Introduction
Gastric cancer is one of the most frequent types of
malignancy in Korea as well as in East Asia, and a number of
factors contribute to its development, including genetic or
epigenetic events. To date, numerous regulatory genes, including
adenomatous polyposis coli gene (APC), P14, P16, death-associated
protein (DAP)-kinase (DAPK), human mutL homolog 1 (hMlH1) and
runt-related transcription factor 3 (RUNX3), have been shown to be
frequently inactivated by promoter hypermethylation in gastric
adenocarcinoma tissues (1–3). Previous studies using allelic loss
mapping have identified a homozygous deletion in the 3p21.3 region
of lung, breast or kidney tumor cells (4,5).
Furthermore, certain tumor suppressor genes in this locus are known
to be commonly inactivated by promoter hypermethylation, which
results in transcription inhibition (6).
Among them, Ras-associated factor 1A (RASSF1A) has
been the most extensively investigated, and is known to be
frequently methylated in numerous types of cancer (7). Exogenous RASSF1A in RASSF1A
expression-negative cancer cell lines may promote apoptosis and
cell cycle arrest, and inhibit cell proliferation and
tumorigenicity. The RASSF1A protein forms a complex with protein
modulator of apoptosis-1 (MAP-1), which induces a conformational
change in proapoptotic proteins, such as B-cell lymphoma-associated
X, as well as mitochondrial membrane perforation and cytochrome c
release (8). Transfection of
clones stably expressing RASSF1A was shown to increase the binding
capacity of p120E4F, a transcriptional repressor, to the
cyclin A2 promoter, which in turn regulates cell cycle progression
(9). Numerous other studies have
demonstrated the molecular mechanisms underlying the regulation of
multiple biological processes by RASSF1A (8,10,11).
A number of methylation studies have demonstrated
aberrant CpG island promoter hypermethylation of RASSF1A in gastric
adenocarcinoma tissues. These studies were based on the use of
conventional methylation-specific polymerase chain reaction (MSP)
analysis. However, the methylation rate among gastric cancer tissue
samples was variously reported as low (0–7.5%) (12,13)
or moderate to high (58.7–66.7%) (3,14–16).
Furthermore, significant differences in the methylation ratio of
the RASSF1A promoter between gastric cancer and pre-cancerous
tissues adjacent to cancerous tissues (intestinal metaplasia) have
not been consistently demonstrated. An in vitro study
described an alteration of tumorigenicity, including changes in
cell proliferation, cell cycle progression and apoptosis, following
introduction of RASSF1A into expression-negative gastric cancer
cell lines (17).
The present study aimed to investigate whether
promoter methylation of RASSF1A occurred frequently in various
gastric cancer cell lines, using conventional MSP as well as
bisulfite sequencing. In addition, the demethylating agent
5-aza-2′-deoxycytidine (5-Aza-dc) was used to examine the
restoration of gene expression through alteration of methylation
status. Furthermore, the effect of transfection of RASSF1A
expression-negative gastric cancer cell lines with an
RASSF1A-expressing plasmid on cell growth and cell cycle machinery
was investigated. Finally, the difference in expression and
methylation levels of RASSF1A in pre-cancerous tissues adjacent to
gastric cancer and gastric cancer tissues was examined using
quantitative reverse transcription polymerase chain reaction
(qRT-PCR) and MSP methods.
Materials and methods
Human gastric tissues and gastric cancer
cell lines
A total of 14 samples of gastric mucosa tissue from
patients with chronic gastritis or dyspepsia (NL group), 32
pre-cancerous gastric tissue samples adjacent to cancerous tissues
from patients with gastric cancer (NT group) and 21 gastric
adenocarcinoma tissue samples (GC group) were obtained using
forceps biopsy during esophagogastroduodenal endoscopic
examination. Written informed consent was obtained from all
patients and healthy volunteers. In addition, the clinical
characteristics of gastric cancer patients were investigated,
including T stage (T1 vs. T2~4), differentiation (differentiated
vs. undifferentiated) and Lauren’s classification (intestinal vs.
diffuse). Tissue specimens were maintained in 1 ml RNA-stabilizing
reagent (RNAlater; Qiagen, Valencia, CA, USA) at room temperature
overnight and then stored at −70°C prior to use. The human gastric
cancer cell lines SNU-16, SNU-638, SNU-719, MKN-28, MKN-45,
KATO-III and AGS as well as the cervical cancer cell line Hela were
obtained from the Korean Cell Line Bank (Seoul National University,
Seoul, Korea). All cell lines were cultured in RPMI (Gibco-BRL,
Invitrogen Life Technologies, Carlsbad, CA, USA) supplemented with
10% heat-inactivated fetal bovine serum (Gibco-BRL) and
penicillin/streptomycin (1.0%; Gibco-BRL). The use of human gastric
mucosal tissue in this study was approved by the Ethics Committee
of Korea University College of Medicine, Guro Hospital (Seoul,
Korea).
RT-PCR and qRT-PCR
Total RNA was extracted from each cell line or
tissue using Trizol™ (Invitrogen Life Technologies) following the
manufacturer’s instructions. cDNA was subsequently produced using a
high capacity cDNA RT kit (Applied Biosystems, Foster City, CA,
USA) and treatment with 1 U DNAse (Promega Corp., Madison, WI,
USA). In order to analyze the expression of RASSF1A in gastric
cancer cell lines, RT-PCR was conducted by modifying a previously
described method (18). Briefly,
20 ng prepared cDNA was used to generate 25 μl PCR product using
Econo Taq® PLUS Green Master Mix (Lucigen Co.,
Middleton, WI, USA). PCR was conducted using the following
conditions: Initial denaturation at 94°C for 2 min, followed by 40
cycles of denaturation at 94°C for 15 sec, annealing at 55°C for 15
sec, extension at 72°C for 15 sec and a final extension at 72°C for
10 min. In order to detect specific gene expression of the A
isoform among the RASSF1 splicing variants (A-H), the following
primers were used: Forward, 5′-AGTGCGCGCATTGCAAGTT-3′, which
crossed the 1st and 2nd exon boundary, and reverse,
5′-GCTCGTCCACGTTCGTGTC-3′, which crossed the 2nd and 3nd exon
boundary (GenBank accession no. NM_007182). These primers were
specific to isoform A (19) and
produced products of 123 bp. GAPDH was used as a reference gene for
each sample and the primer sequences used were as follows: Forward,
5′-GGTCTCCTCTGACTTCAACA-3′ and reverse, 5′-AGCCAAATTCGTTGTCATAC-3′
(19). All primers were designed
by ourselves, with the exception of the primers for GAPDH, MSP-2
and bisulfite sequencing, and all primers were supplied by
Macrogen, Seoul, Korea. Five microliters of PCR products were
loaded on a 2% agarose gel, and positive bands were obtained by
staining with ethidium bromide (Amresco, Solon, OH, USA).
In order to compare the expression levels of the
RASSF1A gene in gastric tissues samples from the NL, NT and GC
groups, qRT-PCR was conducted as described previously (11). Briefly, following RT as described
above, 100 ng cDNA was used as a DNA template and qRT-PCR was
performed using a Takara SYBR Premix Ex Taq (Takara Bio, Inc.,
Otsu, Japan). Primer sequences were the same as those used for
RT-PCR and the PCR condition was as follows: 30 sec of initial
denaturation at 95°C, followed by 40 cycles of denaturation (95°C
for 5 sec) and annealing (60°C for 30 sec). Ct values were obtained
following PCR and normalized to those of GAPDH for quantitative
analysis.
Conventional MSP
Genomic DNA was extracted using QIAamp genomic DNA
kit (Qiagen). Bisulfite-modified genomic DNA was subsequently
produced using commercial bisulfite kit (EZ DNA methylation kit;
Zymo Research, Irvine, CA, USA) according to the manufacturer’s
instructions. Forward and reverse primers for MSP were designed to
partially cover the CpG island of RASSF1A, which is located from
+91 to +300 bp, based on the transcription initiation site (GenBank
accession number AC_002481; Fig.
1A, MSP-1). The following primers were used: Forward,
5′-TAGCGTTTAAAGTTAGCGAAGTAC-3′ and reverse,
5′-GAACTAAAAACGATAACCACGAC-3′ for methylation-specific sequences
and forward, 5′-AGTGTTTAAAGTTAGTGAAGTATGG-3′ and reverse,
5′-CAAACTAAAAACAATAACCACAAC-3′ for unmethylation-specific
sequences. The following PCR conditions were used: Initial
denaturation at 94°C for 2 min, followed by 35 cycles of
denaturation at 94°C for 30 sec, annealing at 60°C for 30 sec,
extension at 72°C for 30 sec and a final extension at 72°C for 10
min. Each primer set was able to amplify 207 bp of PCR product.
Samples were loaded on a 2% agarose gel, and visualized as
described above for RT-PCR.
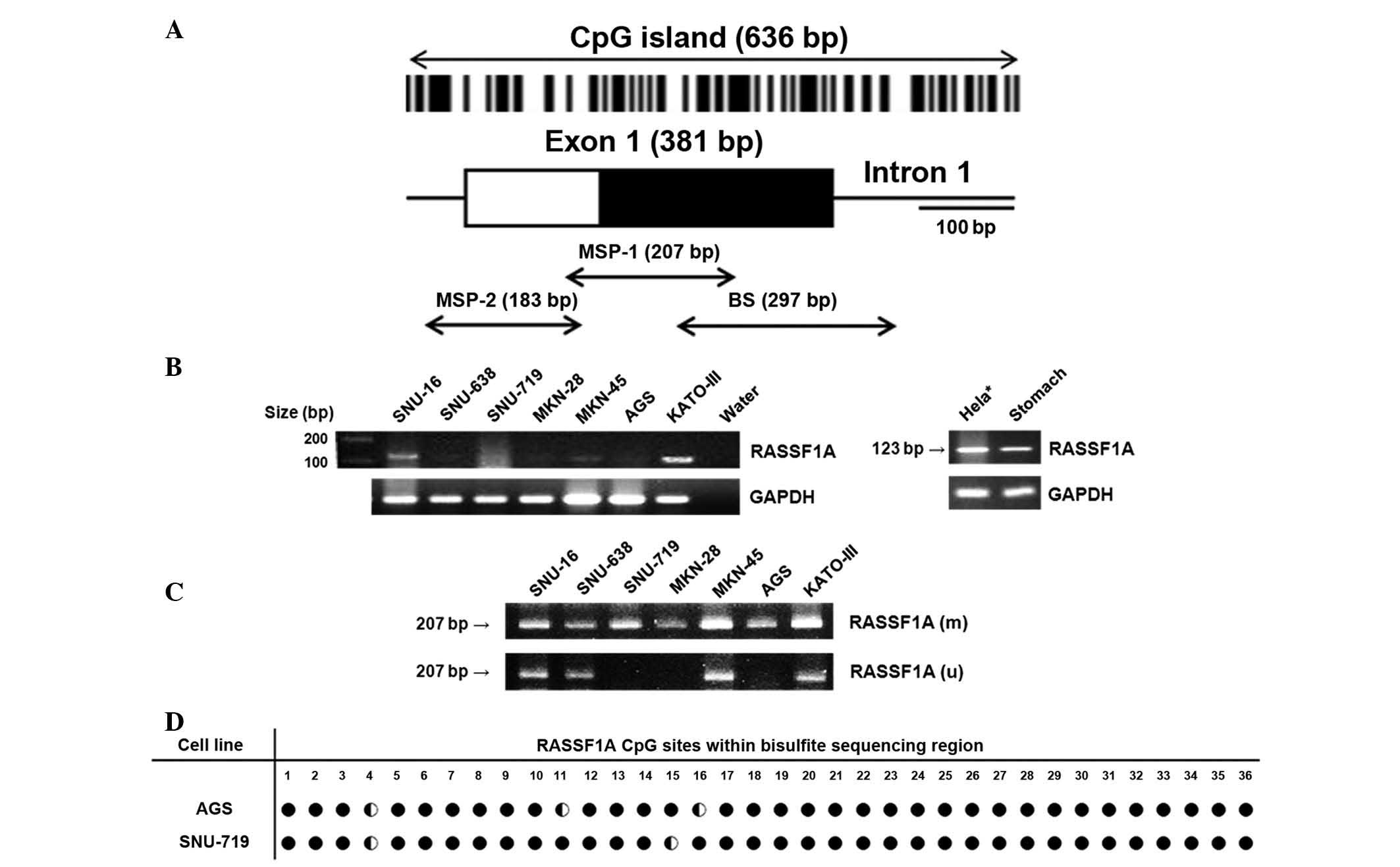 | Figure 1Gene expression and methylation of
RASSF1A in gastric carcinoma cell lines. (A) Schematic
representation of the 5′-region of human RASSF1A gene. Box
indicates exon, including coding (white) and non-coding (black)
regions. Vertical bars show CpG sites and arrows indicate regions
subject to MSP-1 (+91-+300, based on transcription initiation
site), MSP-2 (−73–+109) and bisulfite sequencing (+137–+443). (B)
Reverse transcription polymerase chain reaction showed RASSF1A gene
expression to be negative in SNU-638, SNU-719, MKN-28 and AGS cells
and positive or weakly positive in SNU-16, MKN-45 and KATO-III
cells. (C) Conventional MSP demonstrated that all gastric carcinoma
cell lines were partially (SNU-16, SNU-638, MKN-45, KATO-III) or
fully (SNU-719, MKN-28, AGS) methylated. (D) Methylation status of
RASSF1A in AGS and SNU-719 cells. Numbers show CpG sites within
bisulfite sequencing region, and ● indicates fully methylated
cytosine, whereas ◐ indicates partially methylated cytosine.
*Hela was used as a positive control for RASSF1A
expression. M, methylation-specific band; U, unmethylation-specific
band; RASSF1A, Ras association domain family 1A; MSP,
methylation-specific polymerase chain reaction. |
In order to perform conventional MSP using gastric
tissues, primers used previously were modified (Fig. 1A, MSP-2) (14,16).
The following primers were used: Forward, 5′-GGGTTTTGCGAGAGCGCG-3′
and reverse, 5′-GCTAACAAACGCGAACCG-3′ for methylation-specific
sequences and forward, 5′-GGGGTTTTGTGAGAGTGTG-3′ and reverse,
5′-CACTAACTTTAAACACTAAC-3′ for unmethylation-specific sequences.
The following PCR conditions were used: Initial denaturation at
94°C for 2 min, followed by 45 cycles of denaturation at 94°C for
30 sec, annealing at 55°C for 30 sec, extension at 72°C for 30 sec
and a final extension at 72°C for 10 min. The primers were able to
amplify 169 and 183 bp of PCR products, respectively. Samples were
reported as exhibiting positive methylation if a clear bands of the
correct size was produced by the methylation-specific primers.
Peripheral blood mononuclear cells for methylated and unmethylated
control genomic DNA were obtained from the healthy volunteers in
the present study.
Demethylation with 5-Aza-dc
SNU-719, MKN-28 and AGS cells were seeded at a
density of 1×106 cells/ml in a 100-mm diameter dish for
24 h. The following day, cells were treated with 5 μmol/l of the
DNA demethylating agent, 5-Aza-dc (Sigma-Aldrich, St. Louis, MO,
USA) and this was continued for four consecutive days. Cells were
then harvested in order to extract total RNA, genomic DNA and
protein for RT-PCR, MSP and western blotting, respectively.
Bisulfite sequencing
Bisulfite-modified DNA was amplified by primers
anchoring from +137 to +443 bp relative to the transcription
initiation site (Fig. 1A; BS). The
following primer sequence were used: Forward,
5′-GGGGAGTTTGAGTTTATTGAGTT-3′ and reverse,
5′-CTACCCCTTAACTACCCCTTCC-3′. The resulting 297 bp PCR products
were cloned into a pCR2.1-TOPO vector (Invitrogen Life
Technologies, Carsbad, CA, USA) and three to five clones were
randomly obtained for the subsequent sequencing analysis.
Sequencing reactions were performed in an MJ Research PTC-225
Peltier Thermal Cycler using an ABI PRISM® BigDye™
Terminator Cycle Sequencing kit with AmpliTaq® DNA
polymerase (FS enzyme; Applied Biosystems) according to the
manufacturer’s instructions.
Transfection
The pCIneoFLAG-His6-RASSF1A plasmid was purchase
from Adgene (#37016; Cambridge, MA, USA). The backbone of the
plasmid vector pCIneoFLAG-His6 was modified by digesting the
pCIneoFLAG-His6-RASSF1A plasmid with EcoRI (5) and SaI (3), and then ligating with DNA polymerase,
which were all purchase from Promega Corp. For the transfection,
AGS and SNU-719 cells were cultured for 24 h until they reached
70–80% confluence and transfected with 2 μg plasmid using
Lipofectamine 2000 (Invitrogen Life Technologies) according to the
manufacturer’s instructions. Cells were collected at the indicated
time-points for further functional analyses.
Water-soluble tetrazolium salt-1 (WST-1)
cell proliferation assay
In order to quantify the inhibitory effect of
RASSF1A expression on cell proliferation, a commercial WST-1 assay
kit (EZ-CYTOX, Dogen, Seoul, Korea) was used according to the
manufacturer’s instructions (20).
Briefly, 1×104 AGS and SNU-719 cells per well were
cultured in 96 wells at 37°C for 24 h, prior to transfection with
RASSF1A-plasmid or empty plasmid for 24, 48 or 72 h. Untransfected
cells were also cultured for the same time period as a control.
Following transfection, each well was treated with 10 μl WST for 4
h and absorbance at 450 nm was measured using an ELISA reader
(Epoch, serial no. 1212265; BioTek Instruments, Seoul, Korea). All
experiments were performed in triplicate.
Western blot analysis
A mouse monoclonal antibody against human RASSF1A
(NBP2-03644) was obtained from Novus Biologicals (Littleton, CO,
USA), and rabbit polyclonal antibodies against cyclin D1 (sc-718),
p27 (sc-527), p-Rb (Ser 608; sc-56174) and β-actin (sc-47778) were
obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). A
total of 80–100 μg cytoplasmic proteins were extracted using
CelLytic-M (C2978; Sigma-Aldrich) with a protease inhibitor
cocktail (Complete Mini; Roche Diagnostics, Manneheim, Germany).
Primary antibodies were diluted at 1:1,000 in Tris-buffered saline
with Tween20 (Biosesang Inc., Seongnam, Korea) containing 5%
non-fat milk (BD DifcoTM, Sparks, MD, USA). Probed
membranes were incubated overnight at 4°C with the above primary
antibodies, each at a dilution of 1:1,000. The membranes were then
incubated with 1:1,000 goat anti-mouse or anti-rabbit IgG secondary
antibodies (170-6516 and 170-6515; Bio-Rad, Hercules, CA, USA) for
1 h at room temperature. Protein bands were detected by exposing
the membrane to enhanced chemiluminescence (Western Lightning
Plus-ECL; PerkinElmer, Inc., Waltham, MA, USA) for 1 min.
Statistical analysis
Relative mRNA (mRNA) expression levels of RASSF1A
were normalized to those of GAPDH. Continuous data are presented as
the median ± interquartile range (log2 ratio) and categorical data
as the percentage (frequency) of methylation. Stayistical analysis
was performed using SPSS 19.0 software (International Business
Machines, Armonk, NY, USA). Analysis of variance was performed for
continuous data and Pearson’s χ2 method was used for
categorical data. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression and methylation status of
RASSF1A in gastric carcinoma cell lines
Expression of the RASSF1A gene in seven gastric
carcinoma cell lines was investigated using RT-PCR. Among the cell
lines examined, four (SNU-638, SNU-719, MKN-28 and AGS) had no
detectable expression of RASSF1A. By contrast, SNU-16 and KATO-III
cells exhibited positive expression and MKN-45 cells exhibited
weakly positive expression (Fig.
1B). The methylation status in the seven gastric carcinoma cell
lines was then investigated using a conventional MSP method. Only
methylation-specific bands were detected in SNU-719, MKN28 and AGS
cells (indicating full methylation of the promoter). These cell
lines did not express RASSF1A. By contrast, SNU-16, MKN-45 and
KATO-III cells exhibited methylation- and unmethylation-specific
bands (indicating partial methylation). These cell lines all
exhibited positive or weakly positive expression of RASSF1A.
SNU-638 cells also exhibited methylation- and
unmethylation-specific bands. However, this cell line did not
express detectable levels of RASSF1A (Fig. 1C). These results indicated that the
methylation status of the CpG island promoter is associated with
undetectable or reduced expression of RASSF1A.
In order to validate the promoter hypermethylation
of RASSF1A in gastric cancer cell lines, AGS and SNU-719 cells,
which did not express detectable levels of RASSF1A and exhibited
only methylation-specific band in the MSP experiments, were
selected and used to conduct bisulfite sequencing, using primers
targeting regions adjacent to MSP-1 primers. Bisulfite sequencing
demonstrated that the majority of CpG sites were densely methylated
in AGS and SNU-719 cell lines (Fig.
1D).
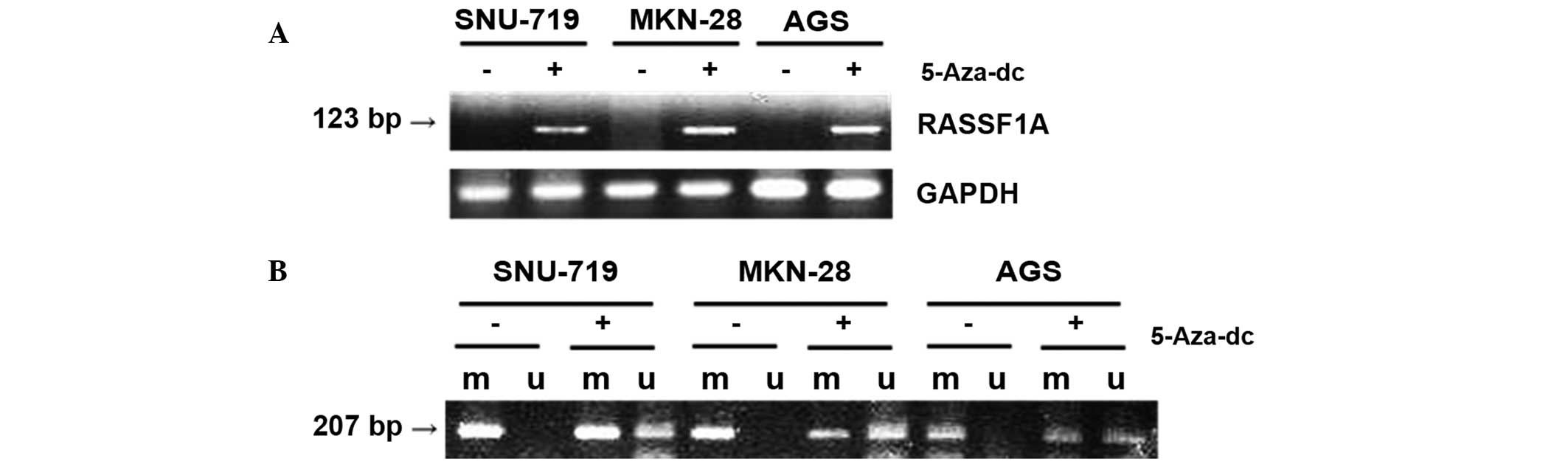
Altered expression and MSP pattern of
RASSF1A following treatment with 5-Aza-dc in gastric carcinoma cell
lines
Three gastric cancer cell lines (SNU-719, MKN-28 and
AGS) were selected, which were negative for RASSF1A expression, as
detected using RT-PCR, and only exhibited methylation-specific
bands according to MSP analysis (Fig.
1). These cells were treated with 5 μmol/l 5-Aza-dc for four
days. Following treatment, RT-PCR was used to demonstrate that
RASSF1A was now expressed in these cells (Fig. 2A). MSP performed following
treatment with 5-Aza-dc demonstrated unmethylation-specific bands
in all three cell lines and also thinner methylation-specific bands
in the MKN-28 and AGS cell lines, compared with the untreated
control (Fig. 2B). These findings
also supported the hypothesis that aberrant methylation of RASSF1A
induced transcription inhibition in gastric carcinoma cell
lines.
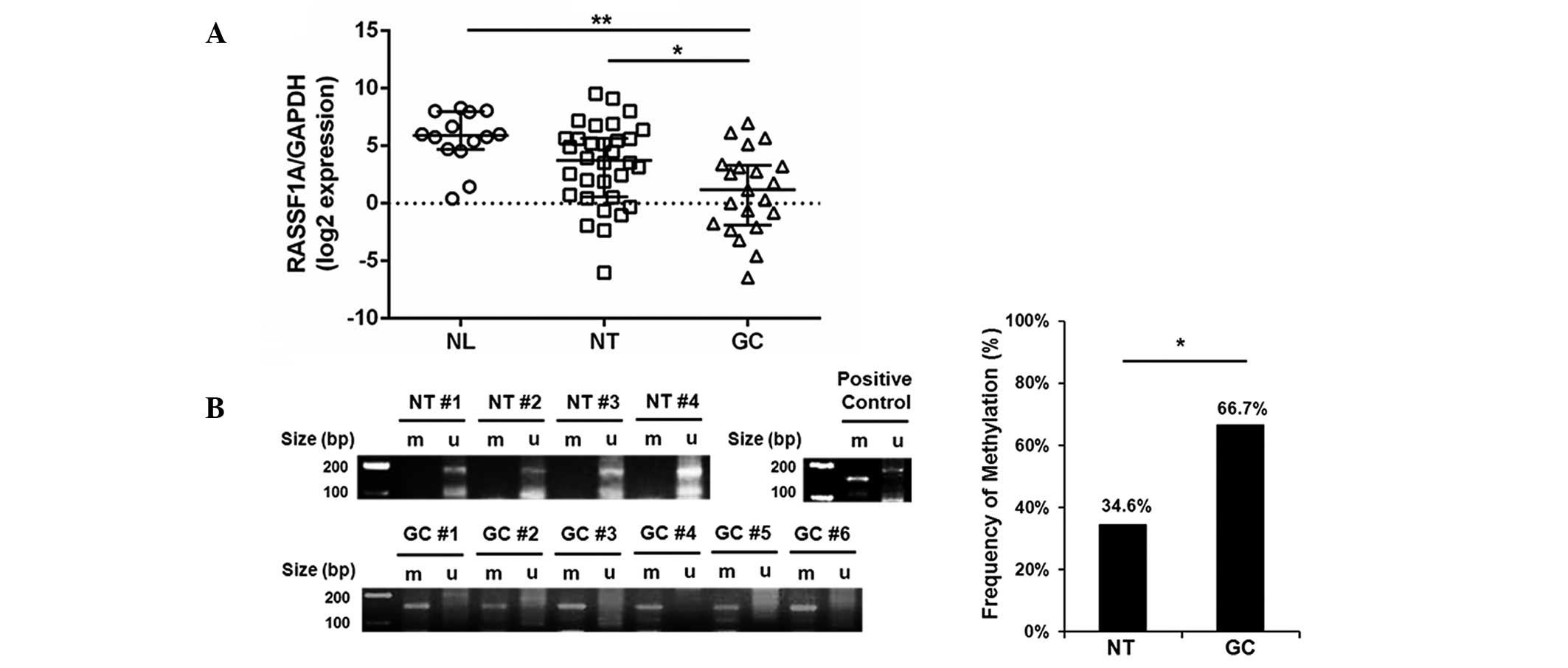
Reduced gene expression and increased
methylation rate in gastric carcinomas
In order to investigate RASSF1A expression and
methylation patterns in gastric tissues, the mRNA levels of RASSF1A
in the NL, NT and GC groups was compared using qRT-PCR. All tissues
in the NL group displayed evidence of chronic gastritis only;
however, those in the NT group had intestinal metaplasia, a
pre-cancerous stage of gastric adenocarcinoma. As hypothesized,
RASSF1A expression was significantly reduced in the GC group
compared with that in the NL and NT groups (P=0.001 and 0.032,
respectively; Fig. 3A).
Furthermore, 14 out of 21 gastric carcinoma tissue samples (66.7%)
in the GC group were methylation-positive, whereas only 9 out of 26
non-tumorous tissue samples (34.6%) were positive in the NT group
(P=0.029; Fig. 3B). These results
indicated that RASSF1A expression was significantly reduced in
association with promoter hypermethylation in gastric carcinoma
compared with that in pre-cancerous adjacent tissues. This process
may contribute to gastric tumorigenesis.
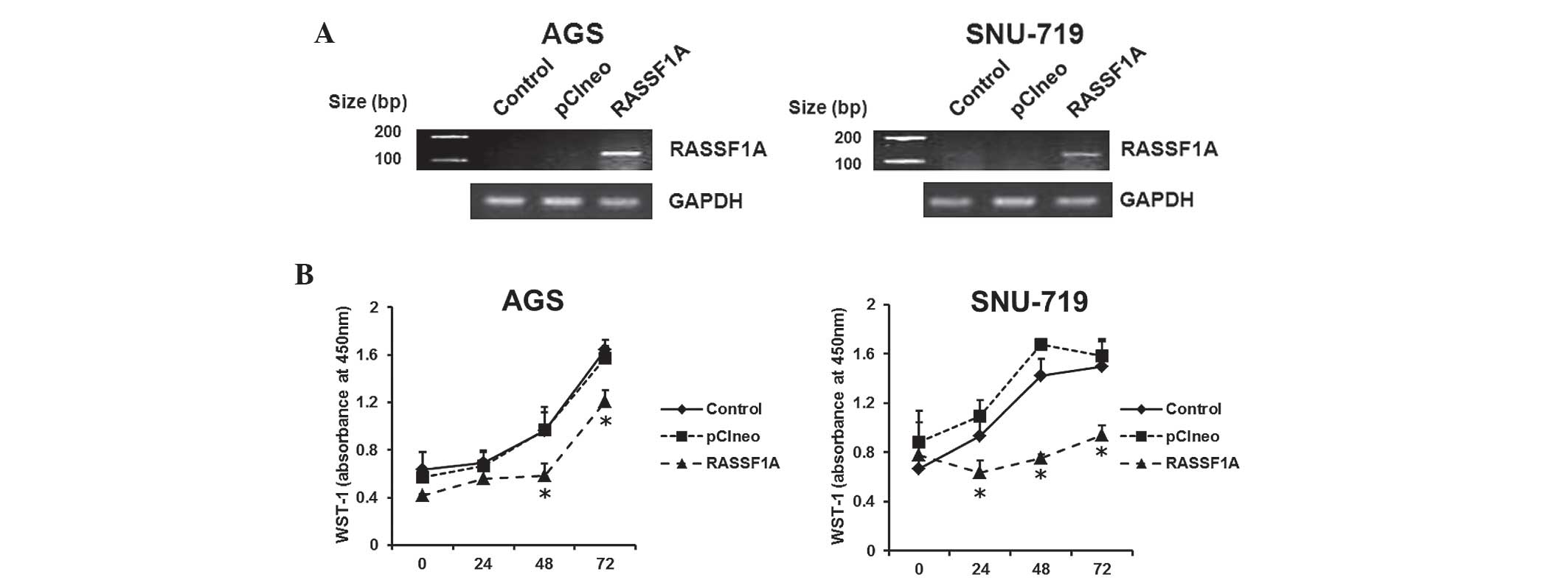
Exogenous RASSF1A expression inhibits
proliferation of gastric carcinoma cell lines
Two RASSF1A expression-negative cell lines (AGS and
SNU-719) were selected in order to investigate the
anti-proliferative effects of RASSF1A in gastric carcinoma cells.
Following transfection of the RASSF1A expression plasmid for two
days, a renewed expression of RASSF1A was demonstrated in AGS and
SNU-719 cells by RT-PCR. This effect was not observed in untreated
cells or cells transfected with the backbone-modified plasmid
(Fig. 4A). RASSF1A expression
plasmids were then transfected into AGS and SNU-719 cells for 24,
48 or 72 h. A WST-1 assay was then conducted, which demonstrated
significant inhibition of cell proliferation at two days (for AGS)
or one day (for SNU-719) following transfection, compared with that
of untreated cells or cells transfected with the backbone plasmid
(Fig. 4B). These findings
indicated that epigenetic silencing of RASSF1A may induce cell
proliferation in gastric carcinoma cell lines.
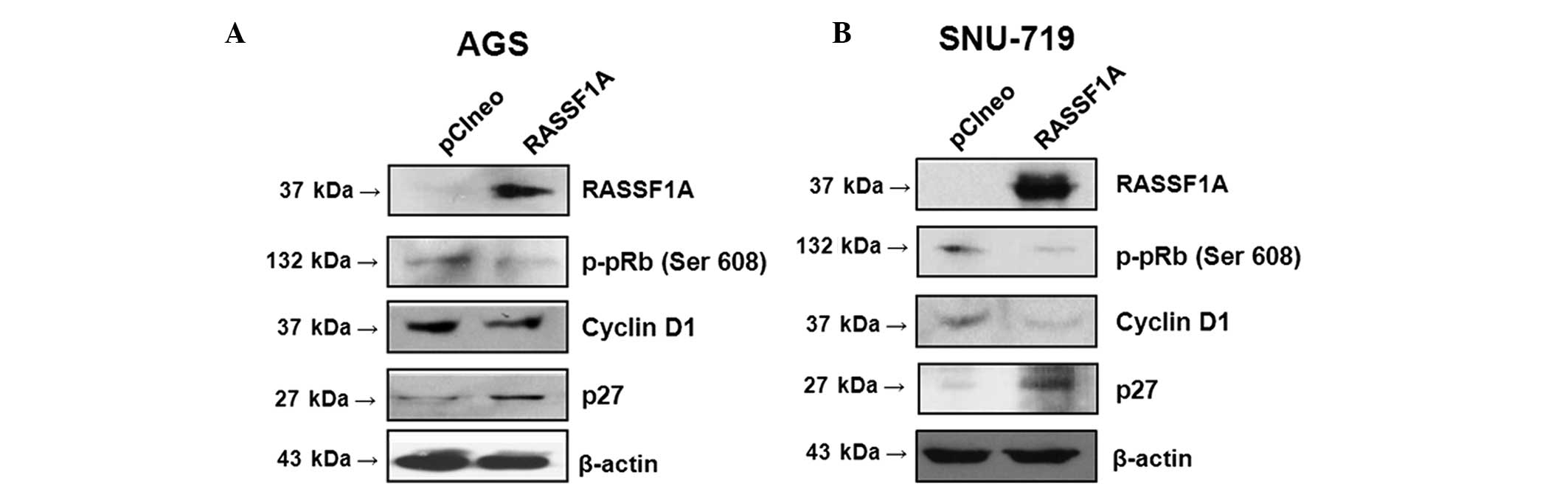
Exogenous RASSF1A expression modulates
cell cycle machinery proteins in gastric carcinoma cell lines
A western blot assay was performed in order to
investigate the effect of RASSF1A on the regulation of cell cycle
machinery proteins. AGS and SNU-719 cell lines, which were
transfected with RASSF1A expression plasmid, upregulated the
expression of p27, an inhibitory regulator of the G1/S
transition, and downregulated that of cyclin D1, an inducer of
G1/S cell cycle progression, as well as that of
phosphorylated retinoblastoma protein (p-pRb), which is
phosphorylated and inactivated by the cyclin D1-cyclin-dependent
kinase 4 (CDK4) complex (Fig. 5A and
B) (21). These findings
suggested that RASSF1A may inhibit the Ras/Raf kinase pathway, in
turn promoting cell cycle arrest in G1 phase and
inhibiting cell cycle progression in gastric carcinoma cell
lines.
Discussion
In the present study, ~2/3 of the samples in the GC
group exhibited methylation, which was significantly higher than
the percentage in the NT group. This was correlated with the
downregulation of RASSF1A expression, which was significantly
reduced in the GC group compared with that in the NL or NT group. A
number of studies investigating the hypermethylation of RASSF1A in
gastric carcinoma reported a higher frequency of promotor
methylation in gastric cancer tissues than that in the surrounding
non-cancerous tissues (3,16). However, other studies have failed
to reproduce these (12,13,22).
The latter studies reported a relatively low methylation rate in
the gastric carcinoma tissues which were examined (0–7.5%) as well
as in adjacent non-cancerous tissues. The results of the present
study support the hypothesis that promoter methylation frequently
occurs in gastric carcinoma tissues and that levels of methylation
are higher than those in non-cancerous tissues. The present study
also examined differences in various characteristics of gastric
carcinoma, including tumor invasion (T1 vs. T2–4), differentiation
(differentiated vs. undifferentiated) or Lauren’s classification
(intestinal or diffuse) (23),
between methylated and unmethylated samples. However, no
significant differences were detected (data not shown), which may
have partly been a result of the small sample size. A previous
study demonstrated significantly reduced expression of the RASSF1A
gene in advanced stage and undifferentiated gastric carcinomas
(24). However, a separate study
of methylation using surgical specimens of gastric carcinoma did
not detect any significant differences in the frequency of
methylation according to tumor invasion, Lauren’s classification or
distant metastasis (3). Further
research is required to assess the association between
clinico-pathological characteristics and RASSF1A promoter
hypermethylation in gastric carcinoma.
At the in vitro level, a number of gastric
carcinoma cell lines were shown to be RASSF1A expression-negative.
These lines all exhibited a methylation-specific band only in the
MSP analysis. Gastric carcinoma cell lines that exhibited partial
methylation of the RASSF1A promoter were undifferentiated and
derived from metastatic carcinoma (SNU-16, SNU-638, MKN-45 and
KATO-III), whereas cells that exhibited full methylation were
predominantly well-differentiated (SNU-719 and MKN-28) or from a
primary carcinoma that was not known to have metastasized (SNU-719
and AGS). The role of RASSF1A in the differentiation or metastasis
of gastric carcinoma requires further investigation. The advantages
of the present study were that it was able to demonstrate renewed
expression in association with an altered methylation pattern of
RASSF1A in gastric carcinoma cell lines by utilizing a
demethylating agent. Numerous in vitro studies have reported
an altered MSP pattern of RASSF1A following treatment with 5-Aza-dc
in other cancer cell lines (25,26).
However, few studies have demonstrated this finding in various
gastric carcinoma cell lines, and, to the best of our knowledge,
only one previous study has shown the effect of 5-Aza-dc and sodium
butyrate on the expression of RASSF1A in gastric cancer cell lines
(27). Following treatment with
the demethylating agent, a change in the MSP pattern in all three
fully methylated gastric carcinoma cell lines (SNU-719, MKN-28,
AGS) was observed, and this was correlated with gene expression.
This suggested that transcription inhibition of RASSF1A is closely
associated with CpG island promoter hypermethylation in gastric
carcinoma cells.
Numerous studies have investigated the function of
RASSF1A in cancer cell lines, and it has been shown that RASSF1A
inhibited cell proliferation and tumor growth (28). The two common pathways which
RASSF1A is hypothesized to modulate in cancer cells are apoptosis
and cell cycle progression. Within these pathways, cyclin D1 has
been identified as a cell cycle activator, which is regulated by
RASSF1A (29,30). It has been suggested that the
amplification of cyclin D1 may remove p27 from CDK2 and contribute
to cell cycle progression. pRb is also linked to, and inactivated
by cyclin D1 (31). The present
study demonstrated that exogenous RASSF1A significantly inhibited
cell proliferation in AGS and SNU-719 cells, as expected. It was
also shown to upregulate p27 and downregulate cyclin D1 and p-pRb.
To the best of our knowledge, this was a novel finding, which
indicated that cell cycle machinery proteins, in particular p27,
may be modulated by RASSF1A in gastric cancer cell lines. Given
that p27 is neither inactivated nor hypermethylated in
gastrointestinal cancer cell lines (32,33),
unlike inhibitor of CDK4 family proteins, such as p16 (34,35),
it is likely that p27 expression was restored by the exogenous
introduction of RASSF1A into expression-negative gastric carcinoma
cell lines.
In conclusion, the results of the present study
indicated that the CpG island promoter hypermethylation of RASSF1A
commonly occurs in gastric carcinoma cell lines as well as in
gastric carcinoma tissues. This was supported by the observation
that treatment with the demethylating agent 5-Aza-dc altered the
expression and methylation pattern of RASSF1A in RASSF1A-negative
cell lines. Exogenous expression of RASSF1A by plasmid transfection
significantly inhibited cell proliferation and modulated the
expression of a cell cycle activator (cyclin D1) and an inhibitor
(p27). Future studies are required to elucidate the function of
RASSF1A in gastric carcinogenesis by investigating
clinicopathological variables influenced by RASSF1A expression and
hypermethylation in gastric carcinoma tissues, as well as by
identifying target genes through establishing stably
RASSF1A-expressing gastric cell lines.
Acknowledgements
This study was supported by grants from Korea
University (grant no. R1111971) and PacificPharma Corp. (grant no.
Q1307141; Seoul, Korea).
Abbreviations:
|
RASSF1A
|
Ras association domain family 1A
gene
|
|
MSP
|
methylation-specific polymerase chain
reaction
|
|
5-Aza-dc
|
5-Aza-2′-deoxycytidine
|
|
qRT-PCR
|
quantitative reverse transcription
polymerase chain reaction
|
|
WST
|
water-soluble tetrazolium salt
|
References
|
1
|
Kang GH, Shim YH, Jung HY, Kim WH, Ro JY
and Rhyu MG: CpG island methylation in premalignant stages of
gastric carcinoma. Cancer Res. 61:2847–2851. 2001.PubMed/NCBI
|
|
2
|
Bernal C, Aguayo F, Villarroel C, et al:
Reprimo as a potential biomarker for early detection in gastric
cancer. Clin Cancer Res. 14:6264–6269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ksiaa F, Ziadi S, Amara K, Korbi S and
Trimeche M: Biological significance of promoter hypermethylation of
tumor-related genes in patients with gastric carcinoma. Clin Chem
Acta. 404:128–133. 2009. View Article : Google Scholar
|
|
4
|
Lerman MI and Minna JD: The 630-kb lung
cancer homozygous deletion region on human chromosome 3p21.3:
identification and evaluation of the resident candidate tumor
suppressor genes. The international lung cancer chromosome 3p213
tumor suppressor gene consortium. Cancer Res. 60:6116–6133.
2000.PubMed/NCBI
|
|
5
|
Hesson LB, Cooper WN and Latif F:
Evaluation of the 3p21.3 tumour-suppressor gene cluster. Oncogene.
26:7283–7301. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qu Y, Dang S and Hou P: Gene methylation
in gastric cancer. Clinica Chim Acta. 424:53–65. 2013. View Article : Google Scholar
|
|
7
|
van der Weyden L and Adams DJ: The
Ras-association domain family (RASSF) members and their role in
human tumourigenesis. Biochim Biophys Acta. 1776:58–85.
2007.PubMed/NCBI
|
|
8
|
Baksh S, Tommasi S, Fenton S, et al: The
tumor suppressor RASSF1A and MAP-1 link death receptor signaling to
Bax conformational change and cell death. Mol Cell. 18:637–650.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ahmed-Choudhury J, Agathanggelou A, Fenton
SL, et al: Transcriptional regulation of cyclin A2 by RASSF1A
through the enhanced binding of p120E4F to the cyclin A2 promoter.
Cancer Res. 65:2690–2697. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Korah R, Healy JM, Kunstman JW, et al:
Epigenetic silencing of RASSF1A deregulates cytoskeleton and
promotes malignant behavior of adrenocortical carcinoma. Mol
Cancer. 12:872013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Palakurthy RK, Wajapeyee N, Santra MK, et
al: Epigenetic silencing of the RASSF1A tumor suppressor gene
through HOXB3-mediated induction of DNMT3B expression. Mol Cell.
36:219–230. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kang GH, Lee S, Kim JS and Jung HY:
Profile of aberrant CpG island methylation along the multistep
pathway of gastric carcinogenesis. Lab Invest. 83:635–641. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zou XP, Zhang B, Zhang XQ, Chen M, Cao J
and Liu WJ: Promoter hypermethylation of multiple genes in early
gastric adenocarcinoma and precancerous lesions. Hum Pathol.
40:1534–1542. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ye M, Xia B, Guo Q, Zhou F and Zhang X:
Association of diminished expression of RASSF1A with promoter
methylation in primary gastric cancer from patients of central
China. BMC Cancer. 7:1202007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou SL, Cui J, Fan ZM, et al:
Polymorphism of A133S and promoter hypermethylation in Ras
association domain family 1A gene (RASSF1A) is associated with risk
of esophageal and gastric cardia cancers in Chinese population from
high incidence area in northern China. BMC Cancer. 13:2592013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo W, Dong Z, Chen Z, et al: Aberrant CpG
island hypermethylation of RASSF1A in gastric cardia
adenocarcinoma. Cancer Invest. 27:459–465. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deng ZH, Wen JF, Li JH, Xiao DS and Zhou
JH: Activator protein-1 involved in growth inhibition by RASSF1A
gene in the human gastric carcinoma cell line SGC7901. World J
Gastroenterol. 14:1437–1443. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Feng Y, Zhang S and Huang X: A robust
TALENs system for highly efficient mammalian genome editing. Sci
Rep. 4:36322014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rivera MN, Kim WJ, Wells J, et al: The
tumor suppressor WTX shuttles to the nucleus and modulates WT1
activity. Proc Natl Acad Sci USA. 106:8338–8343. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang Z, Lee H, Lee E, Kang SK, Nam JM and
Lee M: Responsive nematic gels from the self-assembly of aqueous
nanofibres. Nat Commun. 2:4592011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kitagawa M, Higashi H, Jung HK, et al: The
consensus motif for phosphorylation by cyclin D1-Cdk4 is different
from that for phosphorylation by cyclin A/E-Cdk2. EMBO J.
15:7060–7069. 1996.PubMed/NCBI
|
|
22
|
To KF, Leung WK, Lee TL, et al: Promoter
hypermethylation of tumor-related genes in gastric intestinal
metaplasia of patients with and without gastric cancer. Int J
Cancer. 102:623–628. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu B, El Hajj N, Sittler S, Lammert N,
Barnes R and Meloni-Ehrig A: Gastric cancer: Classification,
histology and application of molecular pathology. J Gastrointes
Oncol. 3:251–261. 2012.
|
|
24
|
Byun DS, Lee MG, Chae KS, Ryu BG and Chi
SG: Frequent epigenetic inactivation of RASSF1A by aberrant
promoter hypermethylation in human gastric adenocarcinoma. Cancer
Res. 61:7034–7038. 2001.PubMed/NCBI
|
|
25
|
Reu FJ, Leaman DW, Maitra RR, et al:
Expression of RASSF1A, an epigenetically silenced tumor suppressor,
overcomes resistance to apoptosis induction by interferons. Cancer
Res. 66:2785–2793. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jung Y, Park J, Kim TY, et al: Potential
advantages of DNA methyltransferase 1 (DNMT1)-targeted inhibition
for cancer therapy. J Mol Med (Berl). 85:1137–1148. 2007.
View Article : Google Scholar
|
|
27
|
Shen WJ, Dai DQ, Teng Y and Liu HB:
Regulation of demethylation and re-expression of RASSF1A gene in
gastric cancer cell lines by combined treatment of 5-Aza-CdR and
NaB. World J Gastroenterol. 14:595–600. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Agathanggelou A, Cooper WN and Latif F:
Role of the Ras-association domain family 1 tumor suppressor gene
in human cancers. Cancer Res. 65:3497–3508. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shivakumar L, Minna J, Sakamaki T, Pestell
R and White MA: The RASSF1A tumor suppressor blocks cell cycle
progression and inhibits cyclin D1 accumulation. Mol Cellular Biol.
22:4309–4318. 2002. View Article : Google Scholar
|
|
30
|
Agathanggelou A, Bieche I, Ahmed-Choudhury
J, et al: Identification of novel gene expression targets for the
Ras association domain family 1 (RASSF1A) tumor suppressor gene in
non-small cell lung cancer and neuroblastoma. Cancer Res.
63:5344–5351. 2003.PubMed/NCBI
|
|
31
|
Arteaga CL: Cdk inhibitor p27Kip1 and
hormone dependence in breast cancer. Clin Cancer Res. 10:368S–371S.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shin JY, Kim HS, Park J, Park JB and Lee
JY: Mechanism for inactivation of the KIP family cyclin-dependent
kinase inhibitor genes in gastric cancer cells. Cancer Res.
60:262–265. 2000.PubMed/NCBI
|
|
33
|
Xiong H, Chen ZF, Liang QC, et al:
Inhibition of DNA methyltransferase induces G2 cell cycle arrest
and apoptosis in human colorectal cancer cells via inhibition of
JAK2/STAT3/STAT5 signalling. J Cell Mol Med. 13:3668–3679. 2009.
View Article : Google Scholar
|
|
34
|
Sun Y, Deng D, You WC, et al: Methylation
of p16 CpG islands associated with malignant transformation of
gastric dysplasia in a population-based study. Clin Cancer Res.
10:5087–5093. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu J, Xie YS, Wang FL, Zhang LJ, Zhang Y
and Luo HS: Cytotoxicity of 5-Aza-2′-deoxycytidine against gastric
cancer involves DNA damage in an ATM-P53 dependent signaling
pathway and demethylation of P16 (INK4A). Biomed Pharmacother.
67:78–87. 2013. View Article : Google Scholar
|