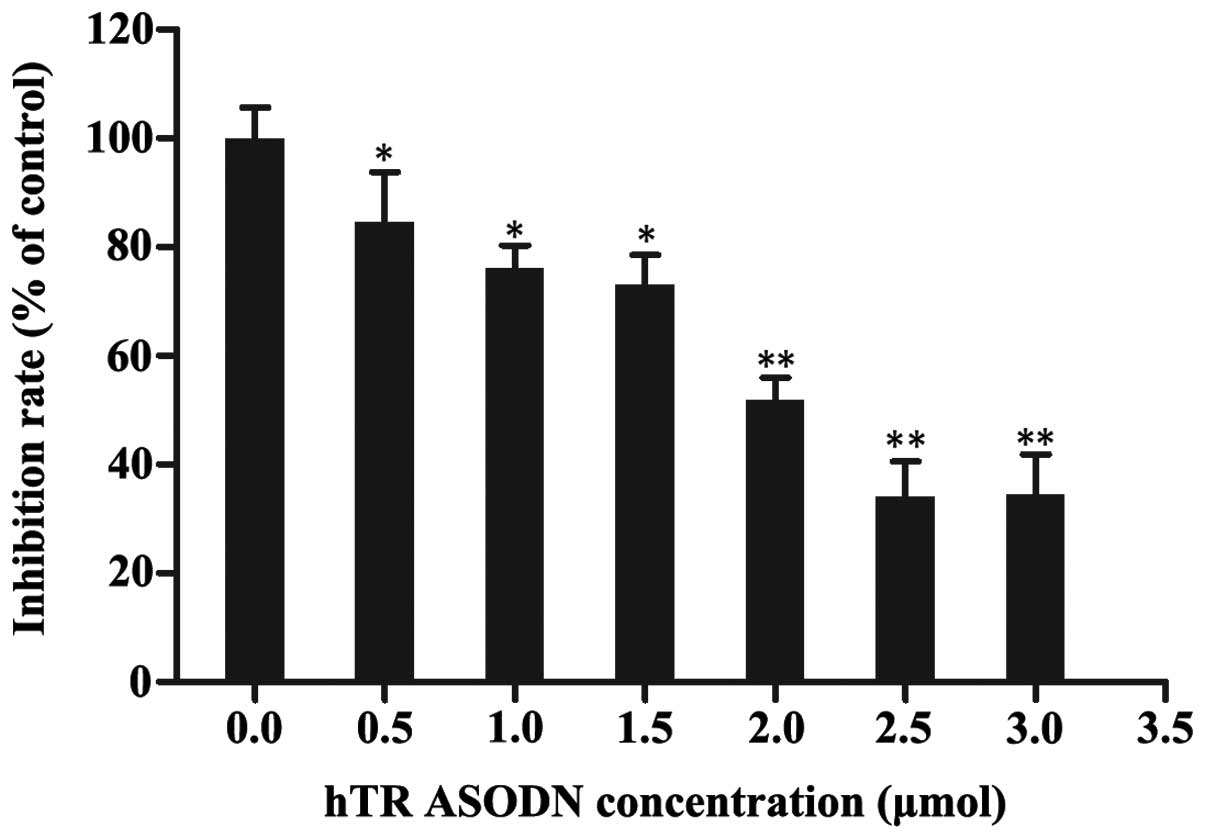
hTR ASODN inhibits the growth of CNE-2
cells
To determine the inhibitory effects of hTR ASODN on
human NPC cells, the growth of CNE-2 cells was measured using an
MTT assay. The CNE-2 cells were transfected with 0.5, 1.0, 1.5,
2.0, 2.5 or 3.0 μmol DNA/5×106 cells
(Lipofectamine®:DNA, 3:1). The growth of CNE-2 cells was
markedly inhibited 48 h post-transfection, as compared with the
control cells. The growth inhibitory rate of the cells transfected
with hTR ASODN (2.5 μmol) was 73±0.69% (Fig. 3). These results suggest that hTR
ASODN exhibits potential cytotoxic activity against human NPC
cells.
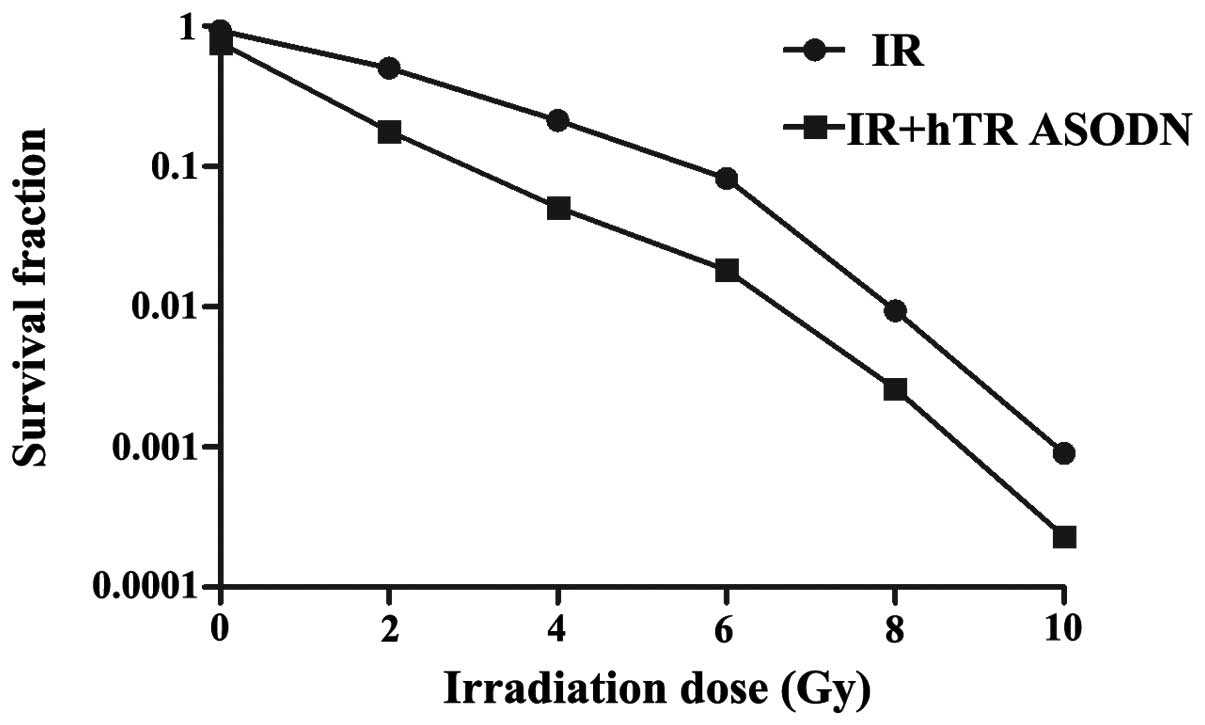
hTR ASODN enhances the cytotoxic effects
of X-ray irradiation on CNE-2 cells
To determine the effects of hTR ASODN on
radiosensitization of CNE-2 cells, a colony forming assay was
performed on the cells either treated with irradiation, or
transfected with hTR ASODN (2.5 μmol) followed by irradiation. The
cells transfected with hTR ASODN followed by radiation exposure
exhibited a greater inhibition of colony formation, as compared
with the control cells (Fig. 4,
P<0.01). Further analysis of clonogenic survival indicated that
the sensitization enhancement ratio (SER) was 1.479. These results
suggest that hTR ASODN may be capable of sensitizing NPC cells to
the cytotoxic effects of radiation treatment.
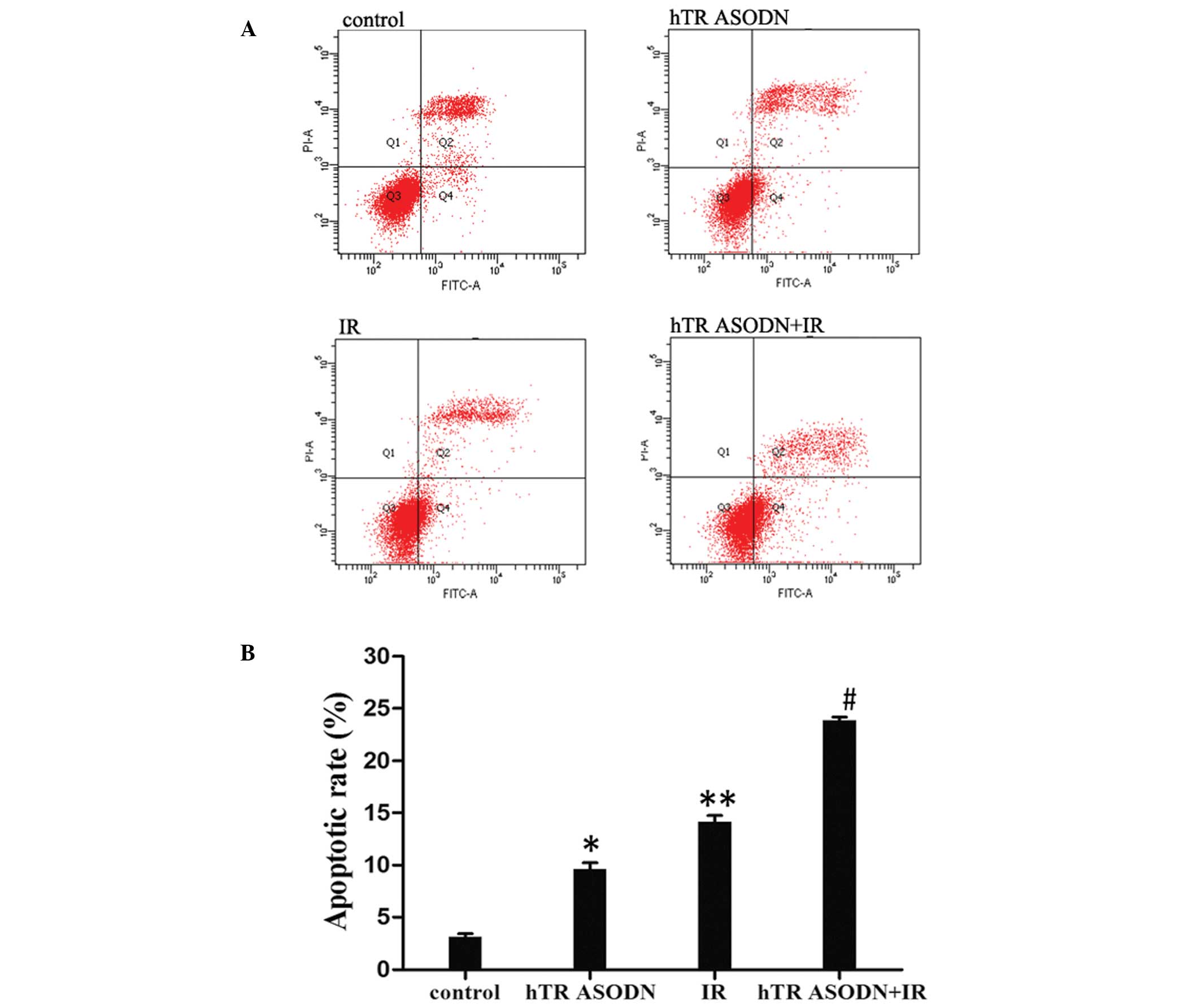
hTR ASODN enhances the pro-apoptotic
effects of X-ray irradiation in CNE-2 cells
To investigate the underlying mechanisms of the
inhibition of colony survival following transfection of the CNE-2
cells with hTR ASODN (2.5 μmol) and radiation (6 Gy), the cells
were analyzed by flow cytometry. The rate of apoptosis was
significantly increased by transfection with hTR ASODN or
irradiation alone, as compared with the control group. Combined
treatment of hTR ASODN with irradiation significantly increased the
rate of cell apoptosis, as compared with either hTR ASODN or
irradiation treatment alone (Fig.
5A). The apoptotic rate of the untreated controls and the cells
treated with irradiation, hTR ASODN and hTR ASODN combined with
irradiation were 3.2±0.6, 9±2.2, 14.2±2.1 and 23.8±1.87%,
respectively. Combined treatment of hTR ASODN with irradiation led
to increased cell apoptosis of CNE-2 cells (Fig. 5B, P<0.05). In addition, the
results of a western blot analysis suggested that combined
treatment of hTR ASODN with irradiation led to increased protein
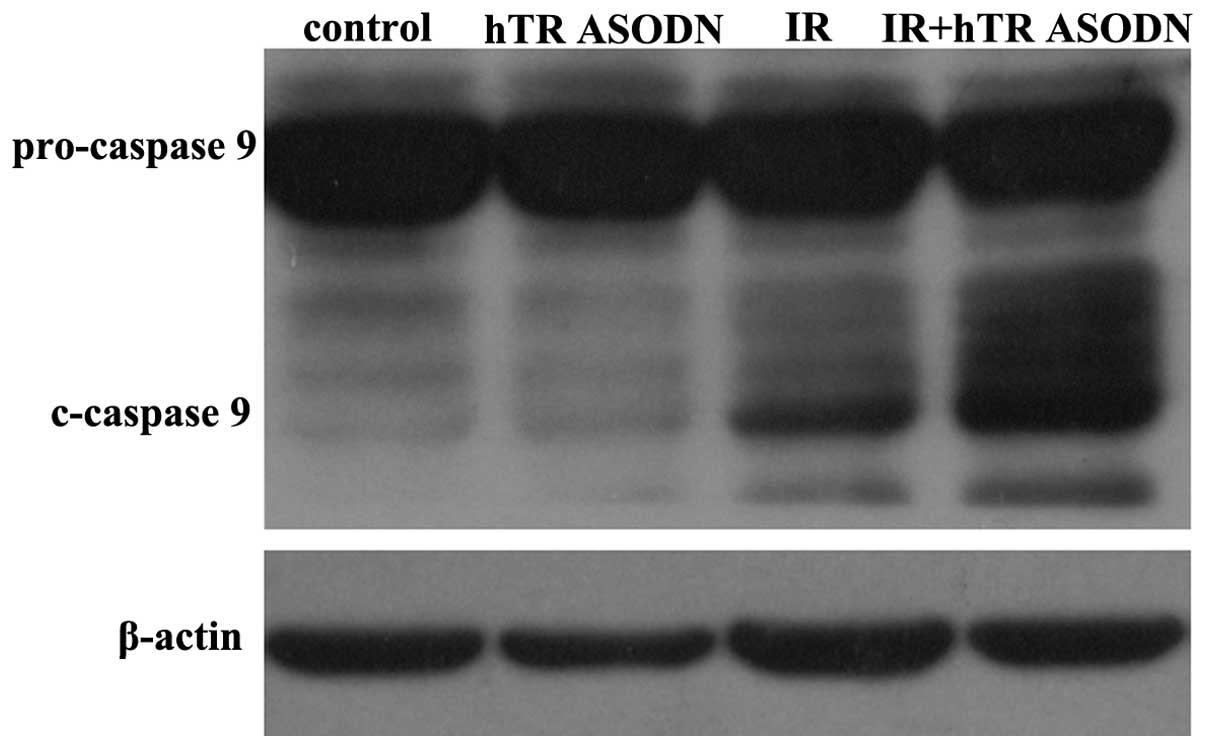
expression levels of cleaved caspase 9 in the CNE-2 cells, thus
indicating an increased rate of cell apoptosis (Fig. 6).
Discussion
The present study explored the effects of hTR ASODN
on the CNE-2 NPC cell line. Transfection with hTR ASODN
significantly reduced the telomere length, inhibited the
proliferation of NPC cells and enhanced the anti-tumor efficacy of
radiation by inducing cell apoptosis. This is the first study, to
the best of our knowledge, to determine the effects of hTR ASODN in
combination with radiation on NPC.
The results of the present study demonstrated that
hTR ASODN was efficiently transfected into CNE-2 cells using
Lipofectamine® 2000. The transfection efficiency reached
82.6% when the cells were transfected with 2.5 μmol
DNA/5×106 cells. Furthermore, transfection with hTR
ASODN significantly reduced the growth of the CNE-2 cells. Human
telomerase consists of the hTR and the human reverse transcriptase
catalytic subunit (hTERT) (15).
Since telomerase activity is present in ~90% of human cancer cells,
but not in the majority of normal human somatic cells, telomerase
inhibition is considered to be a potent molecular target in cancer
therapeutics (16). A previous
study detected frequently increased levels of telomerase activity
in NPC, as compared with normal nasopharyngeal tissue (17). Furthermore, previous studies have
observed tumor inhibitory effects by targeting telomerase. Wang
et al (18) demonstrated
that short hairpin RNA targeting hTERT inhibited cell viability in
NPC cells. In addition, ASODN targeting telomerase were also shown
to inhibit the growth of NPC cells (19). Therefore, inhibition of telomerase
activity by hTR ASODN may be a potential therapeutic strategy in
NPC.
Since radiotherapy is currently the main treatment
for NPC, the present study investigated the combined effects of
radiation and hTR ASODN on CNE-2 cells. The results demonstrated
that transfection with hTR ASODN significantly enhanced the
radiosensitivity of CNE-2 cells, with a SER of 1.479. These results
were concordant with the findings of previous studies. The
shortening of telomeres induced by GRN163L, an oligonucleotide
targeting the RNA component of telomerase, was previously shown to
significantly enhance the effects of radiation in breast cancer
cells (13). Treatment with
GRN163L in combination with radiation and temozolomide also
exhibited a marked effect on cell survival, and activated the DNA
damage response pathway (20).
Notably, a previous study demonstrated that high doses of radiation
(2, 4 and 8 Gy) resulted in decreased telomerase activity (down to
30% of untreated controls), which subsequently resulted in
increased cell death, thus suggesting that inhibition of telomerase
activity by high doses of radiation may have a role in
radiation-induced cell death (21). The present study determined the
rate of apoptosis of the untreated cells and those treated with
irradiation, hTR ASODN and hTR ASODN combined with irradiation, and
the rates were 3.2±0.6, 9±2.2, 14.2±2.1, 23.8±1.87%, respectively.
These results suggest that transfection with hTR ASODN combined
with irradiation significantly increased the rate of cell
apoptosis, as compared with either hTR ASODN or irradiation
treatment alone. In addition, the results of a western blot
analysis demonstrated that transfection with hTR ASODN combined
with irradiation significantly increased the protein expression
levels of cleaved caspase 9. Therefore, it may be hypothesized that
hTR ASODN combined with radiation may induce cell apoptosis by
synergistically reducing telomerase activity.
In conclusion, the present study demonstrated that
hTR ASODN could inhibit the proliferation of NPC cells and enhance
the anti-tumor effects of radiation by inducing cell apoptosis.
These data indicate that hTR ASODN may be a potential adjuvant
agent for the treatment of NPC in combination with radiation
therapy, and this finding is of translational importance.
Acknowledgements
The present study was supported by the National
Nature Science Foundation of China (grant no. 81201736), the
Science and Technology Planning Project of Guangdong Province,
China (grant nos. KZ 0710, KZ 1021 and 2007B031516001) and the
Science and Technology Innovation Project of Guangdong Medical
College, China (grant no. TD1124).
References
|
1
|
Wei WI and Sham JS: Nasopharyngeal
carcinoma. Lancet. 365:2041–2054. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu MC and Yuan JM: Epidemiology of
nasopharyngeal carcinoma. Semin Cancer Biol. 12:421–429. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feng BJ, Huang W, Shugart YY, et al:
Genome-wide scan for familial nasopharyngeal carcinoma reveals
evidence of linkage to chromosome 4. Nat Genet. 31:395–399.
2002.PubMed/NCBI
|
|
4
|
Lee N, Xia P, Quivey JM, et al:
Intensity-modulated radiotherapy in the treatment of nasopharyngeal
carcinoma: an update of the UCSF experience. Int J Radiat Oncol
Biol Phys. 53:12–22. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang GD, Huang TJ, Peng LX, et al:
Epstein-Barr Virus_Encoded LMP1 upregulates microRNA-21 to promote
the resistance of nasopharyngeal carcinoma cells to
cisplatin-induced Apoptosis by suppressing PDCD4 and Fas-L. PLoS
One. 8:e783552013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ruden M and Puri N: Novel anticancer
therapeutics targeting telomerase. Cancer Treat Rev. 39:444–456.
2013. View Article : Google Scholar
|
|
7
|
Ouellette MM, Wright WE and Shay JW:
Targeting telomerase-expressing cancer cells. J Cell Mol Med.
15:1433–1442. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ruden M and Puri N: Novel anticancer
therapeutics targeting telomerase. Cancer Treat Rev. 39:444–456.
2013. View Article : Google Scholar
|
|
9
|
Röth A, Harley CB and Baerlocher GM:
Imetelstat (GRN163L) - telomerase-based cancer therapy. Recent
Results Cancer Res. 184:221–234. 2010. View Article : Google Scholar
|
|
10
|
Shay JW and Keith WN: Targeting telomerase
for cancer therapeutics. Br J Cancer. 98:677–683. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu Q, Zhang Z, Zhang P and Chen W:
Antisense oligonucleotides and all-trans retinoic acid have a
synergistic anti-tumor effect on oral squamous cell carcinoma. BMC
Cancer. 8:1592008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goldblatt EM, Gentry ER, Fox MJ, Gryaznov
SM, Shen C and Herbert BS: The telomerase template antagonist
GRN163L alters MDA-MB-231 breast cancer cell morphology, inhibits
growth, and augments the effects of paclitaxel. Mol Cancer Ther.
8:2027–2035. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gomez-Millan J, Goldblatt EM, Gryaznov SM,
Mendonca MS and Herbert BS: Specific telomere dysfunction induced
by GRN163L increases radiation sensitivity in breast cancer cells.
Int J Radiat Oncol Biol Phys. 67:897–905. 2007. View Article : Google Scholar
|
|
14
|
Xu Z, Fang S, Zuo Y, et al: Combination of
pigment epithelium-derived factor with radiotherapy enhances the
antitumor effects on nasopharyngeal carcinoma by downregulating
vascular endothelial growth factor expression and angiogenesis.
Cancer Sci. 102:1789–1798. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Blackburn EH: Switching and signaling at
the telomere. Cell. 106:661–673. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kyo S and Inoue M: Complex regulatory
mechanisms of telomerase activity in normal and cancer cells: how
can we apply them for cancer therapy? Oncogene. 21:688–697. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Y, Zhang H, Zhai Y, et al: A
functional tandem-repeats polymorphism in the downstream of TERT is
associated with the risk of nasopharyngeal carcinoma in Chinese
population. BMC Med. 9:1062011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Duan HG, Chen SM, Xiao BK, Cheng J
and Tao ZZ: Effect of RNA interference targeting human telomerase
reverse transcriptase on telomerase and its related protein
expression in nasopharyngeal carcinoma cells. J Laryngol Otol.
121:476–482. 2007. View Article : Google Scholar
|
|
19
|
Zhang SZ, Huang PC, Xu Y, Chen J and Cai
KR: Effects of telomerase sense and antisense oligodeoxynucleotides
on growth and differentiation of nasopharyngeal carcinoma cells. Ai
Zheng. 21:493–497. 2002.(In Chinese). PubMed/NCBI
|
|
20
|
Marian CO, Cho SK, McEllin BM, et al: The
telomerase antagonist, imetelstat, efficiently targets glioblastoma
tumor-initiating cells leading to decreased proliferation and tumor
growth. Clin Cancer Res. 16:154–163. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang X, Liu Y, Chow LS, et al: Regulation
of telomerase activity by gamma-radiation in nasopharyngeal
carcinoma cells. Anticancer Res. 20:433–437. 2000.PubMed/NCBI
|