Introduction
Colon cancer is the third most common type of cancer
and the leading cause of cancer-associated mortality in Western
communities (1,2). In China, the incidence is lower than
in Western countries, but nevertheless is a substantial burden
(3). The molecular mechanisms
underlying the development of this type of cancer remain to be
fully elucidated, thus there currently exist limited therapeutic
options (4).
MicroRNAs (miRNAs) are a family of small non-coding
RNA molecules of 18–27 nucleotides in length (5). In general, miRNAs negatively regulate
gene expression by binding to the 3′-untranslated region (3′-UTR)
of their target double-stranded mRNA, leading to degradation of the
mRNA via the Dicer complex (6).
The abnormal expression of certain miRNAs has been observed in
various types of solid tumor, including colon cancer (7). For example, miRNA-21 has recently
emerged as a novel biomarker in colon cancer, with the potential to
be used as a diagnostic and therapeutic target (8). Additionally, several miRNAs have been
reported to regulate colon cell growth, migration and invasion,
including miR-32, -224 and -203 (9–11).
The current study investigated miR-100 using gain-
and loss-of-function experiments, and the effect of up- or
downregulation of miR-100 on the proliferation, migration and
invasion of colon cells was determined.
Materials and methods
Human tissues
A total of 25 pairs of frozen primary colon cancer
samples and corresponding histologically normal mucosa samples were
obtained from the Department of Gastroeterology, Second Affiliated
Hospital of Zhengzhou University (Zhengzhou, China). The diagnoses
of these tissue samples were verified by pathologists. The current
study was approved by the Ethics Committee of the Second Affiliated
Hospital of Zhengzhou University.
Cell culture
The SW480 and HCT116 colon cancer cell lines were
purchased from the cell bank of the Type Culture Collection of The
Chinese Academy of Sciences (Shanghai, China), and cultured in
Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10%
fetal bovine serum, 100 IU/ml penicillin and 100 mg/ml streptomycin
(all from Gibco Life Technologies, Carlsbad, CA, USA).
Cell transfection
miR-100 mimics, antisense oligonucleotides and
negative controls (NCs) were purchased from Shanghai Genepharma
Co., Ltd. (Shanghai, China). Transfections were performed using
Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad, CA,
USA), according to the manufacturer’s instructions.
RNA isolation and quantitative polymerase
chain reaction (qPCR)
RNA was isolated from cells using TRIzol reagent
(Invitrogen Life Technologies), and reverse transcription was
performed with the Takara RNA PCR kit (Takara Biotechnology Co.,
Ltd., Dalian, China), according to the manufacturer’s instructions.
In order to determine the transcripts of the genes of interest,
qPCR was performed using a SYBR Premix Ex Taq master mix (Takara
Biotechnology Co., Ltd.) with an ABI 7500 Real-Time PCR system
(Applied Biosystems Life Technologies, Foster City, CA, USA). PCR
cycling conditions included an initial holding period at 94°C for 5
min, followed by a two-step PCR program consisting of 40 cycles of
94°C for 5 sec and 60°C for 30 sec. Expression of U6 small nuclear
RNA was determined as an internal control. Primer sequences were as
follows: Cyclin D1, F 5′-GCTGCGAAGTGGAAACCATC-3′ and R
5′-CCTCCTTCTGCACACATTTGAA-3′; cyclin E, F 5′-AAGGAGCGGGACACCATGA-3′
and R 5′-ACGGTCACGTTTGCCTTCC-3′.
Bromodeoxyuridine (BrdU) incorporation
assays
For the BrdU incorporation assays, a BrdU cell
proliferation enzyme-linked immunosorbent assay kit (Beyotime
Institute of Biotechnology, Shanghai, China) was used to analyze
the incorporation of BrdU during the S phase of the cell cycle in
the SW480 and HCT116 cells, in accordance with the manufacturer’s
instructions. All experiments were repeated a minimum of three
times in quadruplicate.
MTT assay
The cell viability was determined by assaying the
reduction of 3-(4, 5-dimethylthiazol-2-yl)-2,
5-di-phenyltetrazolium bromide (MTT) to formazan. SW480 and HCT116
cells were cultured in DMEM at a concentration of 60–70%, prior to
the addition of 100 μl MTT (Beyotime Institute of Biotechnology)
for 4 h. The cell viability in the different samples was measured
using a Raman spectrophotometer (B&W Tek, Inc., Newark, DE,
USA) at a wavelength of 470 nm.
Cell migration and invasion assays
Subsequent to transfection of the SW480 and HCT116
cells with miR-100 mimics, antisense or NC for 24 h, cell migration
and invasion were analyzed. The cells were seeded in Transwell
migration or extracellular matrix-coated invasion chambers (Tumor
Cell Transendothelial Migration Assay kit and ECMatrix Cell
Invasion Assay kit, respectively; EMD Millipore, Temecula, CA, USA)
and incubated for a further 24 h. Subsequently, the Transwell
migration assay or invasion assay was conducted according to
manufacturer’s instructions. Cell migration and invasion were
quantified with an iMark Microplate Absorbance Reader (#168–1130;
Bio-Rad Laboratories, Hercules, CA, USA) at 570 nm, according to
the manufacturer’s instructions.
Luciferase reporter assay
Total cDNA from SW480 cells was obtained from mRNA
using a Reverse Transcription System (Promega Corporation, Madison,
WI, USA) according to the manufacturer’s protocols. The cDNA was
used to amplify the 3′-UTR of Lgr5 by PCR. The Lgr5 3′UTR was
cloned into pMir-Report (Ambion Life Technologies, Shanghai,
China), yielding pMir-Report-Lgr5. Mutations were introduced in
potential miR-100 binding sites using the QuikChange site-directed
mutagenesis kit (Agilent Technologies, Shanghai, China). Cells were
transfected with the pMir-Report vectors containing the 3′-UTR
variants with miR-100 mimics or antisense for 36 h. The pRL-TK
vector (Promega Corporation) carrying the Renilla luciferase
gene was used as an internal control to normalize the transfection
efficiency. Luciferase values were determined using the
Dual-Luciferase Reporter Assay System (Promega Corporation).
miRWalk software (www.umm.uni-heidelberg.de/apps/zmf/mirwalk/) was used
for analysis.
Western blot
Following transfection of mimics, antisense or NC,
tissues or SW480 cells were lysed with RIPA buffer (Beyotime
Institute of Biotechnology). The protein (40 μg) was subjected to
7.5% SDS-PAGE (Shanghai Sangong Pharmaceutical Co., Ltd., Shanghai,
China) on a PowerPAC HC High-Current Power Supply electrophoresis
machine (Bio-Rad Laboratories), and separated proteins were
transferred to nitrocellulose membranes (EMD Millipore). The
membranes were incubated overnight at 4°C with the following
antibodies: Monoclonal mouse anti-human β-actin (1:1,000;
sc-130065); polyclonal rabbit anti-human
leucine-rich-repeat-containing G protein-coupled receptor 5 (Lgr5;
1:2,000; sc-135238); monoclonal rabbit anti-human β-catenin
(1:2,000; sc-376841); and polyclonal goat anti-human histone H1
(1:1,000; sc-247158) (all Santa Cruz Biotechnology, Inc., Dallas,
TX, USA). The immunoreactive bands were detected with a ChemiGlow
West Chemiluminescence Substrate kit (ProteinSimple, Santa Clara,
CA, USA) with the FluorChem FC2 system (NtureGene Corporation,
Beijing, China).
Statistical analysis
Data are presented as the mean ± standard error.
Statistical analysis was performed with SPSS, version 13.0 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-100 is downregulated in colon cancer
tissues
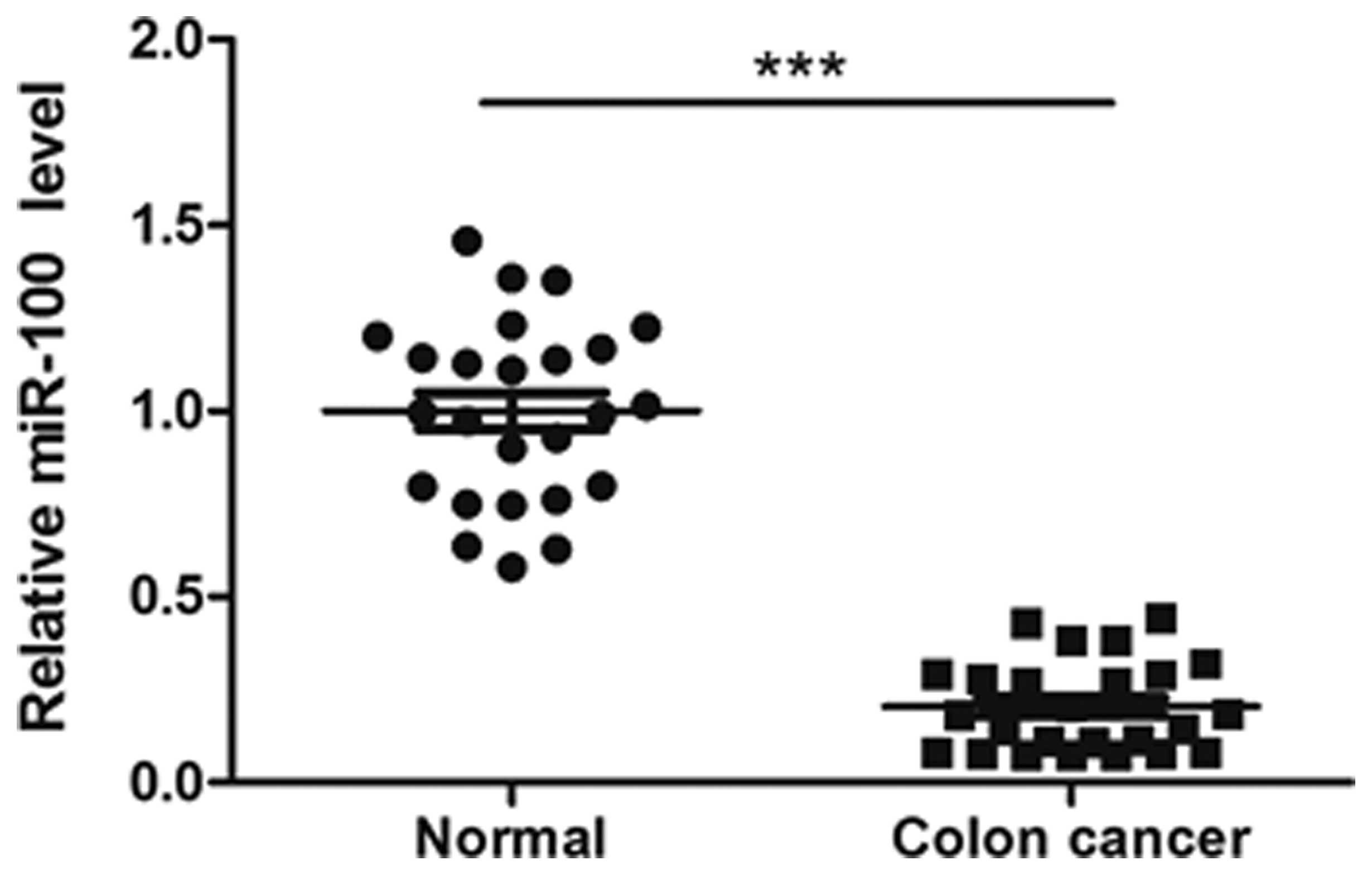
The expression level of miR-100 in 25 pairs of human
tissues was measured by RT-qPCR. As demonstrated in Fig. 1, the miR-100 expression level was
significantly lower in colon cancer tissues compared with the level
in matched adjacent normal tissues (P<0.001).
miR-100 reduces the viability,
proliferation, migration and invasion of colon cancer cells
To investigate the function of miR-100 in
tumorigenesis, gain- and loss-of-function experiments involving the
introduction of mimics or antisense oligonucleotides into SW480 and
HCT116 cells were performed. Scramble sequences were used for the
NC group. As observed with the MTT and BrdU incorporation assays,
cell viability and proliferation levels were significantly reduced
in SW480 cells in which miR-100 was overexpressed, but enhanced by
miR-100 antisense (Fig. 2A and B).
Additionally, miR-100 mimics significantly inhibited the in
vitro migration and invasion abilities of SW480 cells, whereas
its antisense enhanced these processes (Fig. 2C and D). Similar results were also
observed in the HCT116 cells (Fig.
3).
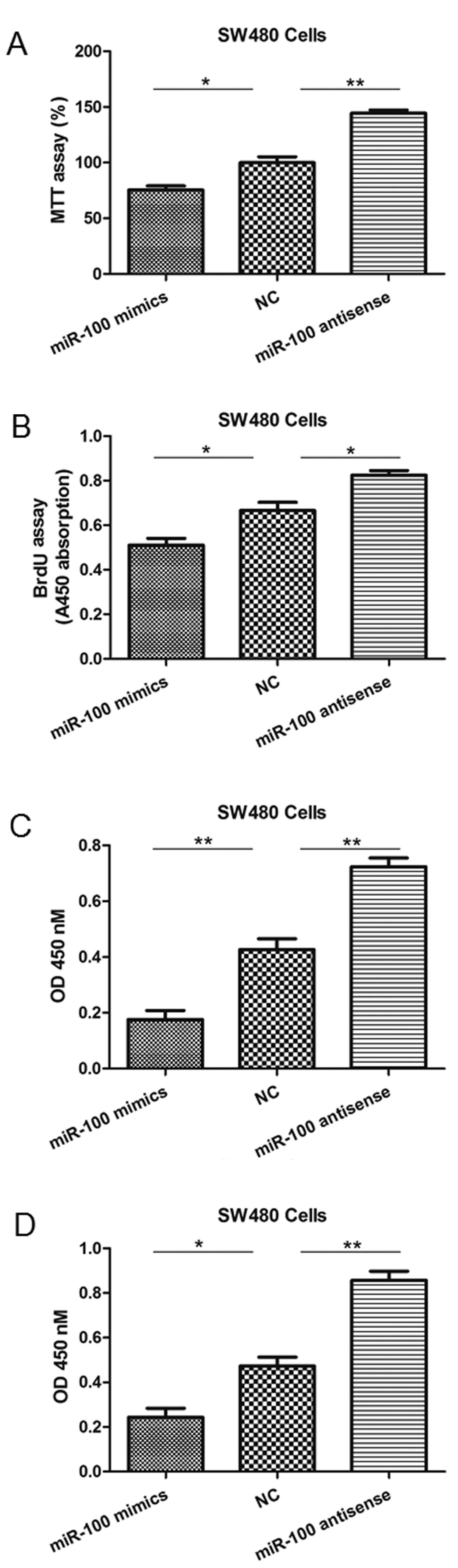 | Figure 2miR-100 mimics or antisense
oligonucleotides regulate SW480 cell viability, proliferation,
migration and invasion. (A) The MTT assay indicated a reduction or
increase in cell viability following transfection of mimics or
antisense, respectively. (B) The BrdU assay indicated a reduction
or increase in proliferation following transfection of mimics or
antisense oligonucleotides, respectively. (C) The Transwell
migration assay indicated a reduction or increase in migration
following transfection of mimics or antisense oligonucleotides,
respectively. (D) The invasion assay indicated a reduction or
increase in invasion capabilities following transfection of mimics
or antisense oligonucleotides, respectively. *P<0.05
and **P<0.01 vs. NC. miR-100, microRNA-100; BrdU,
bromodeoxyuridine; NC, negative control; OD, optical density. |
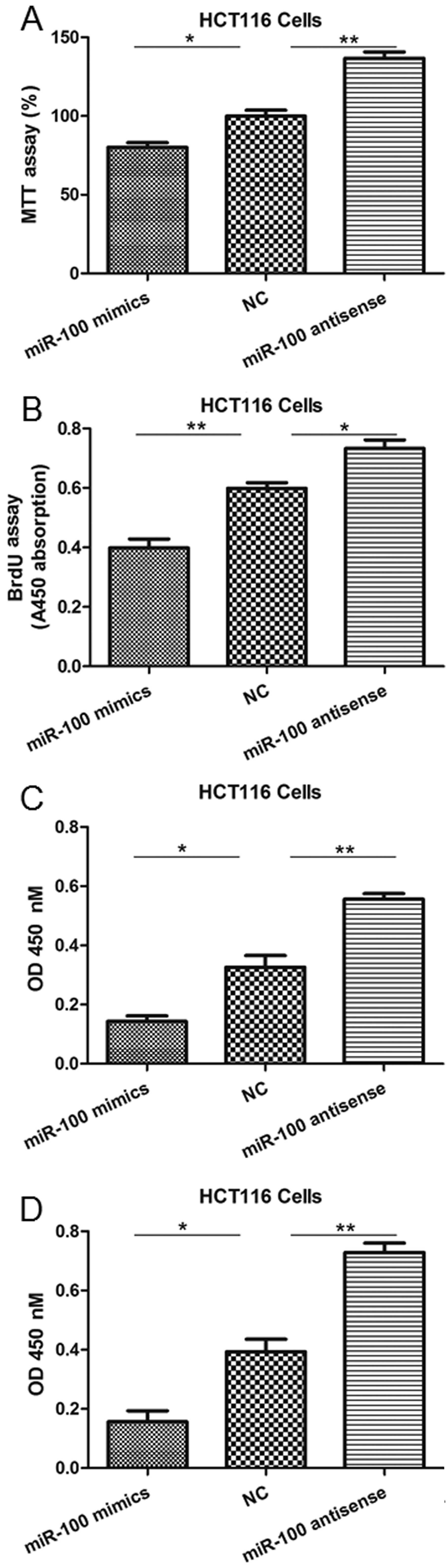 | Figure 3miR-100 mimics or antisense
oligonucleotides regulate HCT116 cell viability, proliferation,
migration and invasion. (A) The MTT assay indicated a reduction or
increase in cell viability following transfection of mimics or
antisense oligonucleotides, respectively. (B) The BrdU assay
indicated a reduction or increase in proliferation following
transfection of mimics or antisense oligonucleotides, respectively.
(C) The Transwell migration assay indicated a reduction or increase
in migration following transfection of mimics or antisense
oligonucleotides, respectively. (D) The invasion assay indicated a
reduction or increase in cell viability following transfection of
mimics or antisense oligonucleotides, respectively.
*P<0.05 and **P<0.01 vs. NC. miRNA-100,
microRNA-100; BrdU, bromodeoxyuridine; NC, negative control; OD,
optical density. |
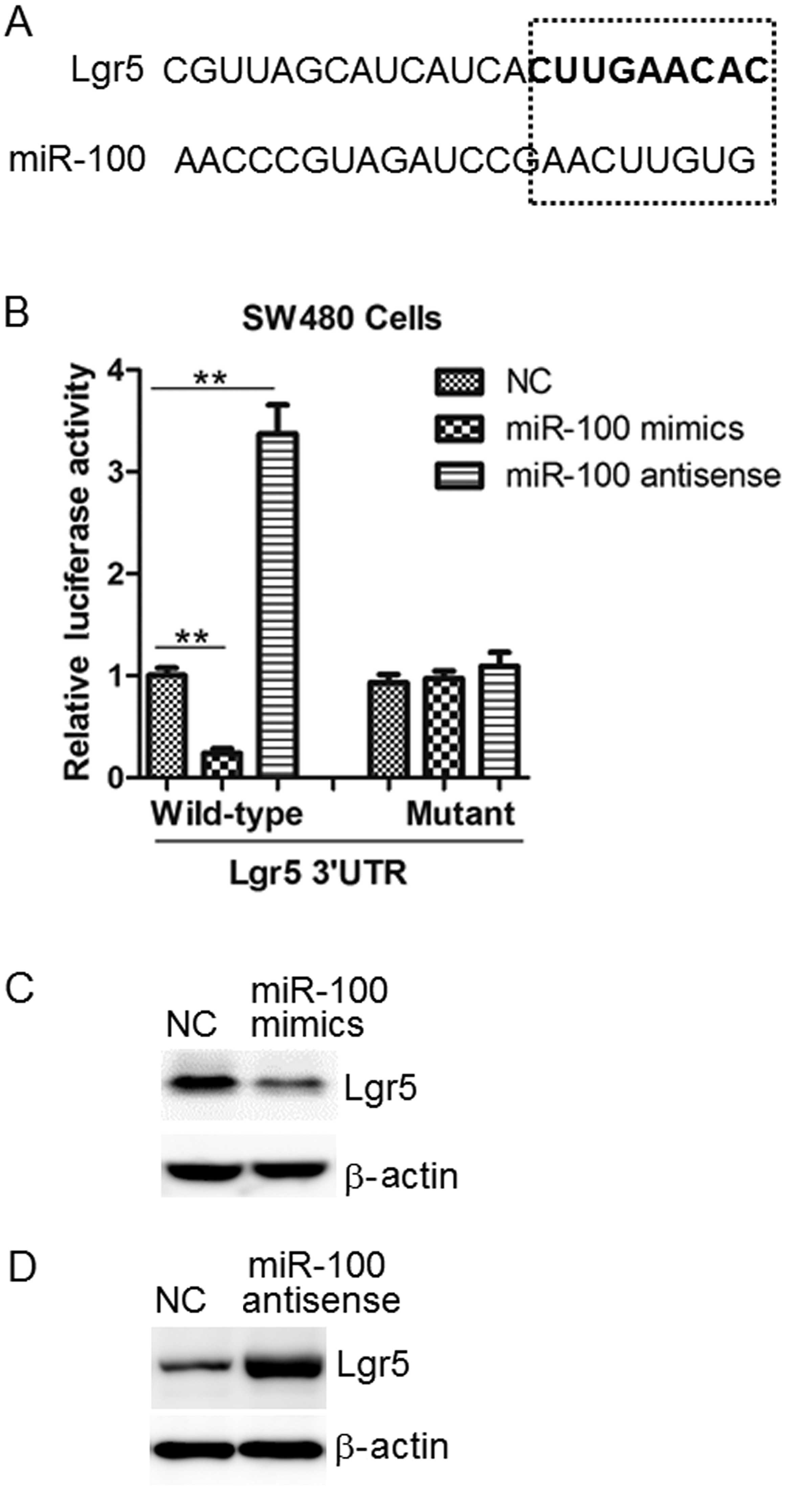
Lgr5 is a target of miR-100 in colon
cancer cells
Bioinformatics software (miRWalk) was used to screen
for the target gene of miR-100 in colon cancer cells. The results
indicated Lgr5 to be a target of miR-100 (Fig. 4A). The luciferase activity assay
established that miR-100 mimics significantly suppressed the
activity of the wild-type 3′-UTR, while its antisense upregulated
it. However, this effect was not observed in the mutant in SW480
cells (Fig. 4B).
Protein levels of Lgr5 and β-catenin are
altered by miR-100
Consistent with the bioinformatic analysis and
luciferase assay results, western blot analysis indicated that
miR-100 mimics reduced Lgr5 protein levels compared with NC levels
in SW480 cells, whilst miR-100 antisense produced the opposite
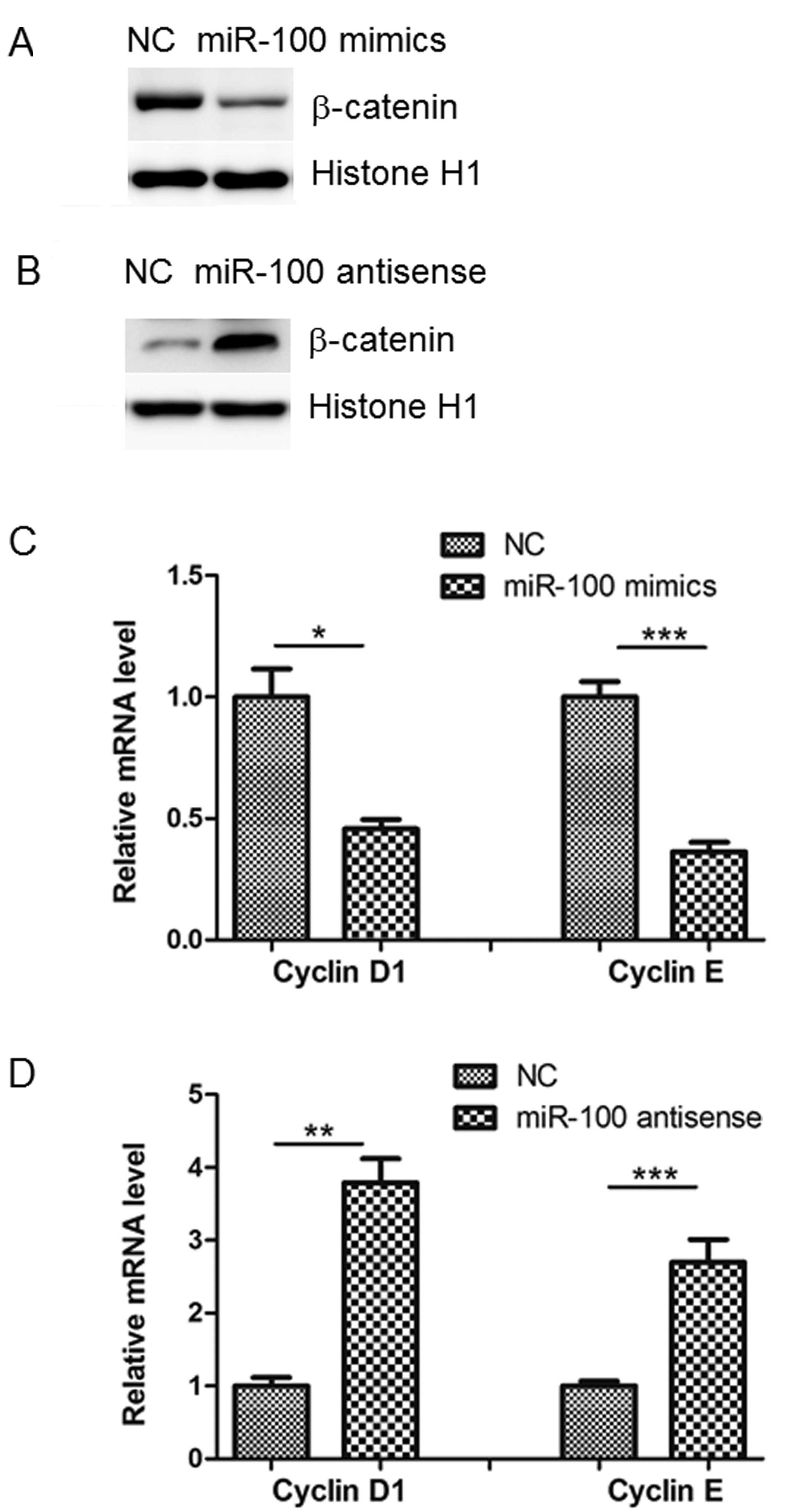
effect (Fig. 4C and D). A previous
study demonstrated that Lgr5 promotes tumor growth and progression
through the activation of Wnt/β-catenin signaling (12). In agreement with this, it was
observed in the current study that miR-100 mimics inhibited, while
its antisense promoted, nuclear β-catenin accumulation in SW480
cells (Fig. 5A and B). The results
of the present study were in accordance with a previous study that
demonstrated that cyclin D1 and E were reduced or increased by
transfection of miR-100 mimics or antisense, respectively (13). This indicates that downstream
targets of β-catenin are also influenced by miR-100 (Fig. 5C and D).
Discussion
Identification of cancer-specific miRNAs may be
important for understanding the role they perform in tumorigenesis
and exploring novel therapeutic targets. Previous studies have
demonstrated that miR-100 is downregulated in several types of
malignancy, including osteosarcoma, acute myeloid leukemia, and
lung and hepatocellular carcinoma (14–17).
miR-100 has been identified as a potential molecular marker of
non-small cell lung cancer, and functions as a tumor suppressor by
targeting polo-like kinase 1 (17). miR-100 has also been demonstrated
to inhibit breast cancer proliferation and survival through the
suppression of insulin-like growth factor 2 and β-tubulin (18). This indicates a fundamental
function for miRNA as a tumor suppressor. However, the function of
miR-100 in colon cancer biology remains unclear.
In the current study, the expression and potential
functions of miR-100 in the regulation of the biological properties
of colon cancer cells were investigated. miR-100 expression was
demonstrated to be downregulated in colon cancer tissues compared
with adjacent normal tissues. The subsequent gain- and
loss-of-function studies suggested that miR-100 was able to reduce
colon cancer cell viability, proliferation, migration and invasion
in vitro.
To further explore the molecular mechanisms involved
in miR-100-mediated effects on biological properties, Lgr5 was
selected for further study as it was predicted to be a target of
miR-100 by bioinformatics analysis. Lgr5, also known as G
protein-coupled receptor 49, is a regulator of Wnt signaling
(19). Consistent with the
dysregulation of Wnt/β-catenin signaling, Lgr5 is overexpressed in
hepatocellular carcinoma, colon and ovarian cancer, basal cell
carcinoma and esophageal adenocarcinoma (20–22).
This suggests an important function for Lgr5 in tumorigenesis.
Adenomatous polyposis coli mutations observed exclusively in
Lgr5-positive cells have been identified to be able to promote
adenomatous growth in the colon of mice (23). In addition to patients, the
overexpression of Lgr5 has been demonstrated to correlate with poor
survival of colon cancer in mice (24). However, the precise molecular
mechanisms underlying the upregulation of Lgr5 in colon cancer
remain to be fully elucidated, and thorough investigation is
required.
Additionally, nuclear localization of β-catenin and
expression of its down-stream target genes, including cyclin D1 and
cyclin E were demonstrated to be regulated by miR-100. Thus, the
downregulation of miR-100 may be an important mechanism for the
aberrant activation of Wnt signaling in human cancer.
To the best of our knowledge, the results from the
current study, for the first time explore the function of miR-100
in the progression of colon cancer. Future studies, including the
generation of miR-100 knockout mice, are required to establish the
physiological function of miR-100 in tumorigenesis.
References
|
1
|
Des Guetz G, Uzzan B, Bouillet T, et al:
Impact of physical activity on cancer-specific and overall survival
of patients with colorectal cancer. Gastroenterol Res Pract.
2013:3408512013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Søgaard M, Thomsen RW, Bossen KS, Sørensen
HT and Nørgaard M: The impact of comorbidity on cancer survival: a
review. Clin Epidemiol. 5(Suppl 1): 3–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chiu BC, Ji BT, Dai Q, Gridley G,
McLaughlin JK, Gao YT, Fraumeni JF Jr and Chow WH: Dietary factors
and risk of colon cancer in Shanghai, China. Cancer Epidemiol
Biomarkers Prev. 12:201–208. 2003.PubMed/NCBI
|
|
4
|
Walker AS, Zwintscher NP, Johnson EK,
Maykel JA, Stojadinovic A, Nissan A, Avital I, Brücher BL and
Steele SR: Future directions for monitoring treatment response in
colorectal cancer. J Cancer. 5:44–57. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun K and Lai EC: Adult-specific functions
of animal microRNAs. Nat Rev Genet. 14:535–548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ameres SL and Zamore PD: Diversifying
microRNA sequence and function. Nat Rev Mol Cell Biol. 14:475–488.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu WK, Law PT, Lee CW, Cho CH, Fan D, Wu
K, Yu J and Sung JJ: MicroRNA in colorectal cancer: from benchtop
to bedside. Carcinogenesis. 32:247–253. 2011. View Article : Google Scholar
|
|
8
|
Kjaer-Frifeldt S, Hansen TF, Nielsen BS,
Joergensen S, Lindebjerg J, Soerensen FB, dePont Christensen R and
Jakobsen A: The prognostic importance of miR-21 in stage II colon
cancer: a population-based study. Br J Cancer. 107:1169–1174. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu W, Yang J, Feng X, Wang H, Ye S, Yang
P, Tan W, Wei G and Zhou Y: MicroRNA-32 (miR-32) regulates
phosphatase and tensin homologue (PTEN) expression and promotes
growth, migration, and invasion in colorectal carcinoma cells. Mol
Cancer. 12:302013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yuan K, Xie K, Fox J, Zeng H, Gao H, Huang
C and Wu M: Decreased levels of miR-224 and the passenger strand of
miR-221 increase MBD2, suppressing maspin and promoting colorectal
tumor growth and metastasis in mice. Gastroenterology. 145:853–864.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schetter AJ, Leung SY, Sohn JJ, Zanetti
KA, et al: MicroRNA expression profiles associated with prognosis
and therapeutic outcome in colon adenocarcinoma. JAMA. 299:425–436.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Haegebarth A and Clevers H: Wnt signaling,
lgr5, and stem cells in the intestine and skin. Am J Pathol.
174:715–721. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wilson C: Diabetes: Human β-cell
proliferation by promoting Wnt signalling. Nat Rev Endocrinol.
9:5022013. View Article : Google Scholar
|
|
14
|
Huang J, Gao K, Lin J and Wang Q:
MicroRNA-100 inhibits osteosarcoma cell proliferation by targeting
Cyr61. Tumour Biol. 35:1095–1100. 2014. View Article : Google Scholar
|
|
15
|
Zheng YS, Zhang H, Zhang XJ, Feng DD, et
al: MiR-100 regulates cell differentiation and survival by
targeting RBSP3, a phosphatase-like tumor suppressor in acute
myeloid leukemia. Oncogene. 31:80–92. 2012. View Article : Google Scholar :
|
|
16
|
Chen P, Zhao X and Ma L: Downregulation of
microRNA-100 correlates with tumor progression and poor prognosis
in hepatocellular carcinoma. Mol Cell Biochem. 383:49–58. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu J, Lu KH, Liu ZL, Sun M, De W and Wang
ZX: MicroRNA-100 is a potential molecular marker of non-small cell
lung cancer and functions as a tumor suppressor by targeting
polo-like kinase 1. BMC Cancer. 12:5192012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gebeshuber CA and Martinez J: miR-100
suppresses IGF2 and inhibits breast tumorigenesis by interfering
with proliferation and survival signaling. Oncogene. 32:3306–3310.
2013. View Article : Google Scholar
|
|
19
|
Leushacke M and Barker N: Lgr5 and Lgr6 as
markers to study adult stem cell roles in self-renewal and cancer.
Oncogene. 31:3009–3022. 2012. View Article : Google Scholar
|
|
20
|
Fukuma M, Tanese K, Effendi K, Yamazaki K,
Masugi Y, Suda M and Sakamoto M: Leucine-rich repeat-containing G
protein-coupled receptor 5 regulates epithelial cell phenotype and
survival of hepatocellular carcinoma cells. Exp Cell Res.
319:113–121. 2013. View Article : Google Scholar
|
|
21
|
Zeki SS, Graham TA and Wright NA: Stem
cells and their implications for colorectal cancer. Nat Rev
Gastroenterol Hepatol. 8:90–100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schuijers J and Clevers H: Adult mammalian
stem cells: the role of Wnt, Lgr5 and R-spondins. EMBO J.
31:2685–2696. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lewis A, Segditsas S, Deheragoda M, et al:
Severe polyposis in Apc(1322T) mice is associated with submaximal
Wnt signalling and increased expression of the stem cell marker
Lgr5. Gut. 59:1680–1686. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Z, Dai W, Jiang L and Cheng Y:
Over-expression of LGR5 correlates with poor survival of colon
cancer in mice as well as in patients. Neoplasma. 61:177–185. 2014.
View Article : Google Scholar
|