Introduction
Cervical cancer is the second most common malignant
disease of the female genital tract, and its incidence increases
with advancing age. Patients with cancer exhibit weaker immune
surveillance capability and a variety of immunological
abnormalities, including the impairment of CD4+ T
cell-mediated immunity, cytokine dysregulation in peripheral blood
and a lack of dendritic cells at the tissue level (1,2). An
impaired immune state is further disrupted by surgical stress,
anesthesia and postoperative pain (3). Postoperative immune dysfunction is a
problem for patients undergoing surgery for malignant tumors, as it
affects the rate of infectious complications, clinical outcomes and
the growth of disseminated tumor cells (4–6).
Particularly in the elderly with cancer, improved postoperative
immunity may result in more favorable long-term oncological results
(7,8). Therefore, it is critically important
to improve the surgery-induced immune suppression and restore the
immune function following surgery in patients with cancer.
The mechanisms underlying immunity depression
include the imbalance of immune cells and associated cytokines.
Previous studies have focused on defining the cytokine secretion
profiles of CD4+ T cells, including helper T cells type
1 (Th1), Th2, Th17 and Treg, as they are involved in immunological
disturbances (9). Th1, Th2, Th17
and Treg cells produce positive or negative effects in the
maintenance of normal immune function by secreting various
cytokines. It is established that Th1 cells are principal effectors
of the cell-mediated immune response, as they secrete IL-2 and
IFN-γ, which increase anti-tumor immunity. However, Th2 cells
produce the humoral immune response-related cytokines IL-4, IL-5
and IL-10, which are involved in suppressing anti-tumor immunity
(10). Th17 cells produce potent
pro-inflammatory effects through the production of IL-17, IL-21 and
IL-23 (11,12), while TGF-β-producing Treg cells
negatively regulate the activation and proliferation of T cells
(13). A previous study reported
that the imbalance of Th1/Th2 cytokines due to the increased
production of IL-10 was closely associated with the weakened immune
function of the body (13). The
current study indicated that the imbalance of Th17/Treg status may
lead to infection, inflammatory response and autoimmune disorders
(9). These observations confirmed
that alterations to Th1, Th2, Th17 and Treg cell cytokines are
potential factors promoting the development of host immune
dysfunction, and the fine balance of these cells is crucial for the
maintenance of normal immune homeostasis. However, the contribution
of these cells to postoperative immunological suppression in
patients with cervical cancer remains elusive.
There is strong evidence that effective analgesia is
able to relieve surgery-induced immune suppression, increase host
resistance to tumor metastasis and improve clinical outcomes
(14). Non-steroidal
anti-inflammatory drugs (NSAIDs) are used to treat pain, and act by
inhibiting the cyclooxygenase enzyme responsible for the release of
inflammatory mediators (15).
Parecoxib, the first selective cyclooxygenase (COX)-2 inhibitor
available for intravenous injection, is able to efficiently and
rapidly cross the blood-brain barrier (16,17)
and produce peripheral and central antihyperalgesic (18) and immunoregulatory effects
(19). An animal and clinical
study suggested that the perioperative use of parecoxib
significantly enhanced systemic Th1 immune responses by increasing
the plasma level of IFN-γ in rat brain tumors (20) and attenuated IL-6 and IL-8
production following surgery for colorectal cancer (21,22).
This implies that parecoxib exerts protective effects on
surgery-induced immune responses. However, little information is
currently available regarding the effects of parecoxib on
postoperative immune function in patients with cervical cancer.
Based on the above observations, the present study
hypothesized that perioperative multiple-dose parecoxib ameliorates
the surgery-induced immunosuppression in patients with cervical
cancer by modulating the balance of Th1, Th2, Th17 and Treg
cytokines. Therefore, the aim of the present study was to evaluate
the mRNA and protein expression levels of Th1, Th2, Th17 and Treg
cytokines in the peripheral blood of patients with cervical cancer
following laparoscopy, and to investigate the effects of parecoxib
on postoperative immune status.
Materials and methods
Patient selection
Between May 2012 and May 2013, a total of 356
patients were diagnosed with cervical cancer in the Cancer
Hospital, Harbin Medical University (Heilongjiang, China). Of
these, 101 patients met the inclusion criteria, but 21 refused to
take part in the study. Finally, 80 patients of ages 18–65 years,
with American Society of Anesthesiology (ASA) stages I-III, that
were accepted for laparoscopic radical hysterectomy for stages IB
and IIA cervical cancer, were evaluated in the current randomized,
double-blind controlled trial. The diagnosis of cervical cancer was
in accordance with the International Federation of Gynecology and
Obstetrics (FIGO) classification. The exclusion criteria were as
follows: Severe heart, hepatic or renal disease; coagulopathy;
bronchial asthma; a history of chronic pain or regular opioid
consumption; morbid obesity (BMI >35 kg/m2); and any
contraindication to NSAIDs. The protocol of the current study was
approved by the Ethics Committee of Heilongjiang University of
Chinese Medicine (Harbin, China), and written informed consent was
obtained from all subjects.
Anesthesia procedures
Phenobarbital sodium (2 mg/kg; (Tianjin Jinyao Amino
Acid Co., Ltd., Tianjin, China) was administered intramuscularly 30
min before surgery. Prior to the induction of anesthesia, standard
monitors were applied to record non-invasive blood pressure, heart
rate and the electrocardiogram. The bispectral index system (BIS)
was used to monitor the depth of anesthesia. Anesthesia was induced
intravenously with midazolam (0.05 mg/kg; Jiangsu Nhwa
Pharmaceutical Co., Ltd., Jiangsu, China), fentanyl (0.03 mg/kg;
Yichang Humanwell Pharmaceutical Co., Ltd., Hubei, China) and
propofol (2.5–3.0 mg/kg; AstraZeneca UK Limited, Macclesfield, UK),
and intubation was performed using vecuronium (0.08 mg/kg; Zhejiang
Xianju Pharmaceutical Co., Ltd., Zhejiang, China). The lungs were
ventilated mechanically (Aestiva 3000; GE Datex-Ohmeda
Instrumentarium Corp., Helsinki, Finland) with a tidal volume of
8–10 ml/kg and ventilator frequency of 12–14 bpm to achieve an
end-tidal carbon dioxide tension of 35–45 mm Hg. Anesthesia was
maintained with nitrous oxide (60%; Harbin Qing Hua Industrial Gas
Co., Heilongjiang, China) and isofluorane (end-tidal concentration
1–3%; Abbott Laboratories, Chicago, IL, USA) to a BIS score of
40–50. To maintain core body temperature normothermia, a
circulating warm water blanket (Tianjin Medical Instrument Research
Institute, Tianjin, China) was applied. Ringer’s acetate (Hunan
Kelun Pharmaceutical Co., Ltd., Hunan, China) was administered at a
rate of 6–8 ml/kg/h in order to maintain basal fluid requirements.
No blood transfusion was given intra-operatively. All patients were
operated on by one surgical team, with the same operative protocol
under a standardized anesthesia protocol. The surgery was performed
as described previously (23) and
anesthesia was performed as described previously (24) with small modifications (nitrous
oxide concentration adjusted to 60%).
Experimental design
An anesthetist not involved in the data collection
performed the randomization with a computer-generated random list
with coded sealed envelopes. Patients were randomized into the
parecoxib-treated (group I; n=40) or control (group II; n=40)
groups. Group I received parecoxib (40 mg; Dynastat, Pfizer, Inc.,
New York, NY, USA) 30 min prior to surgery and then each 12 h
subsequent to surgery until the 60 h time point, while group II
received normal saline as a placebo at the corresponding time
points. Intravenous tramadol (100 mg) was prescribed for
postoperative pain relief as required.
A syringe containing parecoxib or saline was
prepared by a third party and labeled ‘study drug’. All patients,
surgeons, anesthesiologists and nurses involved in recording
postoperative data were blinded to the syringe contents.
Determination of cytokine mRNA expression
by quantitative polymerase chain reaction (qPCR)
Blood samples were obtained prior to parecoxib or
saline administration (basal) and at 24, 48 and 72 h subsequent to
surgery. Total RNA was extracted using TRIzol® reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA). The
concentration of purified RNA was determined by spectrophotometry
(SmartSpec Plus; Bio-Rad, Hercules, CA, USA). Reverse transcription
(RT) was performed on the total RNA (1 μl) to synthesize cDNA. This
was then added into a 20 μl GoldScript one-step RT-PCR kit
(Invitrogen Life Technologies), placed in 42°C water for 60 min and
heated in 70°C water for 15 min to deactivate the reverse
transcriptase. The primers were designed and synthesized by the
Shanghai Institute of Biochemistry (Shanghai, China). The sequences
of the primers used for PCR amplification were as follows: IL-2,
forward (F) 5′-GACGCTGGAAATTTCATCAGCA-3′ and reverse (R)
5′-GCTCATCATCGAATTGGCACTC-3′ (91 bp); IFN-γ, F
5′-AGGCCATCAGCAACAACATAAGTG-3′ and R 5′-GACAGCTTTGTGCTGGATCTGTG-3′
(140 bp); IL-4, F 5′-TGCACCGAGATGTTTGTACCAGA-3′ and R
5′-TTGCGAAGCACCCTGGAAG-3′ (92 bp); IL-10, F
5′-CAGACCCACATGCTCCGAGA-3′ and R 5′-CAAGGCTTGGCAACCCAAGTA-3′ (141
bp); IL-17, F 5′-AACTCATCCATCCCCAGTTG-3′ and R
5′-CCGGTTATGGATGTTCAGGT-3′ (198 bp); IL-23, F
5′-GCACTGCTGAATGTCCCA-3′ and R 5′-CATGCCTAGTGCGTTTGC-3′ (311 bp);
TGF-β, F 5′-ATGAGCACTGAAAGCATGATC-3′ and R
5′-TCACAGGGCAATGATCCCAAAGTAGACCTGCCC-3′ (262 bp); GAPDH, F
5′-GGCACAGTCAAGGCTGAGAATG-3′ and R 5′-ATGGTGGTGAAGACGCCAGTA-3′ (143
bp). PCR amplification was performed on a DNA Engine thermal cycler
(96 well alpha unit with hot bonnet; Bio-Rad) using a SYBR Green I
(SYBR Green PCR Master Mix; Invitrogen Life Technologies) probe (20
μl DNA per assay). The mRNA expression levels of target genes were
normalized against the reference gene GAPDH, and relative mRNA
levels of each cytokine were calculated. All samples were run in
triplicates for each experiment.
Determination of cytokine protein
expression levels by enzyme-linked immunosorbent assay (ELISA)
Plasma was separated by centrifugation at 2,000 × g
for 15 min at 4°C and immediately stored in aliquots at 80°C. The
concentrations of cytokines (IL-2, IFN-γ, IL-4, IL-10, IL-17, IL-23
and TGF-β) were determined by ELISA using commercially available
kits (Shanghai Senxiong Technology Industry Co., Ltd., Shanghai,
China) according to the manufacturer’s instructions. Briefly,
flat-bottomed 96-well microtiter plates (Sigma-Aldrich, St. Louis,
MO, USA) were coated with a monoclonal mouse anti-human
anti-cytokine antibody (50 μl/well; 1:100; Senxiong Science and
Technology Company, Shanghai, China) diluted in coating buffer
(Shanghai Yuanmu Biological Science and Technology Co., Ltd,
Shanghai, China) and incubated overnight. The optical density was
measured on a plate reader (Bio-Rad) at 490 nm. The results are
expressed in pg/ml, and the optical density of the samples was
compared to the standard curves.
Variables measured during the study
period
Postoperative pain at rest and following movement
were assessed by a visual analogue scale (VAS) between 0 (pain
free) and 10 (worst possible pain) prior to parecoxib or saline
administration (basal) and at 2, 6, 12, 18, 24, 36, 48, 60 and 72 h
subsequent to surgery. Mean arterial pressure (MAP) and heart rate
(HR) were measured every 6 h for three days after surgery. The
immune parameters and pain scores were considered the primary
endpoint. Secondary outcomes included the incidence of
postoperative pain (visceral, parietal and shoulder pain),
analgesic relief, the ocurrence of adverse events and the length of
hospital stay.
Sample sizes and statistical
analysis
The sample size was calculated based on the
preliminary experiment comparing an immune variable (IL-2 protein
level) on postoperative day 2 between the two groups and yielded a
sample size of n=21 (type I error=0.05 and type II error=0.2) for
each group. To accommodate for participants that may not complete
the study, 40 patients were enrolled in each group.
The normality of quantitative variables was tested
using the Kolmogorov-Smirnov test. Inter-group differences were
assessed by Student’s t-test. Inter-group comparison of the mean
VAS scores at each measurement point was performed with the t-test.
Quantitative variables were analyzed using χ2 or
Fisher’s exact test. Statistical analyses were performed using SPSS
version 13.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
In the present study, 8 patients were excluded, due
to being converted to laparotomy (n=3; 1 from group I, and 2 from
group II) and incomplete data collection (n=5; 3 from group I, and
2 from group II). A total of 72 patients (n=36 in each group) were
included in the data analysis.
Demographic and surgical information
Demographic and surgical data are presented in
Table I. The mean ages of the
patients were 52.5±11.8 years in group I and 54.4±13.1 years in
group II, which were not statistically different (P>0.05). Other
features of patients, including weight, ASA physical status, FIGO
stage and histology were not significantly different between the
two groups (P>0.05); duration of surgical procedure did not
differ between groups I and II (248.5±65.1 vs. 261.2±57.3 min,
respectively; P>0.05). Patients in group I and II were similar
with respect to blood loss, fluids and lymph node resection
(P>0.05).
 | Table IDemographic and clinical
characteristics of patients. |
Table I
Demographic and clinical
characteristics of patients.
| Characteristic | Group I | Group II |
|---|
| Age (years) | 52.5±11.8 | 54.4±13.1 |
| Weight (kg) | 63.8±9.9 | 58.5±7.5 |
| ASA (I/II/III) | 4/29/3 | 7/22/7 |
| FIGO stage
(Ib/IIa) | 14/22 | 16/20 |
| Histology |
| Squamous | 29 | 32 |
|
Adenocarcinoma | 6 | 4 |
| Adenosquamous | 1 | 0 |
| Other | 0 | 0 |
| Duration of surgery
(min) | 248.5±65.1 | 261.2±57.3 |
| Blood loss
(ml) | 299.0±37.0 | 320.0±48.0 |
| Fluids (ml) | 2080.0±350.0 | 2220.0±430.0 |
| Lymph nodes
resected | 17.5±2.5 | 20.5±3.5 |
Postoperative pain intensity and
incidence
In order to understand the immunoregulatory effect
of parecoxib, its effect on postoperative pain following
gynecologic laparoscopy was evaluated. Parecoxib is an effective
analgesic that acts by inhibiting the synthesis of cyclooxygenase
(COX)-2/prostaglandin E (PGE)2 in the central nervous system. VAS
score is a simple and commonly used method for evaluating
postoperative pain intensity in studies. Pain scores were assessed
using VAS prior to parecoxib or saline administration (basal) and
at 2, 6, 12, 18, 24, 36, 48, 60 and 72 h subsequent to sugery; the
VAS scores are presented in Fig.
1. Compared with group II, VAS scores at rest for group I were
significantly reduced at 2, 6 and 12 h post-surgery (P<0.05;
Fig. 1A). After movement, patients
in group I experienced reduced pain compared with group II at 2, 6,
12, 18 and 24 h post-surgery (P<0.05; Fig. 1B). The results suggest that
parecoxib exerts a stronger analgesic effect following
laparoscopy.
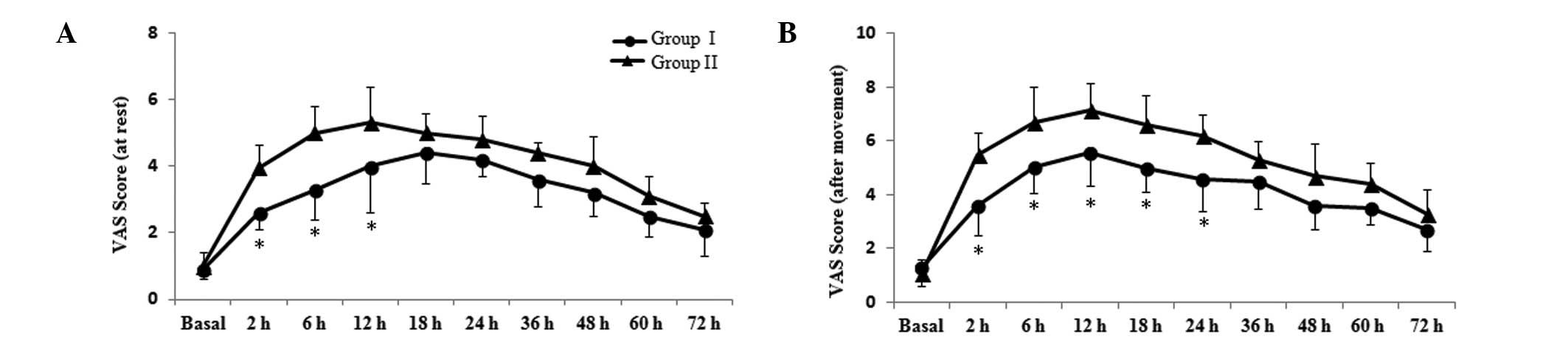 | Figure 1Effects of parecoxib on postoperative
pain scores. Postoperative pain (A) at rest and (B) following
movement were assessed with a VAS (0, no pain; 10, worst possible
pain) prior to parecoxib or saline administration (basal) and at 2,
6, 12, 18, 24, 36, 48, 60 and 72 h subsequent to surgery. Data are
presented as the mean ± standard deviation (n=36 in each group).
*P<0.05 vs. group II. VAS, visual analog scale. |
Early postoperative pain subsequent to laparoscopy
occurs as visceral, parietal and shoulder pain components. The
overall incidences of these pain components in the study were 58.3,
65.3 and 25.0%, respectively (Table
II). The incidences of visceral, parietal and shoulder pain in
group I were lower than those in group II (44.4 vs. 72.2, 52.8 vs.
77.8 and 13.9 vs. 36.1%, respectively; P<0.05). This indicates
that parecoxib may reduce the incidence of these postoperative pain
types following laparoscopy.
 | Table IIIncidence of pain subsequent to
laparoscopy (n, %). |
Table II
Incidence of pain subsequent to
laparoscopy (n, %).
| Group | n | Visceral | Parietal | Shoulder |
|---|
| Group I | 36 | 16 (44.4)a | 19 (52.8)a | 5 (13.9)a |
| Group II | 36 | 26 (72.2) | 28 (77.8) | 13 (36.1) |
| Total | 72 | 42 (58.3) | 35 (65.3) | 18 (25.0) |
Postoperative analgesic relief, length of
hospital stay and occurrence of adverse events
The percentage of patients who required
postoperative analgesic relief was lower in group I compared with
group II (13.9 vs. 69.4%; P<0.01). Neither MAP nor HR differed
between the two groups during the 72-h observation period
(P>0.05). The length of hospital stay in group I was shorter
than group II, but presented no significant difference (7.4±2.2 vs.
9.0±3.1 days; P>0.05). No major complications or adverse events
occurred in the two groups. The incidence of postoperative
nausea/vomiting and infection was significantly lower in group I
than group II (13.8 vs. 33.3% and 5.6 vs. 16.7%; P<0.05); The
incidence of complications, including sedation, headache,
hypotension, hypertension, respiratory-related, gastrointestinal
bleeding and cardiovascular events, during the study period was
similar between the two groups (Table III). This suggests that parecoxib
is safe and well-tolerated for use in pain control for patients
with cervical cancer following laparoscopic surgery.
 | Table IIIPostoperative adverse events (n,
%). |
Table III
Postoperative adverse events (n,
%).
| Adverse event | Group I | Group II |
|---|
|
Nausea/vomiting | 5 (13.8)a | 12 (33.3) |
| Sedation | 3 (8.3) | 2 (5.6) |
| Headache | 1 (2.8) | 2 (5.6) |
| Hypotension | 4 (11.1) | 3 (8.3) |
| Hypertension | 2 (5.6) | 3 (8.3) |
|
Respiratory-related | 1 (2.8) | 2 (5.6) |
| Gastrointestinal
bleeding | 0 | 0 |
|
Cardiovascular-related | 1 (2.8) | 1 (2.8) |
| Postoperative
infection | 2 (5.6)a | 6 (16.7) |
mRNA expression levels of Th1, Th2, Th17
and Treg cytokines
Effective analgesia is able to relieve
surgery-induced immune suppression. Recent experiments suggested
that parecoxib exerts a stronger antihyperalgesic effect and
provides a regulatory effect on the inflammatory immune response.
In the present study, the balance of Th1, Th2, Th17 and Treg cells
was evaluated to determine the immunoregulatory effects of
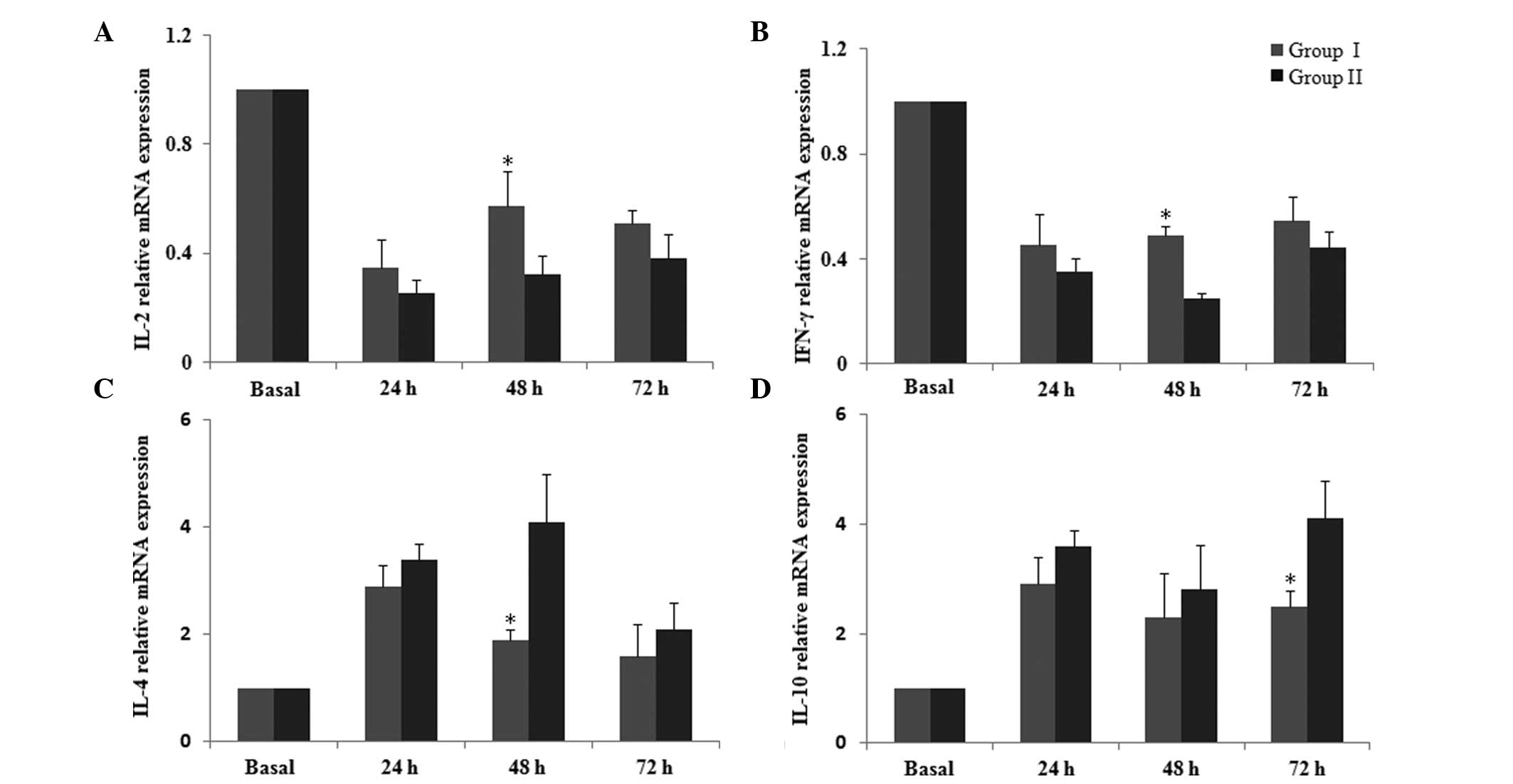
parecoxib. The mRNA levels of Th1/Th2 cytokines quantified by qPCR
are displayed in Fig. 2. The
results indicated that the mRNA expression levels of IL-2 and IFN-γ
were reduced following surgery, and this reduction was
significantly inhibited in group I at postoperative 48 h compared
with group II (P<0.05; Fig. 2A and
B). As presented in Fig. 2C and
D, mRNA expression levels of IL-4 and IL-10 were higher
following surgery, and these increases were markedly suppressed at
48 and 72 h, respectively, in group I compared with group II
(P<0.05). These findings support the hypothesis that
perioperative intravenous administration of multi-dose parecoxib
attenuates postoperative immune suppression.
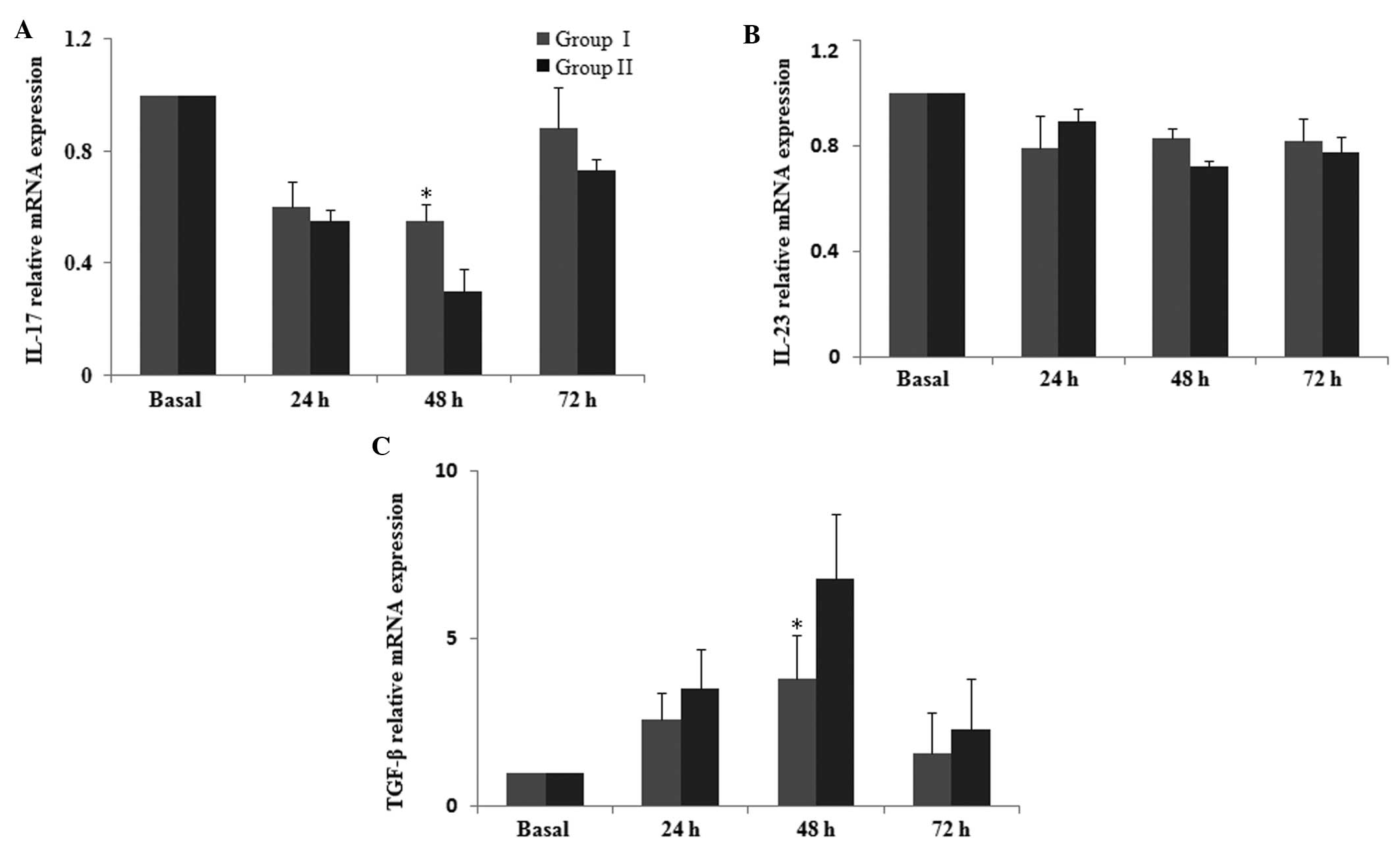
A reduction in the mRNA level of IL-17 subsequent to
surgery was observed, which was then attenuated at 48 h, resulting
in a higher level of IL-17 in group I compared with that of group
II (P<0.05; Fig. 3A). However,
the mRNA level of IL-23 during the postoperative 72-h observation
period was not significantly altered between the two groups
(P>0.05; Fig. 3B). The mRNA
level of TGF-β was increased following surgery, and this increase
was inhibited at 48 h, with a significantly lower level in group I
compared with that of group II (P<0.05; Fig. 3C). These results clearly indicate
that parecoxib is able to attenuate the postoperative immune
impairment by balancing the expression of Th1, Th2, Th17 and Treg
cytokines at the mRNA level.
Protein levels of Th1, Th2, Th17 and Treg
cytokines
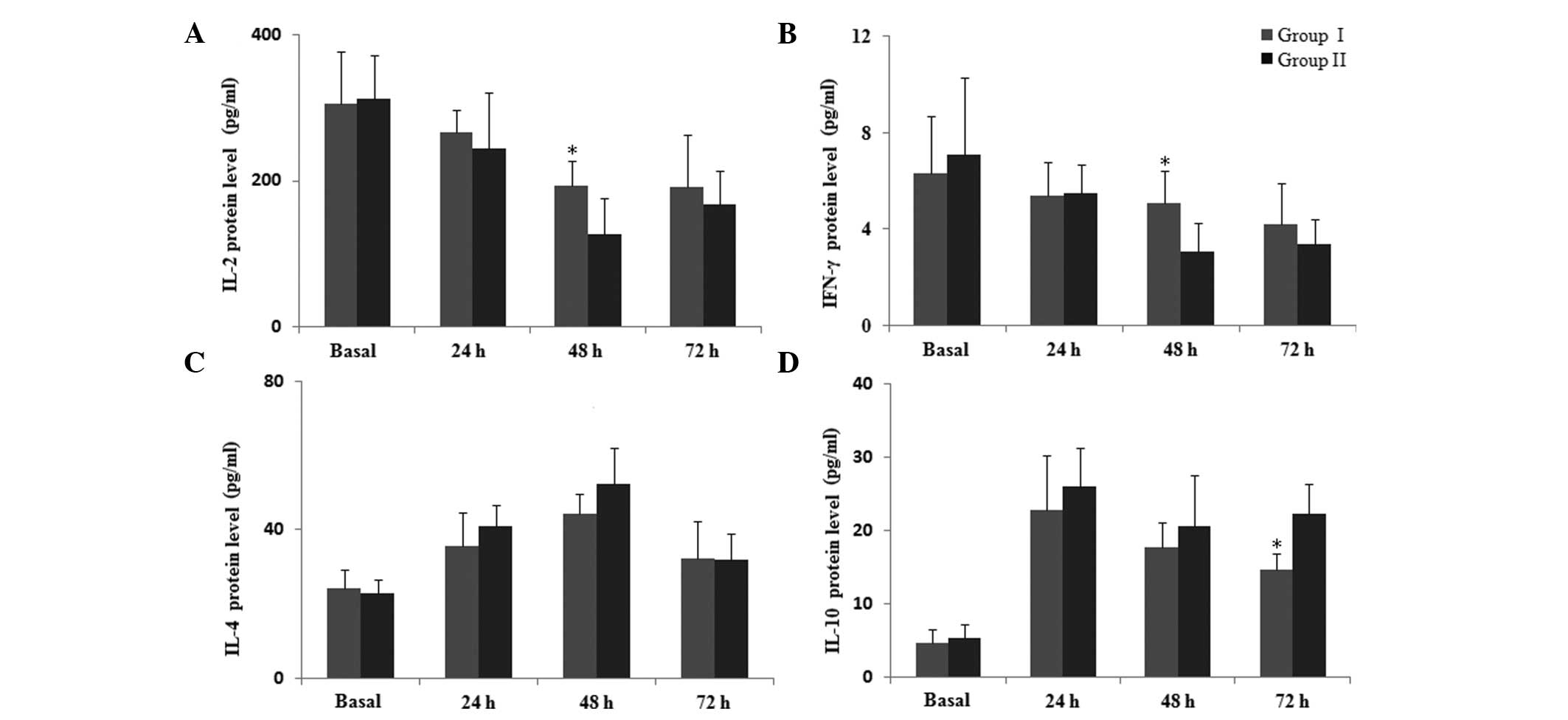
The effects of parecoxib on the protein levels of
Th1, Th2, Th17 and Treg cytokines in peripheral blood were also
evaluated. Similar to the qPCR result, ELISA analysis indicated a
reduction in the protein levels of IL-2 and IFN-γ following
surgery, and this reduction was attenuated at 48 h post-surgery in
group I compared with group II (P=0.045; Fig. 4A and B). The protein concentration
of IL-4 and IL-10 increased following surgery (Fig. 4C and D), and IL-10 production in
group I was significantly lower at 72 h subsequent to surgery
compared with the levels in group II, (P<0.05), while no
difference was identified in the protein level of IL-4 between the
two groups (P>0.05). This suggests that parecoxib may be an
effective agent for restoring perioperative immune competence.
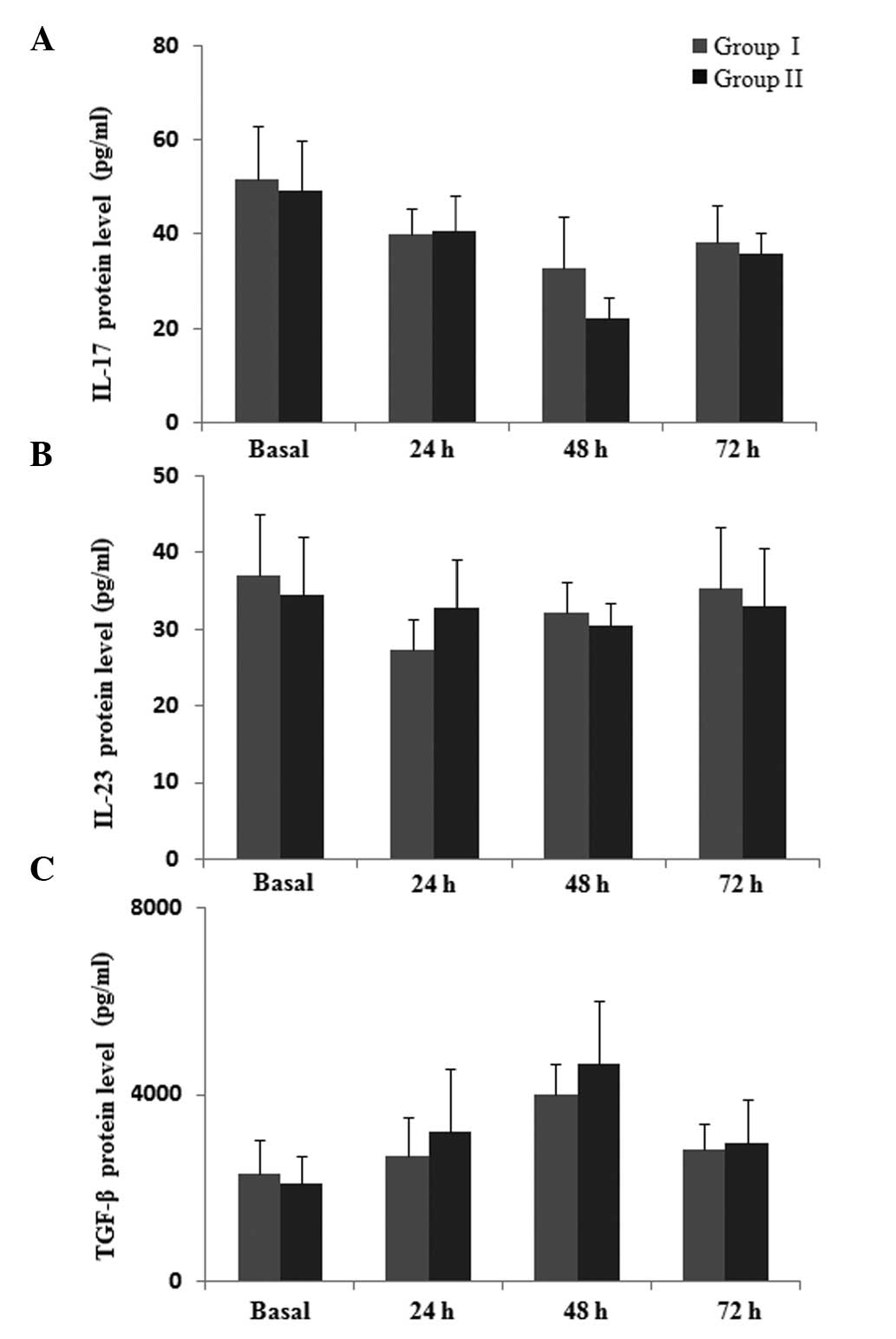
As indicated by Fig.
5, the protein level of IL-17 was slightly higher at 48 h in
group I compared with group II, but the difference was not
significant (P>0.05). There was no significant difference in
levels of IL-23 during the postoperative 72-h observation period
between the two groups (P>0.05). It was observed that the
protein level of TGF-β increased subsequent to surgery, however, no
significant difference was identified between the two groups
(P>0.05). Collectively, these results suggest that parecoxib is
able to attenuate the postoperative immune suppression by
regulating the expression of Th1, Th2, Th17 and Treg cytokines at
the protein level.
Discussion
The principal findings of the present study
demonstrated that the mRNA and protein levels of Th1, Th2, Th17 and
Treg cytokines were imbalanced following laparoscopy in the
peripheral blood of patients with cervical cancer, with reduced
production of IL-2, IFN-γ and IL-17, and an increased expression
level of IL-4, IL-10 and TGF-β. Perioperative multiple-dose
parecoxib markedly enhanced the expression of IL-2, IFN-γ and
IL-17, and suppressed the reduction of IL-4, IL-10 and TGF-β, which
contributed to restore the balance of Th1, Th2, Th17 and Treg
cytokines, and attenuated surgery-related immune suppression. The
findings of the present study also revealed that multiple-dose
parecoxib exerted an analgesic effect and resulted in lower VAS
scores and pain incidence. These observations indicate that
parecoxib, in addition to postoperative pain relief, may ameliorate
surgery-induced immune suppression by balancing the expression of
Th1, Th2, Th17 and Treg cytokines following laparoscopy in patients
with cervical cancer.
Early postoperative pain is the most common
complaint following gynecological laparoscopy. It is a
multifactorial and complex problem, and includes visceral, parietal
and shoulder pain components due to various pain mechanisms
(25). Systematic reviews have
suggested that COX-2 inhibitors are an effective treatment for
acute postprocedural pain (26–28).
In the current study, patients experienced mild-to-moderate
postoperative pain during the 72-h observation period, and the
overall incidences of visceral, parietal and shoulder pain were
58.3, 65.3 and 25.0%, respectively. The data indicated that
administration of multi-dose parecoxib (40 mg every 12 h) relieved
postoperative pain intensity and incidence, and reduced the
requirement for analgesics. These observations are in accordance
with results of other studies that indicated that 40 mg parecoxib
twice daily ameliorated postlaparoscopic pain, and attenuated the
incidence of visceral and shoulder pain with a reduced requirement
for analgesics following gynecological laparoscopy (29,30).
In contrast to the traditional opioid drugs, parecoxib exerts its
analgesic effect without opioid-induced hyperalgesia. It reduces
pain through the inhibition of the COX-2 isoenzyme, which is
important in the synthesis of PGE2 and the prevention of the
wind-up phenomenon of the CNS. In addition, parecoxib was
well-tolerated, as platelets do not contain COX-2 and all synthesis
of thromboxane A2 in the platelet is mediated by COX-1. It was
demonstrated in the current study that parecoxib reduced
postoperative nausea/vomiting and infections, and did not increase
gastrointestinal bleeding and cardiovascular-related adverse
effects. These findings indicated that multiple-dose parecoxib
appears to be effective and safe for use in pain control for
patients with cancer following laparoscopic surgery.
Increasing evidence indicates that patients with
cervical cancer exhibit an imbalance in the functions of Th1 and
Th2 cells, with a Th2-predominant response mode (29), and the Th17/Treg balance has been
noted to be disrupted in peripheral blood from patients with
cervical cancer (13,32,33).
In the present study, in order to improve understanding of the
underlying mechanisms of postoperative immune suppression,
alterations in the levels of Th1, Th2, Th17 and Treg cytokines
following gynecological laparoscopy were observed in patients with
cervical cancer. Previous studies have reported that surgical
trauma induced a Th1/Th2 balance shift in the form of diminished
IL-2 and IFN-γ and increased IL-4 and IL-10 levels, which result in
weakened immunity (13,34). However, there is limited
information on alterations of Th1, Th2, Th17 and Treg cytokine
levels subsequent to gynecological laparoscopy in patients with
cervical cancer. In the current study, the results demonstrated
that the production levels of IL-2, IFN-γ, IL-17 and IL-23 were
markedly reduced following surgery, and the levels of IL-4, IL-10
and TGF-β were significantly increased, implicating surgical trauma
in postoperative immune depression, acting by disrupting the
balance of Th1, Th2, Th17 and Treg cytokines. These data are
consistent with a study by Visser et al (35), which demonstrated that the
increased expression of IL-10 and TGF-β, and frequencies of Treg
cells, influenced the balance between Th17/Treg cells, and promoted
the development of immune suppression, implying that the imbalance
of Th1, Th2, Th17 and Treg cytokines may be involved in the
development of postoperative immune impairment. It provided the
understanding that the imbalance of Th1, Th2, Th17 and Treg
cytokines may be one of the mechanisms leading to postoperative
immune suppression.
A study by Cheng et al (36) indicated that patients with cancer
undergo a series of neuroendocrine and physiological changes in the
body when suffering surgical trauma, stress, postoperative pain and
other external noxious stimulation. Additionally, there is a close
association and mutual influence between neuroendocrine and immune
systems (36). Pain stimulation
subsequent to surgery can directly transmit to the CNS and thus
stimulate the neuro-immune endocrine system. The simultaneous
increase of endogenous catecholamine levels such as corticosteroids
and prostaglandins as a result of the stress response is able to
suppress cellular immune function and increase the tumor cell
transfer probability in the perioperative period (37,38).
Therefore, effective analgesia in the perioperative period is able
to greatly affect the immune system. Other studies suggested that
the cyclooxygenase-2 (COX-2)/PGE2 pathway may be critical in the
analgesic and immune inflammatory response (39,40).
Much remains elusive regarding the effects of parecoxib on immune
function following gynecological laparoscopy in patients with
cervical cancer. In the current study, results indicated that
multi-dose parecoxib can effectively enhance the production of
IL-2, IFN-γ and IL-17, and inhibit the expression of IL-4, IL-10
and TGF-β in patients with cervical cancer. These findings are
consistent with those of a previous study, in which parecoxib
reduced postoperative pain (41),
and another in which it inhibited hippocampal IL-1β and TNF-α
expression through downregulation of the COX-2/PGE2 pathway
(40). A number of studies have
provided data supporting the hypothesis that parecoxib has the
ability to enhance the generation of host immunity, by increasing
the activity of natural killer cells and plasma IFN-γ levels
(42,43). These data indicated that parecoxib
exerts protective effects on postoperative immune function by
regulating the balance of Th1, Th2, Th17 and Treg cytokines.
Another study indicated that the immune status of
the patient may significantly influence the progression and
clinical outcome of the cancer. In the present study, the
attenuation of postoperative immune suppression by administration
of parecoxib may have contributed to the improved postoperative
course with reduced postoperative nausea/vomiting and infections.
Collectively, the results of the current study further demonstrate
that parecoxib can improve the postoperative course, and exert
immunomodulatory effects by balancing the mRNA and protein
expression levels of Th1, Th2, Th17 and Treg cytokines in the
peripheral blood of patients with cervical cancer.
All operations in the present study were performed
without blood transfusion in order to avoid the presence of another
factor that may increase the surgical inflammatory response. The
difference in the immune factors between the two groups is
described at the end-point of treatment with the selected
analgesic. In the present study, perioperative plasma IL-23
concentration was indicated to be unchanged compared with the
pre-operative values, and was unaffected by administration of
multi-dose parecoxib, although previous studies have observed
increases in IL-23 concentration following cataract surgery
(44). However, failure to detect
a change in the production of circulating IL-23 at the specific
time points does not rule out the possibility that this factor may
be released at an earlier time point. This finding may reflect the
complex nature of cytokine homeostasis and the potential regulation
of the interactions between stress hormones and the immune
system.
There were several limitations of the current study.
The effects of parecoxib alone were assessed; but comparison of
parecoxib and other NSAIDs, such as celecoxib or frubiproten, may
aid in the understanding of the specific role of parecoxib in
postoperative immune function. Also, the effects were observed for
just 72 h subsequent to surgery, as the majority of patients did
not request analgesics after this time. A prolonged assessment is
required to clarify the long-term immunoregulatory effects of
parecoxib. Furthermore, the use of tramadol for the relief of
postoperative pain may have intervened in the assessment of
postoperative immune parameters. A previous study reported that
intravenous administration of 100 mg tramadol did not influence the
immune function following laparoscopy (45). Therefore, in order to be clinically
useful, additional studies are required to further elucidate the
immunomodulatory effects of parecoxib on the percentages of
CD4+ T cell subsets, including Th1, Th2, Th17 and Treg
cells.
In conclusion, the current results demonstrated that
perioperative multi-dose parecoxib, in addition to its analgesic
effect, may ameliorate postoperative immune suppression and improve
the clinical course by balancing the expression of Th1, Th2, Th17
and Treg cytokines in the peripheral blood of patients with
cervical cancer following laparoscopy. These findings may
contribute to an alternative therapeutic regime in the management
of postoperative pain and immune responses of patients with
cancer.
Acknowledgements
The authors express their gratitude to Dr Baozhang
Ma (Department of TCM Gynecology, Heilongjiang University of
Chinese Medicine) for his useful comments and criticism; and also
thank the staff and patients that participated in the present
study.
Abbreviations:
|
VAS
|
visual analog scale
|
|
COX-2
|
cyclooxygenase-2
|
|
NSAIDs
|
non-steroidal anti-inflammatory
drugs
|
|
PGE-2
|
prostaglandin E-2
|
|
CNS
|
central nervous system
|
References
|
1
|
Micheli DC, Fernandes PC Jr, Cruvinel JC,
Nomelini ID, Murta EF and Tavares-Murta BM: Circulating cytokines
and nitric oxide are involved in the inhibition of neutrophil
migration in patients with uterine cervical neoplasia. Clin Med
Insights Oncol. 6:233–242. 2012.PubMed/NCBI
|
|
2
|
Kosmaczewska A, Bocko D, Ciszak L, et al:
Dysregulated expression of both the costimulatory CD28 and
inhibitory CTLA-4 molecules in PB T cells of advanced cervical
cancer patients suggests systemic immunosuppression related to
disease progression. Pathol Oncol Res. 18:479–489. 2012. View Article : Google Scholar :
|
|
3
|
Holub Z: Impact of laparoscopic surgery on
immune function. Clin Exp Obstret Gynecol. 29:77–81. 2002.
|
|
4
|
Ling Y, Chen J, Tao M, Chu X and Zhang X:
A pilot study of nimotuzumab combined with cisplatin and 5-FU in
patients with advanced esophageal squamous cell carcinoma. J Thorac
Dis. 4:58–62. 2012.PubMed/NCBI
|
|
5
|
Sah BK, Chen MM, Yan M and Zhu ZG:
Reoperation for early postoperative complications after gastric
cancer surgery in a Chinese hospital. World J Gastroenterol.
16:98–103. 2010.
|
|
6
|
Salo M: Effects of anaesthesia and surgery
on the immune response. Acta Anaesthesiol Scand. 36:201–220. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bürkle A, Caselli G, Franceschi C, et al:
Pathophysiology of ageing, longevity and age related diseases.
Immun Ageing. 4:42007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hou CY, Li XL, Jiang F, Gong RJ, Guo XY
and Yao YQ: Comparative evaluation of surgical stress of
laparoscopically assisted vaginal radical hysterectomy and
lymphadenectomy and laparotomy for early-stage cervical cancer.
Oncol Lett. 2:747–752. 2011.
|
|
9
|
Yang X, Qian F, He HY, et al: Effect of
thymosin alpha-1 on subpopulations of Th1, Th2, Th17, and
regulatory T cells (Tregs) in vitro. Braz J Med Biol Res. 45:25–32.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hildesheim A, Schiffman MH, Tsukui T, et
al: Immune activation in cervical neoplasia: cross-sectional
association between plasma soluble interleukin 2 receptor levels
and disease. Cancer Epidemiol Biomarkers Prev. 6:807–813.
1997.PubMed/NCBI
|
|
11
|
Park H, Li Z, Yang XO, et al: A distinct
lineage of CD4 T cells regulates tissue inflammation by producing
interleukin 17. Nat Immunol. 6:1133–1141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stockinger B and Veldhoen M:
Differentiation and function of Th17 T cells. Curr Opin Immunol.
19:281–286. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen Z, Ding J, Pang N, et al: The
Th17/Treg balance and the expression of related cytokines in Uygur
cervical cancer patients. Diagn Pathol. 8:612013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Page GG: Surgery-induced immunosuppression
and postoperative pain management. AACN Clin Issues. 16:302–309.
416–418. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ling Y, Chen J, Tao M, Chu X and Zhang X:
A pilot study of nimotuzumab combined with cisplatin and 5-FU in
patients with advanced esophageal squamous cell carcinoma. J Thorac
Dis. 4:58–62. 2012.PubMed/NCBI
|
|
16
|
Dembo G, Park SB and Kharasch ED: Central
nervous system concentrations of cyclooxygenase-2 inhibitors in
humans. Anesthesiology. 102:409–415. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mehta V, Johnston A, Cheung R, Bello A and
Langford RM: Intravenous parecoxib rapidly leads to COX-2
inhibitory concentration of valdecoxib in the central nervous
system. Clin Pharmacol Ther. 83:430–435. 2008. View Article : Google Scholar
|
|
18
|
Koppert W, Wehrfritz A, Körber N, Sittl R,
Albrecht S, Schüttler J and Schmelz M: The cyclooxygenase isozyme
inhibitors parecoxib and paracetamol reduce central hyperalgesia in
humans. Pain. 108:148–153. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheer SM and Goa KL: Parecoxib (parecoxib
sodium). Drugs. 61:1133–1143. 2011. View Article : Google Scholar
|
|
20
|
Eberstål S, Badn W, Fritzell S,
Esbjörnsson M, Darabi A, Visse E and Siesjö P: Inhibition of
cyclooxygenase-2 enhances immunotherapy against experimental brain
tumors. Cancer Immunol Immunother. 61:1191–1199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pandazi A, Kapota E, Matsota P,
Paraskevopoulou P, Dervenis C and Kostopanagiotou G: Preincisional
versus postincisional administration of parecoxib in colorectal
surgery: effect on postoperative pain control and cytokine
response. A randomized clinical trial. World J Surg. 34:2463–2469.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peng M, Wang YL, Wang FF, Chen C and Wang
CY: The cyclooxygenase-2 inhibitor parecoxib inhibits
surgery-induced proinflammatory cytokine expression in the
hippocampus in aged rats. J Surg Res. 178:e1–e8. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li G, Yan X, Shang H, Wang G, Chen L and
Han Y: A comparison of laparoscopic radical hysterectomy and pelvic
lymphadenectomy and laparotomy in the treatment of Ib-IIa cervical
cancer. Gynecol Oncol. 105:176–180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Clapcich AJ, Emerson RG, Roye DP Jr, Xie
H, Gallo EJ, Dowling KC, Ramnath B and Heyer EJ: The effects of
propofol, small-dose isoflurane, and nitrous oxide on cortical
somatosensory evoked potential and bispectral index monitoring in
adolescents undergoing spinal fusion. Anesth Analg. 99:1334–1340.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jabbour-Khoury SI, Dabbous AS, Gerges FJ,
Azar MS, Ayoub CM and Khoury GS: Intraperitoneal and intravenous
routes for pain relief in laparoscopic cholecystectomy. JSLS.
9:316–321. 2005.PubMed/NCBI
|
|
26
|
Chen LC, Elliott RA and Ashcroft DM:
Systematic review of the analgesic efficacy and tolerability of
COX-2 inhibitors in post-operative pain control. J Clin Pharm Ther.
29:215–229. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Straube S, Derry S, McQuay HJ and Moore
RA: Effect of preoperative Cox-II-selective NSAIDs (coxibs) on
postoperative outcomes: a systematic review of randomized studies.
Acta Anaesthesiol Scand. 49:601–613. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Desjardins PJ, Grossman EH, Kuss ME,
Talwalker S, Dhadda S, Baum D and Hubbard RC: The injectable
cyclooxygenase-2-specific inhibitor parecoxib sodium has analgesic
efficacy when administered preoperatively. Anesth Analg.
93:721–727. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang H, Shu H, Yang L, et al: Multiple-,
but not single-, dose of parecoxib reduces shoulder pain after
gynecologic laparoscopy. Int J Med Sci. 9:757–765. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Barton SF, Langeland FF, Snabes MC,
LeComte D, Kuss ME, Dhadda SS and Hubbard RC: Efficacy and safety
of intravenous parecoxib sodium in relieving acute postoperative
pain following gynecologic laparotomy surgery. Anesthesiology.
97:306–314. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Warrino DE, Olson WC, Knapp WT, et al:
Disease-stage variance in functional CD4(+) T-cell responses
against novel pan-human leukocyte antigen-D region presented human
papillomavirus-16 E7 epitopes. Clin Cancer Res. 10:3301–3308. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sheu BC, Lin RH, Lien HC, Ho HN, Hsu SM
and Huang SC: Predominant Th2/Tc2 polarity of tumor-infiltrating
lymphocytes in human cervical cancer. J Immunol. 167:2972–2978.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rao PE, Petrone AL and Ponath PD:
Differentiation and expansion of T cells with regulatory function
from human peripheral lymphocytes by stimulation in the presence of
TGF-beta. J Immunol. 174:1446–1455. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Navarro-Zorraquino M, García-Alvarez F,
Martínez-Fernández AR, Pastor C, Larrad L, Salinas JC and Lozano R:
Pharmacological immunomodulation of surgical trauma. J Invest Surg.
20:283–289. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Visser J, Nijman HW, Hoogenboom BN, et al:
Frequencies and role of regulatory T cells in patients with
(pre)malignant cervical neoplasia. Clin Exp Immunol. 150:199–209.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cheng YC, Cheng XB, Li XJ, Wang FZ and Li
ZK: Combined general and regional anesthesia and effects on immune
function in patients with benign ovarian tumors treated by
laparoscopic therapy. Int J Clin Exp Med. 6:716–719.
2013.PubMed/NCBI
|
|
37
|
Hunter JD: Effects of anaesthesia on the
human immune system. Hosp Med. 60:658–663. 1999. View Article : Google Scholar
|
|
38
|
Engers R, Mueller M, Walter A, Collard JG,
Willers R and Gabbert HE: Prognostic relevance of Tiam1 protein
expression in prostate carcinomas. Br J Cancer. 95:1081–1086. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gehling M, Arndt C, Eberhart LH, Koch T,
Krüger T and Wulf H: Postoperative analgesia with parecoxib,
acetaminophen, and the combination of both: a randomized,
double-blind, placebo-controlled trial in patients undergoing
thyroid surgery. Br J Anaesth. 104:761–767. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ang SF, Sio SW, Moochhala SM, MacAry PA
and Bhatia M: Hydrogen sulfide upregulates cyclooxygenase-2 and
prostaglandin E metabolite in sepsis-evoked acute lung injury via
transient receptor potential vanilloid type 1 channel activation. J
Immunol. 187:4778–4787. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bao Y, Fang J, Peng L, Yi Y, Liu K, Li W
and Luo H: Comparison of preincisional and postincisional parecoxib
administration on postoperative pain control and cytokine response
after total hip replacement. J Int Med Res. 40:1804–1811. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sharma S, Zhu L, Yang SC, et al:
Cyclooxygenase 2 inhibition promotes IFN-gamma-dependent
enhancement of antitumor responses. J Immunol. 175:813–819. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kundu N, Walser TC, Ma X and Fulton AM:
Cyclooxygenase inhibitors modulate NK activities that control
metastatic disease. Cancer Immunol Immunother. 54:981–987. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jiang S, Liu X, Luo L, et al: Serum levels
of Th17-related cytokines in Behcet disease patients after cataract
surgery. Mol Vis. 17:1425–1430. 2011.PubMed/NCBI
|
|
45
|
Sacerdote P, Bianchi M, Gaspani L, et al:
The effects of tramadol and morphine on immune responses and pain
after surgery in cancer patients. Anesth Analg. 90:1411–1414. 2000.
View Article : Google Scholar : PubMed/NCBI
|