Introduction
Gastric cancer, also known as stomach cancer, has a
high incidence rate in and is one of the most prominent causes of
cancer-associated mortality in Asia (1). In addition, gastric cancer is the
fourth most prevalent type of cancer worldwide and the second most
frequent cause of cancer-associated mortality worldwide (2,3). The
prognosis of gastric cancer patients is poor and it remains
challenging to cure. Targeted gene therapy is a novel therapeutic
approach for gastric cancer, for which the identification of tumor
suppressors associated with this malignancy is essential.
Polo-like kinase 2 (PLK2) is a member of the
serine/threonine protein kinase family, which includes five
members: PLK1, PLK2 (also termed SNK), PLK3 (also termed Fnk or
Prk), PLK4 (also termed Sak) and PLK5 (4–6).
These kinases have important roles during mitosis and the
centrosome cycle (7). PLK2 is
located in the centrosome and was found to be involved in embryonic
development and cell cycle progression at the G1/S
transition, as well as in skeletal development. Cell cycle analysis
of cultured PLK2−/− embryonic fibroblasts indicated that
these cells proliferated more slowly than cells expressing PLK2 and
exhibited delayed entry into S phase from G1 (8). In central neurons, PLK2 was reported
to be overexpressed in response to synaptic stimulation (9). Decreased expression of PLK2 was
observed in B-cell malignancies; in addition, apoptosis was found
to be induced in Burkitt’s lymphoma cells through the ectopic
expression of PLK2 (10). This
therefore indicated that PLK2 may act as a tumor suppressor gene in
hematologic malignancies (10,11).
Pellegrino et al (12)
showed that PLK2 was a tumor suppressor in hepatocarcinogenesis. In
addition, Kothari et al (13) reported that PLK2 is an outlier
kinase, which was highly expressed in KRAS-dependent pancreatic
cancer cells. The MIA-PaCa-2 pancreatic cancer cell line was used
to assess the effects of small interfering (si)RNA silencing of
PLK2 on cell proliferation; the results showed significant growth
inhibition (13), suggesting that
PLK2 may have an oncogenic role in pancreatic cancer and therefore
may have comparable effects in different types of cancer. However,
the expression and function of PLK2 in gastric cancer remains to be
elucidated. In the present study, siRNA mediated knock-down of PLK2
in SGC-7901 gastric cancer cells was used to examine the expression
and activity of PLK2 in gastric cancer.
Materials and methods
Collection of clinical samples
A total of 24 gastric cancer tissue samples and
adjacent normal tissues were collected from patients undergoing
surgery for gastric cancer at the First and Second Affiliated
Hospitals of the Medical College of Xi’an Jiaotong University
(Shaanxi, China). Samples were placed in liquid nitrogen as soon as
they were obtained and then stored at −80°C until RNA extraction.
The study was approved by the ethics committee of Xi’an Jiatong
University Health Science Centre (Xi’an, China). Written informed
consent was obtained from all patients or their families.
Cell culture and transfection
GES-1 human fetal gastric epithelial cells and
SGC-7901, BGC-823, AGS and MKN-45 gastric cancer cells were
obtained from The Central Laboratory of Biomedical Research of the
Medical College of Xi’an Jiaotong University. Cells were cultured
in Dulbecco’s modified Eagle’s medium (DMEM; Gibco-BRL, Carlsbad,
CA, USA) with 10% fetal bovine serum (FBS; Gibco-BRL), 100 U/ml
penicillin and 100 μg/ml streptomycin (Invitrogen Life
Technologies, Carlsbad, CA, USA), and grown in a 37°C incubator
with 5% CO2. siRNAs targeting PLK2
(5′-GAGCAGCUGAGCACAUCAUDTDT-3′) and the silencer negative controls
(siN05815122147) were purchased from Guangzhou RiboBio Co, Ltd
(Guangzhou, China). Lipofectamine® 2000 (Invitrogen Life
Technologies) was used to transfect PLK2 siRNA and control siRNA
into SGC-7901 cells at a final concentration of 50 nM in all
experiments, according to the manufacturer’s instructions. Cells
were then harvested 24 h post-transfection. The expression of PLK2
mRNA was detected in the SGC-7901, BGC-823, AGS and MKN-45 gastric
cancer cells lines and the control GES-1 cell line, whereas in
subsequent experiments only the SGC-7901 cell line was
analyzed.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analyses
RNA was extracted using TRIzol®
(Invitrogen Life Technologies) according to the manufacturer’s
instructions. Total RNA was used to generate cDNA using a
PrimeScript RT reagent kit (Takara Bio. Inc., Shiga, Japan). The
program used for reverse transcription was as follows: 37°C for 15
min, 85°C for 5 sec. Then the cDNA was used as a template for qPCR.
PLK2 expression in gastric cancer samples and gastric cancer cells
was assessed using qPCR with SYBR premix Ex TaqTM II
(Takara) on a Roche 480 Light cycler (Roche Diagnostics GmbH,
Basel, Switzerland) according to the manufacturer’s instructions.
Primer sequences (Primer Premier 5.0; Premier Biosoft, Palo Alto,
CA, USA) were as follows: Forward: 5′-AATAACTCAGCAACCCAGCAAAC-3′
and reverse: 5′-GTGACCCACTGAAATGATGTGC-3′ for PLK2; and forward:
5′-GAAGGTGAAGGTCGGAGTC-3′ and reverse: 5′-GAAGATGGTGATGGGATTTC-3′
for GAPDH, as used by Radonića et al (14). The PCR program used for
amplification was as follows: 94°C for 3 min, followed by 40 cycles
of 94°C for 20 sec, 60°C for 40 sec and 72°C for 40 sec. qPCR
results were analyzed using the 2−ΔΔCt method.
MTT assay
A cell proliferation assay, using thiazolyl blue
(Sigma-Aldrich, St. Louis, MO, USA) was performed in 96-well
culture plates. A total of 5×104 cells/ml were seeded
into each well and grown at 37°C in 5% CO2 for 3 days.
The growth medium was replaced with serum-free medium prior to
transfection. MTT solution (5 mg/ml; Sigma-Aldrich) was added to
each well at 24, 48 and 72 h and cells were then incubated at 37°C
for 3 h. Following removal of the liquid supernatant, 0.2 ml
dimethyl sulfoxide (Sigma-Aldrich) was added to each well. Optical
density was determined at 490 nm using a FLUOstar/POLARstar OPTIMA
(BMG LABTECH GmbH, Ortenberg, Germany). Data represent the results
of experiments performed at least in triplicate.
Cell cycle analysis
A total of 1×105 cells/well were plated
onto six-well plates and transfected with PLK2 siRNA and control
siRNA. Cells were harvested at 24 h post-transfection, washed with
cold phosphate-buffered saline (PBS) and fixed in ice-cold 70%
alcohol at 4°C overnight. Fixed cells were washed twice in PBS,
then resuspended in 0.5 ml PBS containing propidium iodide (PI; 50
μg/ml) and RNase A (200 μg/ml; Amresco LLC, Solon, OH, USA) for 30
min at room temperature in the dark. Cell cycle analysis was then
performed using a FACSArray (Becton Dickinson, Franklin Lakes, NJ,
USA).
Cell apoptosis analysis
A total of 1×105 cells/well were plated
onto a six-well plate and transfected with PLK2 siRNA and control
siRNA. Cells were harvested at 24 h post-transfection, washed twice
with PBS and then resuspended in 0.5 ml buffer solution (Roche
Diagnostics, Shanghai, China) containing PI and Annexin V (Roche
Diagnostics) at a final concentration of 1 μg/ml, and incubated for
30 min at room temperature in the dark. A FACSArray (Becton
Dickinson) was then used to calculate cell apoptosis rates.
Western blot analysis
Cells (1×105/well) were plated onto a
six-well plate and transfected with PLK2 siRNA and control siRNA.
Cells were then harvested at 24 h post-transfection, washed with
PBS and lysed using radioimmunoprecipitation assay lysis buffer
(Beyotime Institute of Biotechnology, Shanghai, China). Proteins
were separated on 10% SDS polyacrylamide gels and transferred onto
polyvinylidene fluoride (PVDF) membranes (Roche Diagnostics GmbH)
through electroblotting. Membranes were blocked with 5% non-fat
milk and probed with primary antibodies against human PLK2
(Snk/H90, sc-25421; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), GAPDH (6C5, sc-32233; Santa Cruz Biotechnology, Inc.), Bax
(sc-493; ZSGB-BIO, Beijing, China), caspase-3 (sc-65497; ZSGB-BIO),
CDK2 (ab2363; Abcam, Cambridge, MA, USA), and β-actin (KC-5A08;
KangChen Bio-tech, Shanghai, China) (Table I). The membranes were further
probed with horseradish peroxidase-conjugated rabbit anti-mouse and
goat anti-rabbit secondary antibodies (ZDR-5109 and ZDR-5118; 1:50;
ZSGB-BIO). Working solutions of the Enhanced Chemiluminescence
Substrate (Pierce Biotechnology, Inc., Rockford, IL, USA) were
prepared and added to PVDF membranes for 1 min. The membranes were
then removed from the substrates and exposed to ChemiDoc-It 510
(UVP, LLC, Upland, CA, USA).
 | Table IAntibody details. |
Table I
Antibody details.
| Gene | Catalog number | Animal raised in | Animal raised
against | Dilution | Mono/polyclonal |
|---|
| PLK2 | sc-25421 | rabbit | human | 1:500 | polyclonal |
| GAPDH | sc-32233 | mouse | human | 1:1000 | monoclonal |
| Bax | sc-493 | rabbit | human | 1:50 | polyclonal |
| CDK2 | ab2363 | mouse | human | 1:100 | monoclonal |
| caspase 3 | sc-65497 | mouse | human | 1:500 | monoclonal |
| β-actin | KC-5A08 | mouse | human | 1:5000 | monoclonal |
Statistical analysis
Statistical analysis was performed using SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). P-values were calculated
using a one-way analysis of variance. P<0.05 was considered to
indicate a statistically significant difference between values.
Results
Expression of PLK2 mRNA in gastric cancer
samples and gastric cancer cells
A total of 24 matched gastric cancer samples and
adjacent normal tissues were collected from patients with gastric
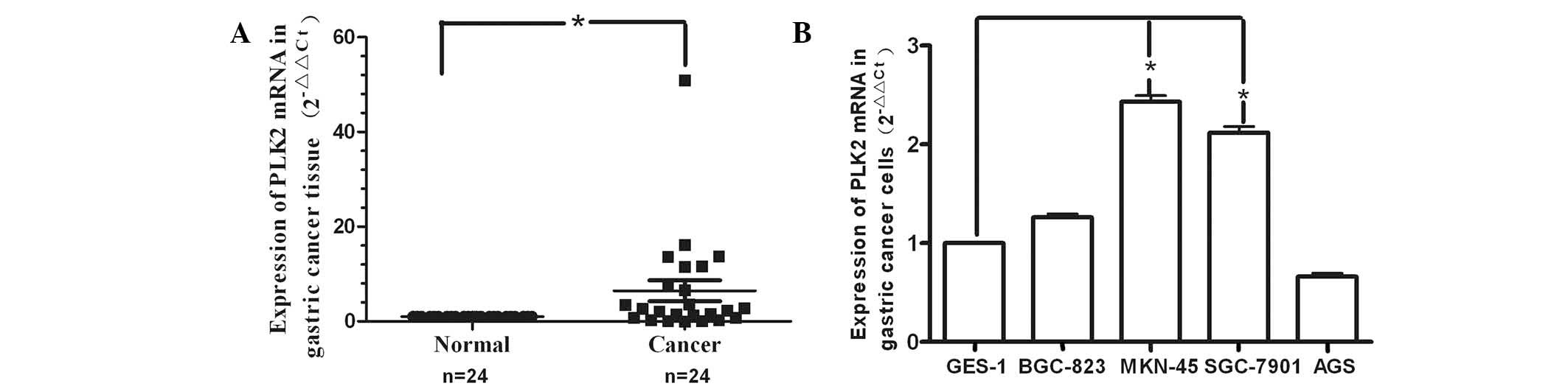
cancer. As shown in Fig. 1A,
RT-qPCR revealed that PLK2 expression was significantly increased
in 17 of the 24 tumor samples compared with that of the
corresponding normal tissue. The expression levels of PLK2 varied
among the 24 gastric cancer patients; however, a general trend
towards increased expression in gastric cancer tissues was
observed. In BGC-823, MKN-45 and SGC-7901 gastric cancer cell lines
(Fig. 1B), PLK2 mRNA expression
was increased to varying degrees compared with that of the GES-1
cells; however, only MKN-45 and SGC-7901 cells showed a significant
increase. By contrast, PLK2 expression was downregulated in AGS
cells.
PLK2 interference effect
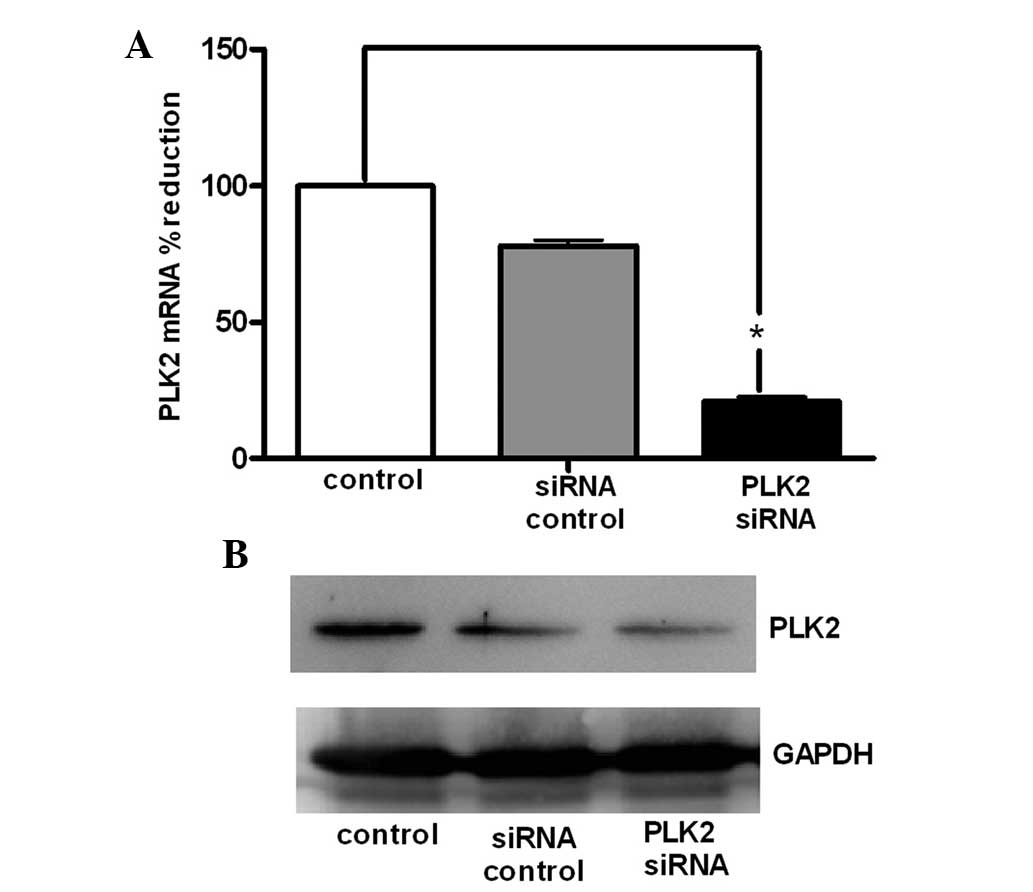
Following transfection of SGC-7901 cells with PLK2
siRNA for 24 h, the cells were collected and RNA and protein were
extracted. As shown in Fig. 2A,
compared with the untransfected and control siRNA control groups,
PLK2 mRNA expression was significantly decreased in the PLK2 siRNA
group. In addition, the results of the western blot analysis were
consistent with those of the RT-qPCR, therefore demonstrating that
PLK2 protein levels were reduced in the PLK2 siRNA group (Fig. 2B).
PLK2 siRNA promotes the growth of
SGC-7901 cells
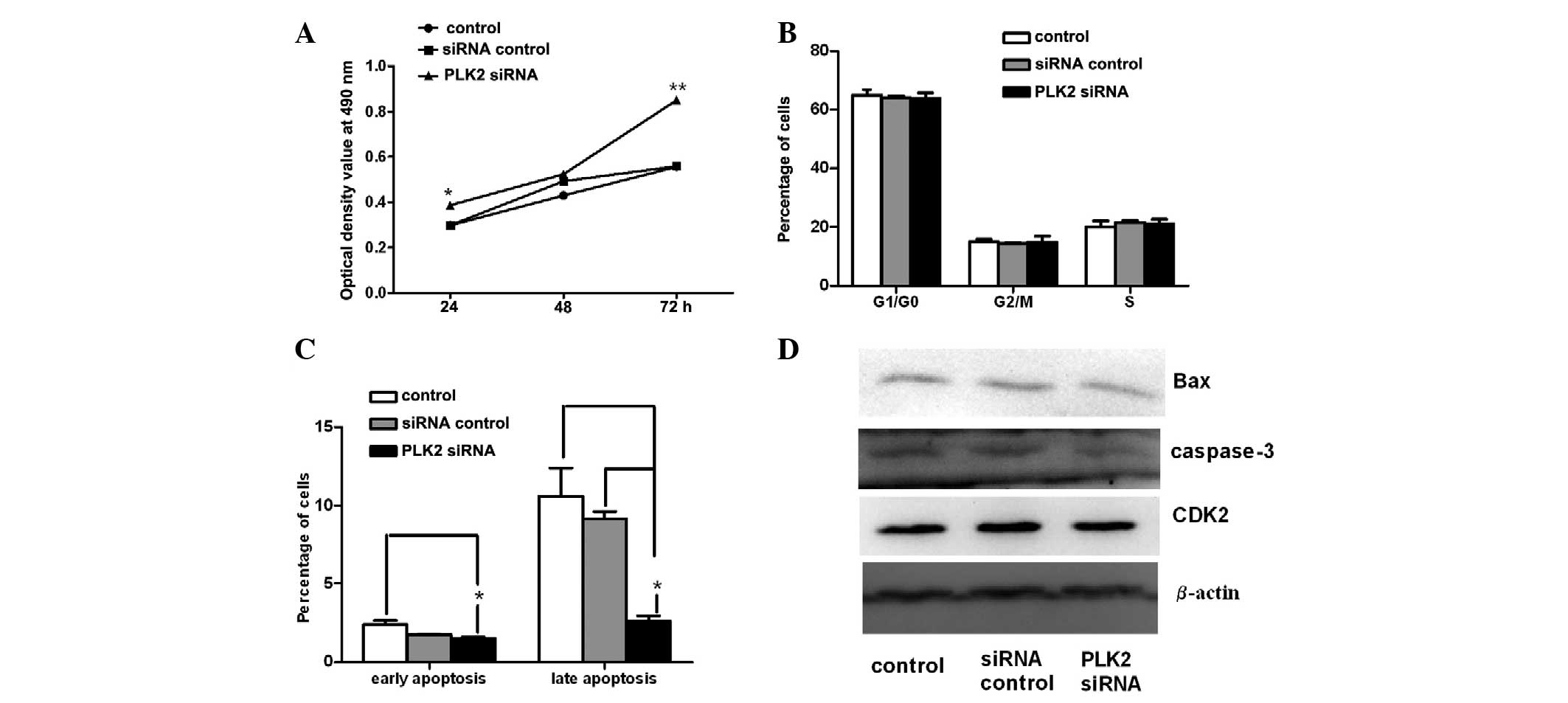
An MTT assay was used to examine the effects of PLK2
siRNA on SGC-7901 cell viability. The growth of SGC-7901 cells was
detected for three days following transfection with PLK2 siRNA. As
shown in Fig. 3A, compared with
the untransfected and siRNA control groups, PLK2 siRNA
significantly promoted the growth of SGC-7901 cells at 24 and 72 h.
This result was consistent with the findings previously reported by
Li et al (15), which
demonstrated that the viability of granulosa cells increased
significantly following PLK2 siRNA treatment and suggested that
PLK2 expression blocks granulosa cell proliferation (15). Pellegrino et al (12) also showed that PLK2 inactivation
led to increased cell viability.
PLK2 siRNA has no effect on SGC-7901 cell
cycle progression
PLK2 is activated close to the G1-to-S phase
transition of the cell cycle (16); therefore, the effect of PLK2
silencing on cell cycle progression was investigated in the present
study. Cell cycle analysis was performed 24 h following treatment
with PLK2 siRNA (Fig. 3B);
however, no significant changes in the ratio of cells at each stage
of the cell cycle were observed among groups at 24 h. These results
differ from those reported by Li et al (15), which showed that PLK2 siRNA reduced
the percentage of cells in the G0/G1 phase as well as increased the
percentage of cells in the G2 phase and S phase. Ma et al
(8) investigated how cell cycle
progression was affected by PLK2 deletion, the results of which
showed that FACS analysis of DNA content revealed a higher number
of PLK2+/− cells than PLK2−/− cells in S
phase. These data suggested that PLK2 may influence G1 progression;
however, unlike PLK1, it is not required for cell division.
PLK2 siRNA decreases apoptosis of
SGC-7901 cells
The results of the Annexin-V/PI assay showed that
the apoptosis of SGC-7901 cells decreased significantly following
PLK2 siRNA treatment for 24 h (Fig.
3C). The percentage of early apoptotic cells in the PLK2 siRNA
group was 1.52%, which was slightly lower than that of the other
two groups at 24 h; however, this was only significantly lower than
the untreated control. By contrast, the percentage of late
apoptotic cells in the PLK2 siRNA group was significantly decreased
compared with that of the two control groups at 24 h. The
percentages of apoptotic cells in the control group, siRNA control
group and PLK2 siRNA group were 10.6, 9.14 and 2.61%, respectively.
These findings suggested that PLK2 overexpression may induce
apoptosis in SGC-7901 cells. In 2006, Syed et al (10) demonstrated that overexpression of
PLK2 in B cell lymphomas led to apoptosis (10). From the results shown in Fig. 3C in the present study, it was
inferred that PLK2 inhibited SGC-7901 cell growth through the
induction of apoptosis.
Western blot analysis of
apoptosis-associated proteins
Due to the obvious effect of PLK2 on apoptosis, the
expression of apoptosis-associated proteins, Bax and caspase-3, was
examined in SGC-7901 cells. The results revealed that PLK2 siRNA
downregulated the expression of Bax and caspase 3 (Fig. 3D). However, expression levels of
the cell cycle-associated protein CDK2 were not altered in response
to PLK2 siRNA (Fig. 3D). These
results were consistent with those of the cell cycle analysis.
Discussion
PLK2 is a member of the polo-like kinase family, the
members of which have previously been reported to regulate the cell
cycle as well as DNA damage-induced checkpoints in mammals
(10,11). PLK2 was classified as an early
growth-response gene due to its increased expression following
growth factor stimulation (17).
PLK2, as a proliferation-associated gene, has been investigated in
association with tumor treatment. In different tumors, PLK2 was
found to have a dual role as an oncogene or tumor suppressor gene.
In the present study, the expression of PLK2 was examined in
gastric cancer and gastric cancer cells. Among the 24 matched
gastric cancer samples, 17 demonstrated PLK2 overexpression. In
addition, PLK2 expression was found to be upregulated in three
gastric cancer cell lines, including BGC-823, MKN-45 and SGC-7901.
These result indicated that PLK2 was overexpressed in the majority
of the gastric cancer samples examined as well as in gastric cancer
cells. The expression levels of PLK2 may be associated with altered
tumor pathological classification and staging; however, future
studies are required in order to further examine this.
In the present study, in order to explore the role
of PLK2 in gastric cancer, the behavior of SGC-7901 cells was
investigated in response to PLK2 siRNA transfection. Cell cycle
analysis showed no differences among the number of cells in G0/G1,
S and G2/M phases between the PLK2 siRNA group and the two control
groups. These results were comparable with those reported by Burns
et al (18), in which no
significant differences were observed in the cell cycle at each
phase between U20S, H460 and HeLa cells transfected with PLK2 siRNA
and controls. This therefore suggested that PLK2 was not required
for normal progression through the cell cycle and mitosis.
Strebhardt (19) reported that the
cell cycle profiles and fractions of cells with sub-G1 DNA content
were not altered following an siRNA-mediated decrease in PLK2
expression in different cell lines; whereas, in the present study,
siRNA-mediated silencing of PLK2 improved the growth of SGC-7901
cells through decreasing apoptosis. These results therefore
indicated that SGC-7901 cell proliferation was not mediated by an
effect of PLK2 on the cell cycle but rather through decreasing
apoptosis. The results of western blot analysis of
apoptosis-associated genes were consistent with those of the cell
cycle analysis. The cell cycle-associated CDK2 protein expression
was not altered and Bax and caspase-3 were slightly
downregulated.
Notably, in the present study, PLK2 functioned as a
tumor suppressor in gastric cancer and its expression was
upregulated. However, these findings were inconsistent with a
previous study that demonstrated high gene expression levels of
PLK1 or PLK2 have been observed in pancreatic cancer (12). Conversely, in a previous study,
PLK2 overexpression in B cell lymphomas was shown to lead to
apoptosis (10). Similarly, PLK2
acted as a tumor suppressor in hepatocellular carcinoma, in which
its expression was significantly decreased (11). Gene expression in cancer is
regulated by oncogenes and tumor suppressor genes. In recent years,
with the identification of microRNAs (miRNAs) and their functions,
Li et al (20) and Miko
et al (21) reported that
PLK2 was a target gene of miR126. miRNAs are a type of small
non-coding RNA, ~22-nucleotides, which downregulate the translation
of target mRNAs (22,23). miR126 was first identified in a
tissue specific mouse screen (24)
and was encoded by intron 7 of the EGF-like domain 7 gene in
mammals and birds (25,26). miR-126 is downregulated and acts as
a tumor suppressor in stomach cancer (27). A previous study by our group also
found that miR-126 acted as a tumor suppressor in gastric
carcinoma, and PLK2 was a target gene of miR-126 (28). This therefore indicated that the
upregulation of PLK2 in gastric cancer may be associated with the
downregulation of miR-126; thus, miR-126 may also have a dominant
role in gastric cancer. However, further studies are required in
order to elucidate the role of miR-126 in gastric cancer.
In conclusion, the results of the present study
demonstrated that PLK2 functioned as a tumor inhibitor in gastric
cancer and may therefore have potential for development as a novel
gene therapy in gastric cancer.
Acknowledgements
The present study was supported by a grant from the
Fundamental Research Funds for the Central Universities of Xi’an
Jiaotong University (no. 08143014).
References
|
1
|
Jemal A, Murray T, Ward E, et al: Cancer
statistics, 2005. CA Cancer J Clin. 55:10–30. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkin DM, Bray FL and Devesa SS: Cancer
burden in the year 2000. The global picture. Eur J Cancer. 37:4–66.
2001. View Article : Google Scholar
|
|
3
|
Parkin DM: International variation.
Oncogene. 23:6329–6340. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Glover DM, Hagan IM and Tavares AA:
Polo-like kinases: a team that plays throughout mitosis. Gene Dev.
12:3777–3787. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barr FA, Sillje HH and Nigg EA: Polo-like
kinases and the orchestration of cell division. Nat Rev Mol Cell
Biol. 5:429–440. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Andrysik Z, Bernstein WZ, Deng L, et al:
The novel mouse polo-like kinase 5 responds to DNA damage and
localizes in the nucleolus. Nucleic Acids Res. 38:2931–2943. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Archambault V and Glover DM: Polo-like
kinases: conservation and divergence in their functions and
regulation. Nat Rev Mol Cell Biol. 10:265–275. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma S, Charron J, Erikson RL, et al: Role
of plk2 (Snk) in mouse development and cell proliferation. Mol Cell
Biol. 23:6936–6943. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kauselmann G, Weiler M, Wulff P, et al:
The polo-like protein kinases Fnk and Snk associate with a Ca(2+)-
and integrin-binding protein and are regulated dynamically with
synaptic plasticity. EMBO J. 18:5528–5539. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Syed N, Smith P, Sullivan A, et al:
Transcriptional silencing of polo-like kinase 2 (SNK/PLK2) is a
frequent event in B-cell malignancies. Blood. 107:250–256. 2006.
View Article : Google Scholar
|
|
11
|
Smith P, Syed N and Crook T: Epigenetic
inactivation implies a tumor suppressor function in hematologic
malignancies for Polo-like kinase 2 but not Polo-like kinase 3.
Cell Cycle. 5:1262–1264. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pellegrino R, Calvisi DF, Ladu S, et al:
Oncogenic and tumor suppressive roles of polo-like kinases in human
hepatocellular carcinoma. Hepatology. 51:857–868. 2010.PubMed/NCBI
|
|
13
|
Kothari V, Wei I, Shankar S, et al:
Outlier kinase expression by RNA sequencing as targets for
precision therapy. Cancer Discov. 3:280–293. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Radonića A, Thulkea S, Mackayb IM, et al:
Guideline to reference gene selection for quantitative real-time
PCR. Biochem Biophys Res Common. 313:856–862. 2004. View Article : Google Scholar
|
|
15
|
Li F, Jo M, Curry TE Jr and Liu J:
Hormonal induction of polo-like kinases (Plks) and impact of Plk2
on cell cycle progression in the rat ovary. PLoS One. 7:e41–e44.
2012.
|
|
16
|
Warnke S, Kemmler S, Hames RS, et al:
Polo-like kinase-2 is required for centriole duplication in
mammalian cells. Curr Biol. 14:1200–1207. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Simmons DL, Neel BG, Stevens R, et al:
Identification of an early-growth-response gene encoding a novel
putative protein kinase. Mol Cell Biol. 12:4164–4169.
1992.PubMed/NCBI
|
|
18
|
Burns TF, Fei P, Scata KA, et al:
Silencing of the novel p53 target gene Snk/Plk2 leads to mitotic
catastrophe in paclitaxel (taxol)-exposed cells. Mol Cell Biol.
23:5556–5571. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Strebhardt K: Multifaceted polo-like
kinases: drug targets and antitargets for cancer therapy. Nat Rev
Drug Discov. 9:643–660. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Z, Lu J, Sun M, et al: Distinct
microRNA expression profiles in acute myeloid leukemia with common
translocations. Proc Natl Acad Sci USA. 105:15535–15540. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miko E, Margitai Z, Czimmerer Z, et al:
miR-126 inhibits proliferation of small cell lung cancer cells by
targeting SLC7A5. FEBS Lett. 585:1191–1196. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee RC, Feinbaum RL and Ambros V: The C.
elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lagos-Quintana M, Rauhut R, Yalcin A, et
al: Identification of tissue-specific microRNAs from mouse. Curr
Biol. 12:735–739. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang S, Aurora AB, Johnson BA, et al: The
endothelial-specific microRNA miR-126 governs vascular integrity
and angiogenesis. Dev Cell. 15:261–271. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fish JE, Santoro MM, Morton SU, et al:
miR-126 regulates angiogenic signaling and vascular integrity. Dev
Cell. 15:272–284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Feng R, Chen X, Yu Y, et al: miR-126
functions as a tumour suppressor in human gastric cancer. Cancer
Lett. 298:50–63. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu LY, Wang W, Zhao LY, et al: miR-126
inhibits growth of SGC-7901 cells by synergistically targeting the
oncogenes PI3KR2 and Crk, and the tumor suppressor PLK2. Int J
Oncol. 45:1257–1265. 2014.PubMed/NCBI
|