Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic
progressive fibrotic lung disease of unknown origin with a median
survival time of <3 years (1,2). To
date, only lung transplantation has been proved to be effective in
the treatment of IPF, due to its poorly understood etiology and
pathogenesis (1,2). Increasing evidence has demonstrated
that recurrent or continuous injury to the lung epithelium
initiates and promotes lung fibrosis (3,4).
Unresolved injury can lead to compromised proliferative capacity of
the alveolar epithelial cells (AECs), increased cell death (either
necrosis or apoptosis), and a phenotypic switch of the surviving
cells (5,6). Apoptosis of AECs has been observed in
lung biopsies of IPF patients (7,8) as
well as in BLM-challenged experimental models of lung fibrosis
(9) in areas adjacent to
fibroblast foci. Transplantation of alveolar epithelial cells,
aimed at protecting cells from endogenous and exogenous injuries
and stimulating regeneration, has been shown to ameliorate
BLM-induced lung fibrosis (10).
In addition, hepatocyte growth factor (HGF) and keratinocyte growth
factor (KGF), which are both capable of promoting development and
proliferation of AECs, can attenuate BLM-induced lung fibrosis
(11–13). Therefore, any treatment capable of
promoting proliferation or reducing apoptosis of AECs may
ameliorate pulmonary fibrosis.
Bone marrow mesenchymal stem cells (BM-MSCs) are a
useful tool in cellular therapy of injured tissues. BM-MSCs can
differentiate into mature non-hematopoietic cells of multiple
tissues that can facilitate the repair of injured organs, such as
the liver, lung, heart and kidney (14). However, numerous studies have shown
that lung epithelial or endothelial cells are rarely derived from
BM-MSCs (15,16), therefore engraftment in the lung as
structural epithelium or endothelium is not currently considered
the mechanism by which BM-MSCs can repair lung tissue (17). Previous evidence has shown however,
that BM-MSCs can migrate to injured tissues, communicate with
injured parenchyma cells, and function in the wound healing process
through the production of paracrine-soluble cytokines and growth
factors, which modulate the regeneration of the epithelium and
endothelium (18).
Previous research has revealed that systemic
infusion of conditioned medium (CM) obtained from mesenchymal stem
cells (MSCs) leads to a hepato-protective response in the acutely
injured liver, specifically by inhibition of cell apoptosis and
stimulation of reparative programs (19). Xu et al (20) showed that BM-MSCs in the lung
suppressed the endotoxin-induced increase in circulating
proinflammatory cytokines by paracrine factors. Additionally,
MSC-CM has been shown to prevent O2-induced alveolar
epithelial cell apoptosis, accelerate alveolar epithelial cell
wound healing, and enhance endothelial cord formation (21). The aim of the present study was to
investigate the protective effects of MSC-CM on BLM-induced
alveolar epithelial injury in vitro, and pulmonary fibrosis
in vivo.
Materials and methods
Isolation and culture of BM-MSCs
Isolation and expansion of MSCs was performed as
described previously (22).
Briefly, BM-MSCs were obtained from 4 week-old male Sprague Dawley
rats by flushing the femur and tibia cavities with
phosphate-buffered saline (PBS). Following isolation, the cell
suspension was centrifuged, and the cells were plated in Dulbecco’s
modified Eagle’s medium (DMEM) (Gibco-BRL, Grand Island, NY, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco-BRL), 2 mm
L-glutamine (Gibco-BRL), 0.1 mmol/l non-essential amino acids
(HyClone, Logan, UT, USA), 100 IU/ml penicillin (Gibco-BRL) and 100
IU/ml streptomycin (Gibco-BRL) in culture dishes (Corning-Costar,
Corning, NY, USA). Non-adherent cells were removed after 30 h of
incubation, and BM-MSCs were recovered by their tight adhesion to
the culture dishes. BM-MSCs were identified phenotypically by their
typical fibroblast-like appearance and by detection of immune cell
surface markers specific for CD45, CD34, CD11b, CD44 and CD29
(Lifespan BioSciences, USA) by flow cytometry. BM-MSCs were
routinely cultured and used at passages 3 and 4 for subsequent
experiments. The use of rats in the present study was approved by
the Ethics Committee of the Beijing Chao-Yang Hospital (Capital
Medical University, Beijing, China).
Preparation of MSC-CM
Once BM-MSCs had reached 80% confluence in 10 cm
diameter culture dishes (~2×106 cells), the cells were
washed thoroughly three times with PBS, following which they were
incubated in 5 ml serum-free DMEM for 24 h. The CM, containing
secreted proteins, was collected and filtered using a 0.45-μm low
molecular weight protein binding membrane. The CM was further
concentrated 25-fold by ultrafiltration (Millipore, Bedford, MA,
USA) with a 3-kDa molecular weight cut-off (19). The concentrated CM was filtered
through a 0.22-μm membrane, for sterilization, and stored at −80°C
prior to use. The CM of 3T3 fibroblasts (FB-CM) was prepared as a
control and using the same methodology as described for the
production of MSC-CM.
Cell proliferation assay
A549 human non-small cell lung cancer epithelial
cells (ATCC, Rockefeller, MD, USA) were seeded into 48-well plates
(10,000 cells/well), and were incubated with serum-free RPMI-1640
medium (Gibco-BRL) and serum-free RPMI-1640 supplemented with
MSC-CM (MSC-CM/RPMI-1640), separately. Different proportions of the
MSC-CM was added to the medium: 1, 2, 4, 8 and 16%. After
incubation for 48 h, the cell growth-promoting activity of the
MSC-CM was detected by MTT assay (Sigma, St. Louis, MO, USA). The
absorbance was read at a wavelength of 492 nm.
Apoptosis analysis by flow cytometry
A549 cells were cultured in RPMI-1640 medium
supplemented with 10% FBS, at 37°C in a humidified incubator with
an atmosphere of 5% CO2/95% air. Following adherence of
the cells to the culture dish, the cultures were washed thoroughly
with PBS three times. A549 cells were cultured in either serum-free
RPMI-1640 (BLM group) or serum-free RPMI-1640 supplemented with 8%
MSC-CM (MSC-CM group) for 24 h. BLM (300 μg/ml) (Nippon Kayaku Co.,
Tokyo, Japan) was added to the cultures, which were then incubated
for a further 24 h. A549 cells cultured in serum-free RPMI-1640 for
48 h were used as a control group. A total of 1×106
cells were prepared from each sample for the apoptosis assay using
Annexin V-PE Apoptosis Detection kit (BioVision, Mountain View, CA,
USA) using an LSR flow cytometer (Becton-Dickinson, Mountain View,
CA, USA) and CellQuest™ software (Becton-Dickinson). The
experiments were repeated independently three times.
Pulmonary fibrosis in vivo studies
All animals used in the present study were
maintained in compliance with the guidlines of the Chinese Ministry
of Public Health. Twenty female Wistar rats, weighing between 200
and 220 g, were used for the pulmonary fibrosis experiment. The
rats were randomly divided into four groups (n=5); a control, BLM,
MSC-CM, and an FB-CM group. The rats were anaesthetized by
intraperitoneal injection of pentobarbital sodium at a dosage of 30
mg/kg body weight. The rats of the BLM, MSC-CM, and FB-CM groups
were intratracheally administered 0.2 ml saline, 0.2 ml MSC-CM, and
0.2 ml FB-CM, respectively, at 6 h and on day 3 following the
intratracheal administration of BLM (5 mg/kg body weight). The rats
of the control group were given the same volume of saline. All of
the rats were sacrificed on day 28 and the lung tissues were
harvested. The experimental protocols were approved by the
Committee on the Ethics of Animal Experiments, of Capital Medical
University (Beijing, China).
Histological assessment
For histological analysis, the right lower lung
lobes were fixed in 10% neutral formaldehyde for 24 h and embedded
in paraffin. Sections of 5-μm thickness were cut and stained with
hematoxylin and eosin (H&E) and Masson’s trichrome, separately.
Histopathological evaluation of pulmonary fibrosis was performed
according to the Ashcroft scoring under a photomicroscope (23).
Hydroxyproline measurement
Following sacrifice of the rats, the right upper
lung lobes were rapidly frozen in liquid nitrogen and stored at
−80°C prior to use. The hydroxyproline content was measured by a
colorimetric-based spectrophotometric assay using a Hydroxyproline
Detection kit (Nanjing Jiancheng Biotechnology Company, Nanjing,
China), according to the manufacturer’s instructions.
Terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling assays (TUNEL)
Apoptotic scores were obtained by TUNEL assay using
an In Situ Cell Death Detection kit (Roche-Diagnostics,
Mannheim, Germany).
Statistical analysis
All of the experiments were repeated three times.
Statistical analysis was performed using SPSS version 15.0 (SPSS
Inc., Chicago, IL, USA). The data are expressed as the means ±
standard deviation and was statistically analyzed using an analysis
of variance. Significant differences (P<0.05) between groups
were assessed using a post hoc analysis. For comparison of
pathologic grades, the Mann-Whitney U-test was used. A P<0.05
was considered to indicate a statistically significant
difference.
Results
Effects of MSC-CM on A549 cells
BM-MSCs were phenotypically identified by their
typical fibroblast-like appearance (Fig. 1A and B), and by absent expression
of the immune markers for CD45, CD34 and CD11b and positive
expression for CD44 and CD29 on the cell surface, as determined by
flow cytometry (Fig. 1C–G). In
order to investigate the proliferative effect of MSC-CM, A549 cells
were treated with serum-free RPMI-1640 containing different
proportions of MSC-CM: 0, 1, 2, 4, 8 and 16%, for 48 h. Results
from the MTT cell viability assay showed that as compared with the
control group (0.17±0.01), the treatment of A459 cells with medium
containing 1, 2, and 4% MSC-CM appeared to have an effect on cell
proliferation, however the differences were not statistically
significant (0.19±0.01, 0.20±0.02 and 0.20±0.03, respectively)
(P>0.05) (Fig. 2). When A549
cells were treated with medium supplemented with 8 and 16% MSC-CM,
the absorption values were 0.24±0.03 and 0.24±0.04 respectively,
which was significantly greater as compared with the control group
(P<0.01). This indicated that MSC-CM had a stimulatory effect on
A549 cell proliferation, and the effect was correlated with the
concentration of MSC-CM in the culture medium.
Inhibition of MSC-CM on A549 cell
apoptosis in vitro
To determine the inhibitory effect of MSC-CM on A549
cell apoptosis, 8% MSC-CM was used on BLM-challenged cells. The
apoptotic rate of A549 cells of the BLM group (38.06±4.32%) was
significantly increased as compared with that of the control group
(9.93±1.20%) (P<0.01) (Fig. 3).
Following treatment of the A549 BLM-challenged cells with 8% MSC-CM
for 48 h, the cell apoptotic rate was reduced to
(23.43±3.76%), this reduction, in comparison to the
apoptotic rate of the BLM group, was statistically significant
(P<0.05) (Fig. 3). These
results indicated that MSC-CM could partially rescue A549 cells
from BLM-induced apoptosis.
Anti-fibrotic effects of MSC-CM in
BLM-induced lung fibrosis
The protective effects of MSC-CM were examined using
a BLM-challenged rat model of lung fibrosis. As compared with the
control group (Fig. 4A and E), the
inflammation and collagen accumulation in the lung tissues was
significantly increased in the BLM group (P<0.05; Fig. 4B and F), and this increase was
attenuated by treatment of the rats with MSC-CM (Fig. 4C and G). The Ashcroft fibrosis
score was significantly increased in the BLM group as compared with
the control group (5.0±0.55 vs. 0.8±0.20, P<0.01, Fig. 4I). As compared with the BLM group,
MSC-CM treatment significantly reduced the fibrotic scores
(3.00±0.31, P<0.05, Fig. 4I),
whereas no difference was observed following FB-CM treatment
(3.20±0.49, P>0.05, Fig.
4I).
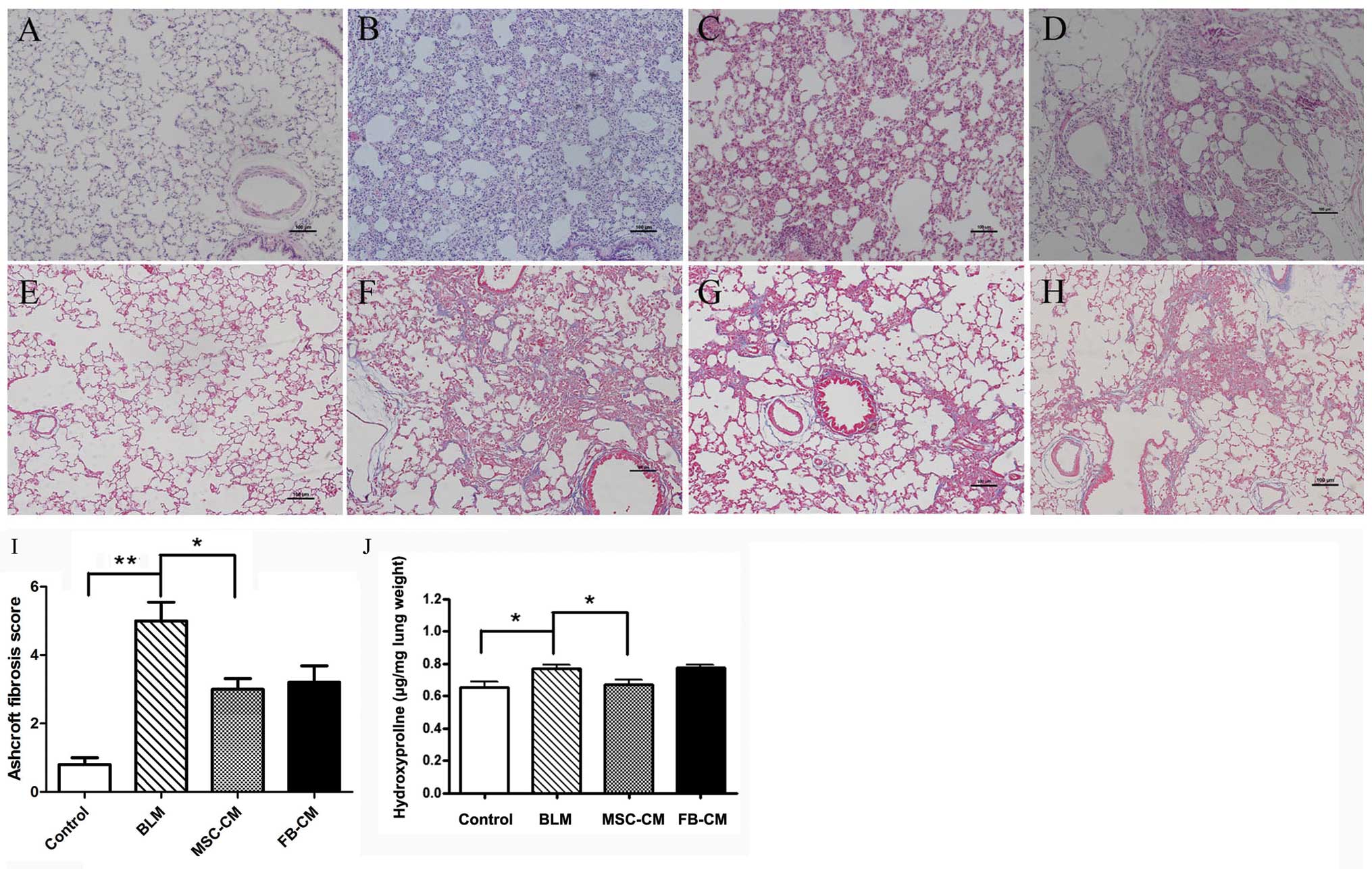 | Figure 4Effects of MSC-CM on lung fibrosis.
MSC-CM attenuated BLM-induced pulmonary injury. Lung sections were
stained with (A–D) hematoxylin and eosin and (E-H) Masson’s
trichrome, and examined under a photomicroscope. (A and E) No
marked pathological changes were detected in the control group. (B
and F) Thickened alveolar septa with increased infiltration of
inflammatory cells and collagen deposition were observed in the BLM
group. (C and G) Attenuated interstitial inflammation and collagen
deposition was observed in the MSC-CM group as compared with the
BLM group. (D and H) In the FB-CM group, interstitial inflammation
and collagen deposition were not relieved. Scale bar = 100 μm;
magnification, ×100. (I) Ashcroft scoring. As compared with the BLM
group, MSC-CM administration attenuated fibrotic lesions. Data are
shown as the means ± standard deviation. *P<0.05,
**P<0.01, n=5. (J) The collagen content was assessed
by hydroxyproline assay. As compared with control rats, BLM
increased collagen deposition. As compared with the BLM group,
MSC-CM administration decreased the hydroxyproline content in the
lung tissues, whereas FB-CM treatment did not attenuate the
collagen content. Data are shown as the means ± standard deviation.
*P<0.05, n=5. BLM, bleomycin; MSC-CM, mesenchymal
stem cell-conditioned media; FB-CM, fibroblast-conditioned
media. |
The collagen content of the rat lung tissue was
assessed by a hydroxyproline assay. The collagen production of lung
tissues was significantly increased in the BLM group (0.77±0.05
μg/mg lung weight), as compared with the control group (0.65±0.09
μg/mg lung weight) (P<0.05, Fig.
4J). In comparison to the BLM group, the hydroxyproline content
of lung tissues in the MSC-CM group was significantly decreased
(0.67±0.07 μg/mg lung weight) (P<0.05, Fig. 4J), whereas FB-CM treatment did not
attenuate the collagen content in rat lung tissues (0.78±0.03 μg/mg
lung weight) (P>0.05) (Fig.
4J).
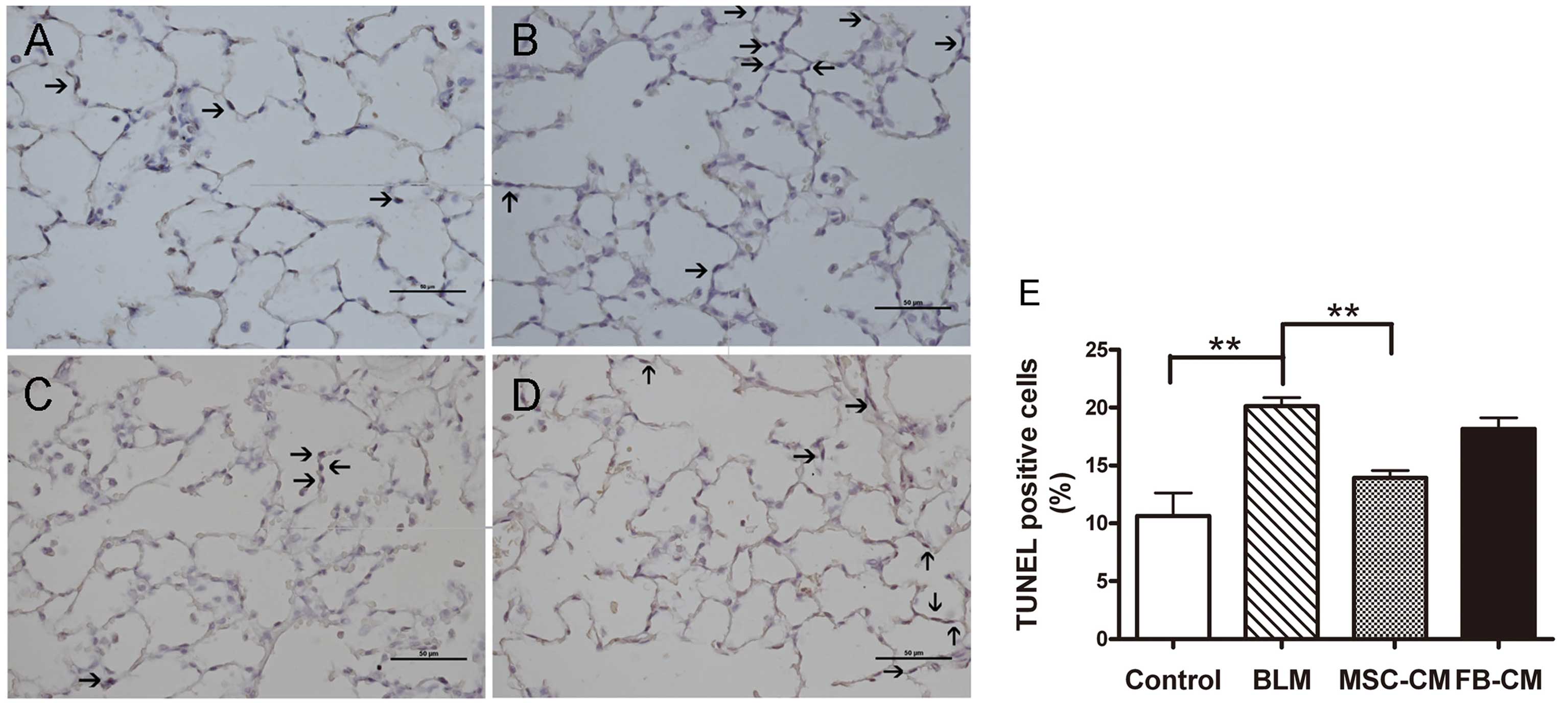
MSC-CM attenuates the apoptosis rate of
AECs in vivo
As shown in Fig. 5,
the proportion of apoptotic AECs in the BLM group (20.14±1.61%) was
significantly higher as compared with the control group
(10.64±4.48%) (P<0.01). As compared with the BLM group, MSC-CM
treatment had a significant protective effect on the apoptotic rate
of AECs (13.92±1.47%) (P<0.01), whereas there was no effect on
the apoptotic rate of AECs following FB-CM treatment (18.17±2.18%)
(P>0.05).
Discussion
IPF is a progressive parenchymal lung disease with a
poor prognosis, as there is thus far no effective treatment.
Unresolved injury-induced impaired proliferative capacity and an
increased apoptotic rate of AECs represents potentially important
mechanisms in the development of lung fibrosis (4,5).
Failed epithelium repair is followed by abnormal wound healing
processes, including epithelial and fibroblast activation, cytokine
production, coagulation pathway activation, neoangiogenesis,
re-epithelialization and fibrosis (4,5). It
is therefore important to develop a method by which to abrogate AEC
injury and to protect AECs from further apoptosis. The present
study investigated whether MSC-CM had a therapeutic effect on
BLM-induced lung epithelial injuries in vitro, and on
pulmonary fibrosis in vivo. The major findings of the study
were: (a) MSC-CM demonstrated the ability to improve viability and
proliferation of A549 cells. (b) MSC-CM inhibited A549 cell
apoptosis induced by BLM in serum-free conditions. (c) MSC-CM
reduced the hydroxyproline content and fibrotic score of rat lung
tissues treated with BLM, for which this protective effect was
shown to be associated with the anti-apoptotic effects of MSC-CM.
The present study provides evidence that MSC-CM has the potential
to reduce apoptosis of AECs, promote AEC regeneration and inhibit
development of lung fibrosis.
MSCs were first identified in the bone marrow in
1976 (24) and have since been
shown to express a broad spectrum of secreted molecules, including
interferon (IFN)-γ, interleukin (IL)-1β, IL-6, IL-10, transforming
growth factor (TGF)-β1, vascular endothelial growth factor (VEGF),
stromal derived factor (SDF)-1, HGF, KGF, prostaglandin PGE2,
amongst others (17,18). Through the action of these soluble
mediators, BM-MSCs have been shown to modulate the activation,
proliferation, and downstream effects of inflammatory and immune
cells in both the innate and adaptive immune systems; including
neutrophils, macrophages and lymphocytes (25,26).
However, it is unknown whether BM-MSCs are capable, through
paracrine properties, of modulating AEC proliferation and
apoptosis. The present study used MSC-CM, containing paracrine
factors secreted from BM-MSCs, to determine the effects of BM-MSCs
on lung epithelial cells. It was observed that MSC-CM enhanced the
proliferation of A549 cells, and this effect was correlated with
the concentration of MSC-CM (Fig.
2). MSC-CM was also shown to have an anti-apoptotic effect on
A549 cells induced by BLM in vitro (Fig. 3). These findings indicate that the
beneficial effects of BM-MSCs on AECs can be exerted by their
paracrine properties. Previous studies have shown that the
paracrine factors of MSCs have anti-apoptotic effects in
vitro on cardiac cells (27,28),
pancreatic β cells (29) and
hepatocytes (19). BM-MSCs are
known to produce several epithelial specific growth factors,
including HGF and KGF (17,18),
which have been proven to initiate a variety of biological
responses including migration, proliferation, and morphogenesis in
AECs (30–33). Therefore it can be speculated that
the protective roles of MSC-CM on AECs may be associated with the
epithelial specific growth factors secreted by BM-MSCs and the
synergistic effects of these factors.
Ineffective AEC repair and increased cell apoptosis
can lead to aberrant fibroblastic responses and end-stage fibrosis
(5). The present in vitro
study observed that BM-MSCs promoted A549 proliferation and
protected against cell apoptosis induced by a BLM challenge. It has
therefore been hypothesized that MSC-CM may attenuate lung fibrosis
in vivo by the rescue of injured AECs. In a BLM challenged
rat model of lung fibrosis, it was demonstrated that lung
inflammation (Fig. 4A–D), collagen
accumulation (Fig. 4E–H),
hydroxyproline content (Fig. 4J)
and the fibrotic score of the rat lung tissues (Fig. 4I) could be significantly decreased
by MSC-CM treatment, whereas FB-CM treatment had little effect. A
possible mechanism explaining the MSC-CM induced reduction in lung
cell apoptosis (Fig. 5) is the
generation and secretion of soluble paracrine cytokines; including
HGF, KGF, PGE2, VEGF (30–33) It may also be speculated that
apoptosis of AECs is mediated by reactive oxygen species, which are
involved in BLM-induced lung injury and IPF (34,35).
It has been shown that MSCs have antioxidative activity and have a
neuroprotective role in experimental autoimmune encephalomyelitis
(36); therefore MSCs may inhibit
the apoptosis of AECs and pulmonary fibrosis by an antioxidant
effect.
The in vivo study showed that BM-MSCs
attenuated lung fibrosis by paracrine properties, which has been
previously demonstrated by other studies (37–39).
In a BLM-induced lung fibrosis model, systemic MSC administration
significantly reduced lung inflammation and damage, collagen
deposition, and other injury and fibrosis markers, while only a
small number of MSCs appeared to have structurally engrafted in the
lung (37). Similarly, Kumamoto
et al (38) and Lee et
al (39) demonstrated that
systemically administered BM-derived MSCs in a BLM model of lung
fibrosis resulted in an improved lung injury score and modulation
of inflammatory cytokine production, by protective paracrine
effects. MSCs of origins other than bone marrow have also been
shown to attenuate lung fibrosis. Moodley et al (40) reported that intratracheal and
systemic infusion of umbilical cord derived MSCs (UC-MSCs) in a BLM
model of lung injury decreased lung collagen accumulation, fibrosis
score and matrix metalloproteinase expression. Based on the
findings of the present study, together with previous studies, it
can be concluded that administration of both MSC-CM (paracrine
factors) and MSCs can attenuate lung fibrosis. The paracrine
factors that mediate the protective effects of MSCs requires
further study.
To our knowledge, the present study was the first to
use MSC-CM, in comparison to MSCs alone, to show that MSCs are
effective in reducing apoptosis of AECs induced by BLM, and
enhancing AECs regeneration, resulting in fewer lung injuries,
lower fibrosis scores, and reduced collagen production in the lung
by their significant paracrine capacity. These effects were
independent of the structural engraftment to the airway or alveolar
epithelium and phenotypic switch to structural lung cells. MSC-CM
may provide a novel approach for the treatment of lung
fibrosis.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (no. 30871120), the
Sci-Tech Development Program of Beijing Municipal Education
Communication (no. KZ201110025028) and the National Basic Research
Program of China 973 Program (no. 2009CB522106).
References
|
1
|
King TE Jr, Pardo A and Selman M:
Idiopathic pulmonary fibrosis. Lancet. 378:1949–1961. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Raghu G, Collard HR, Egan JJ, et al;
ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An
official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis:
evidence-based guidelines for diagnosis and management. Am J Respir
Crit Care Med. 183:788–824. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kropski JA, Lawson WE, Young LR and
Blackwell TS: Genetic studies provide clues on the pathogenesis of
idiopathic pulmonary fibrosis. Dis Model Mech. 6:9–17. 2013.
View Article : Google Scholar :
|
|
4
|
Zoz DF, Lawson WE and Blackwell TS:
Idiopathic pulmonary fibrosis: a disorder of epithelial cell
dysfunction. Am J Med Sci. 341:435–438. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hardie WD, Hagood JS, Dave V, et al:
Signaling pathways in the epithelial origins of pulmonary fibrosis.
Cell Cycle. 9:2769–2776. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sakai N and Tager AM: Fibrosis of two:
Epithelial cell-fibroblast interactions in pulmonary fibrosis.
Biochim Biophys Acta. 1832:911–921. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maher TM, Evans IC, Bottoms SE, et al:
Diminished prostaglandin E2 contributes to the apoptosis paradox in
idiopathic pulmonary fibrosis. Am J Respir Crit Care Med.
182:73–82. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li X, Shu R, Filippatos G and Uhal BD:
Apoptosis in lung injury and remodeling. J Appl Physiol (1985).
97:1535–1542. 2004. View Article : Google Scholar
|
|
9
|
Degryse AL, Tanjore H, Xu XC, et al:
Repetitive intratracheal bleomycin models several features of
idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol.
299:L442–452. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Serrano-Mollar A, Nacher M, Gay-Jordi G,
et al: Intratracheal transplantation of alveolar type II cells
reverses bleomycin-induced lung fibrosis. Am J Respir Crit Care
Med. 176:1261–1268. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bao S, Wang Y, Sweeney P, et al:
Keratinocyte growth factor induces Akt kinase activity and inhibits
Fas-mediated apoptosis in A549 lung epithelial cells. Am J Physiol
Lung Cell Mol Physiol. 288:L36–L42. 2005. View Article : Google Scholar
|
|
12
|
Crestani B, Marchand-Adam S, Quesnel C, et
al: Hepatocyte growth factor and lung fibrosis. Proc Am Thorac Soc.
9:158–163. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Umeda Y, Marui T, Matsuno Y, et al:
Skeletal muscle targeting in vivo electroporation-mediated HGF gene
therapy of bleomycin-induced pulmonary fibrosis in mice. Lab
Invest. 84:836–844. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Krause DS, Theise ND, Collector MI, et al:
Multi-organ, multi-lineage engraftment by a single bone
marrow-derived stem cell. Cell. 105:369–377. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rejman J, Colombo C and Conese M:
Engraftment of bone marrow-derived stem cells to the lung in a
model of acute respiratory infection by Pseudomonas aeruginosa. Mol
Ther. 17:1257–1265. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fritzell JA Jr, Mao Q, Gundavarapu S, et
al: Fate and effects of adult bone marrow cells in lungs of
normoxic and hyperoxic newborn mice. Am J Respir Cell Mol Biol.
40:575–587. 2009. View Article : Google Scholar :
|
|
17
|
Tzouvelekis A, Ntolios P and Bouros D:
Stem cell treatment for chronic lung diseases. Respiration.
85:179–192. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Conese M, Carbone A, Castellani S and Di
Gioia S: Paracrine effects and heterogeneity of marrow-derived
stem/progenitor cells: relevance for the treatment of respiratory
diseases. Cells Tissues Organs. 197:445–473. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van Poll D, Parekkadan B, Cho CH, et al:
Mesenchymal stem cell-derived molecules directly modulate
hepatocellular death and regeneration in vitro and in vivo.
Hepatology. 47:1634–1643. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu J, Woods CR, Mora AL, et al: Prevention
of endotoxin-induced systemic response by bone marrow-derived
mesenchymal stem cells in mice. Am J Physiol Lung Cell Mol Physiol.
293:L131–L141. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
van Haaften T, Byrne R, Bonnet S, et al:
Airway delivery of mesenchymal stem cells prevents arrested
alveolar growth in neonatal lung injury in rats. Am J Respir Crit
Care Med. 180:1131–1142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tögel F, Weiss K, Yang Y, et al:
Vasculotropic, paracrine actions of infused mesenchymal stem cells
are important to the recovery from acute kidney injury. Am J
Physiol Renal Physiol. 292:F1626–F1635. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ashcroft T, Simpson JM and Timbrell V:
Simple method of estimating severity of pulmonary fibrosis on a
numerical scale. J Clin Pathol. 41:467–470. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Phinney DG: Building a consensus regarding
the nature and origin of mesenchymal stem cells. J Cell Biochem
Suppl. 38:7–12. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nauta AJ and Fibbe WE: Immunomodulatory
properties of mesenchymal stromal cells. Blood. 110:3499–3506.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lau AN, Goodwin M, Kim CF and Weiss DJ:
Stem cells and regenerative medicine in lung biology and diseases.
Mol Ther. 20:1116–1130. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sadat S, Gehmert S, Song YH, et al: The
cardioprotective effect of mesenchymal stem cells is mediated by
IGF-I and VEGF. Biochem Biophys Res Commun. 363:674–679. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nakanishi C, Yamagishi M, Yamahara K, et
al: Activation of cardiac progenitor cells through paracrine
effects of mesenchymal stem cells. Biochem Biophys Res Commun.
374:11–16. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu YX, Chen L, Wang R, et al: Mesenchymal
stem cell therapy for diabetes through paracrine mechanisms. Med
Hypotheses. 71:390–393. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gazdhar A, Susuri N, Hostettler K, et al:
HGF expressing stem cells in usual interstitial pneumonia originate
from the bone marrow and are antifibrotic. PLoS One. 8:e654532013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gazdhar A, Temuri A, Knudsen L, et al:
Targeted gene transfer of hepatocyte growth factor to alveolar type
II epithelial cells reduces lung fibrosis in rats. Hum Gene Ther.
24:105–116. 2013. View Article : Google Scholar
|
|
32
|
Chakraborty S, Chopra P, Hak A, Dastidar
SG and Ray A: Hepatocyte growth factor is an attractive target for
the treatment of pulmonary fibrosis. Expert Opin Investig Drugs.
22:499–515. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sakamoto S, Yazawa T, Baba Y, et al:
Keratinocyte growth factor gene transduction ameliorates pulmonary
fibrosis induced by bleomycin in mice. Am J Respir Cell Mol Biol.
45:489–497. 2011. View Article : Google Scholar
|
|
34
|
Inghilleri S, Morbini P, Oggionni T, Barni
S and Fenoglio C: In situ assessment of oxidant and nitrogenic
stress in bleomycin pulmonary fibrosis. Histochem Cell Biol.
125:661–669. 2006. View Article : Google Scholar
|
|
35
|
Teixeira KC, Soares FS, Rocha LG, et al:
Attenuation of bleomycin-induced lung injury and oxidative stress
by N-acetylcysteine plus deferoxamine. Pulm Pharmacol Ther.
21:309–316. 2008. View Article : Google Scholar
|
|
36
|
Lanza C, Morando S, Voci A, et al:
Neuroprotective mesenchymal stem cells are endowed with a potent
antioxidant effect in vivo. J Neurochem. 110:1674–1684. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ortiz LA, Gambelli F, McBride C, et al:
Mesenchymal stem cell engraftment in lung is enhanced in response
to bleomycin exposure and ameliorates its fibrotic effects. Proc
Natl Acad Sci USA. 100:8407–8411. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kumamoto M, Nishiwaki T, Matsuo N, Kimura
H and Matsushima K: Minimally cultured bone marrow mesenchymal stem
cells ameliorate fibrotic lung injury. Eur Respir J. 34:740–748.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee SH, Jang AS, Kim YE, et al: Modulation
of cytokine and nitric oxide by mesenchymal stem cell transfer in
lung injury/fibrosis. Respir Res. 11:162010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Moodley Y, Atienza D, Manuelpillai U, et
al: Human umbilical cord mesenchymal stem cells reduce fibrosis of
bleomycin-induced lung injury. Am J Pathol. 175:303–313. 2009.
View Article : Google Scholar : PubMed/NCBI
|